ABSTRACT
Percutaneous lung biopsy is one of the most common procedures performed in radiology departments and the minimally invasive gold standard for the histopathologic investigation of lung masses. Compared with other percutaneous biopsy procedures, lung biopsy carries a higher risk of potential complications, including occasional reports of death. Radiologists should be able to quickly recognize complications, provide required acute care, manage the patient to complete resolution, and obtain a consultation from colleagues in surgery and medicine when indicated. To this end, standing protocols for the performance of lung biopsy and the management of complications such as pneumothorax should be in place prior to performing percutaneous lung biopsy.
Keywords: Lung biopsy, pneumothorax, complications, chest tube
Percutaneous lung biopsy is considered a safe and effective method for obtaining a tissue diagnosis in patients with lung masses. Reported complications include pneumothorax, hemoptysis, air embolism, seeding of the biopsy tract, and death. The most common complication, pneumothorax, is easily treatable by radiologists and is typically associated with no long-term sequelae. Despite wide variability in the incidence of complications, retrospective studies have uncovered consistent factors that directly affect the risk of adverse sequelae. This article provides a review of those factors most critical to the safe performance of percutaneous lung biopsy. In addition, measures for the recognition and management of complications are discussed.
NONVASCULAR COMPLICATIONS
Pneumothorax
The most common complication of chest biopsy is development of a pneumothorax. The largest retrospective series placed the incidence of pneumothorax at 20.5% and the incidence of pneumothorax requiring chest drainage at 3.1%.1 However, the reported incidence of pneumothorax is highly variable, most likely the result of multiple factors such as differences in patient population, procedural technique, operator experience, and methods of detection. Factors predisposing patients to this complication can be divided into those related to underlying pathology and those related to procedural technique.
Predisposing factors related to underlying pathology of the patient population include bullae, emphysema, ventilatory obstruction as evidenced by increased forced expiratory volume to vital capacity ratio, deep lesions, smaller lesions, and lesions abutting fissures.2,3 Deeper, smaller lesions are associated with higher risk presumably due to the greater degree of manipulation required during needle advancement and the occasional crossing of more than one pleural surface. Prior surgery and pleural thickening may result in fibrous adhesion of the visceral and parietal pleura, which may provide a protective benefit against pleural tears and subsequent pneumothorax.2 When consulted for the performance of lung biopsy, it is imperative to consider alternatives to the percutaneous route when the underlying pathology places the patient at risk, including bronchoscopic biopsy and surgical biopsy via limited thoracotomy. In addition, if pulmonary function tests are not available, an assessment of the patient's ability to tolerate a pneumothorax based on imaging findings such as bullous disease4 is important for the purposes of informed consent and procedure preparation.
Predisposing factors related to procedural technique include a more acute angle between the needle and the pleura,2,3 a greater number of needle passes, and crossing of more than one pleural surface. We recommend an approach that is orthogonal to the pleural surface and crosses the shortest pathway to the lesion. Once the lesion has been accessed, dwell time2 and introducer size appear to have no effect on the rate of pneumothorax, allowing the radiologist to take multiple samples through a larger introducer with relative impunity. For this reason, we routinely pass an 18- to 19-G introducer needle to a point abutting the lesion and then take aspiration and core samples through the introducer using 20- to 21-G needles and biopsy guns. To avoid tearing the pleura, the tip of the introducer needle should never linger at the pleural surface during image-guided advancement. A rapid pass across the pleura is ideal.
When a pneumothorax does occur, it is the responsibility of the radiology staff to diagnose this complication, provide appropriate treatment in the acute setting, and manage the patient to complete resolution. During the course of a busy day in radiology, it is easy to overlook clinical deterioration of these patients. Enlargement of the pneumothorax or development of a tension pneumothorax, even after placement of a chest drain (Fig. 1), can lead to rapid respiratory collapse. Patients should receive immediate oxygen by nasal cannula, typically at a rate of 2 to 4 L/s, and heart rate, respiratory rate, blood pressure, and oxygen saturation should be monitored. Iatrogenic pneumothorax is such a common complication after lung biopsy that its management should be easily within the capabilities of radiologists. Asymptomatic, small (less than 20%) pneumothoraces may be managed with observation alone in an inpatient setting where development of a tension pneumothorax can be quickly addressed. Outpatient management can also be appropriate if: (1) the patient has an interval radiograph (at least 3 hours apart) showing stability or improvement in the pneumothorax, (2) the patient has adequate cardiopulmonary reserve such that he or she would have time to get help should lung collapse progress, and (3) there is an adequate social support system (i.e., a working telephone and a responsible adult to stay with the patient). Complete resolution of the pneumothorax can be confirmed by follow-up chest radiograph.
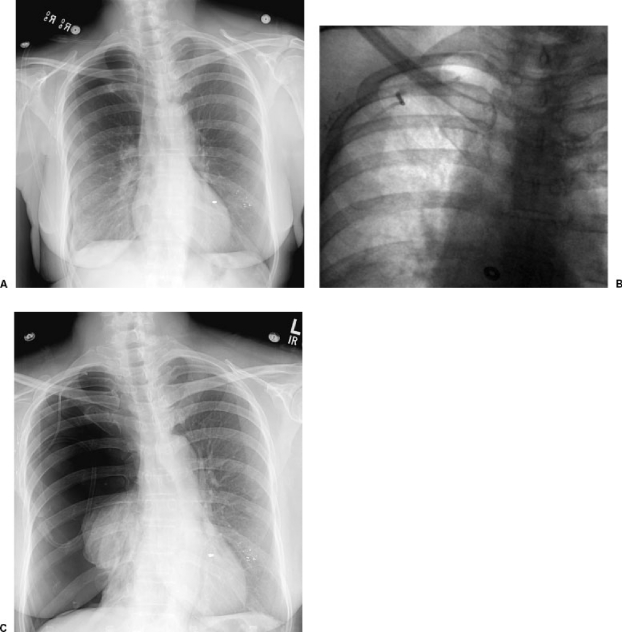
Figure 1.
Development of a tension pneumothorax following chest tube obstruction. (A) Chest radiograph following percutaneous biopsy shows a moderate apical pneumothorax. (B) Follow-up fluoroscopic image following placement of a Turner pigtail catheter (Cook, Bloomington, IN) and reexpansion of the lung. (C) Follow-up radiograph after the patient complained of chest tightness showing a tension pneumothorax. The chest tube was cleared, repositioned, and eventually removed following resolution of the pneumothorax.
Tension pneumothorax or an enlarging pneumothorax can develop as a result of delayed treatment, inadvertent kinking or obstruction of a chest drain (Fig. 1), or misplacement of a chest drain (Fig. 2). The development of tension pneumothorax or collapse greater than 20% should be treated with chest drainage. Small-bore, pigtail, or straight catheters (Fig. 3) placed anterioapically are appropriate and are typically placed between the first and second interspace. Connection to a one-way valve (Heimlich or pneumostat) is often sufficient. For cases requiring suction drainage, after reexpansion of the lung by chest radiograph and termination of the air leak under water seal, the catheter can be placed to one-way valve. In most cases, we obtain a chest radiograph 2 hours after capping the tube to verify stability and the absence of recurrence prior to removing the tube. Safe outpatient management of chest drains requires excellent education and patient understanding that is often difficult to accomplish in one session. Therefore, overnight admission for pain control and tube management is recommended for patients after chest drainage.
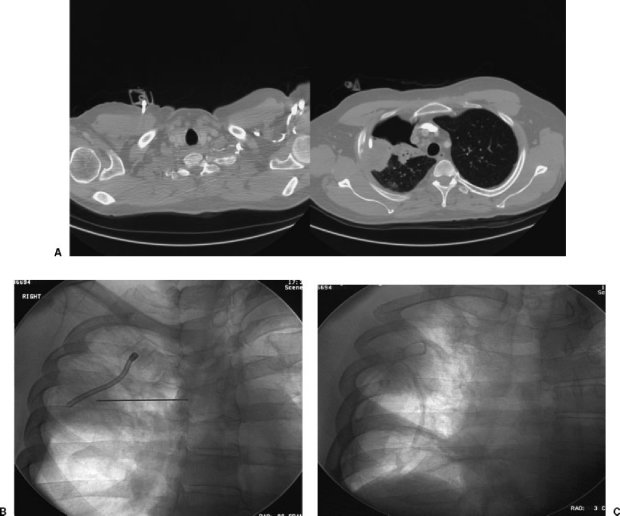
Figure 2.
Bronchoscopic biopsy leading to pneumothorax and inadvertent placement of chest vent into lung tumor without imaging guidance. (A) CT showing large, persistent pneumothorax and the tip of the chest vent tubing terminating within the mass. The chest vent was placed by the pulmonology service in the intensive care unit without the benefit of imaging guidance. (B) The patient was referred to interventional radiology for “chest tube repositioning.” Fluoroscopy shows the existing vent tubing terminating in the center of the mass. A needle has been advanced for placement of a Turner pigtail catheter. (C) Fluoroscopy shows successful placement of a Turner pigtail catheter, removal of the chest vent, and reexpansion of the lung.
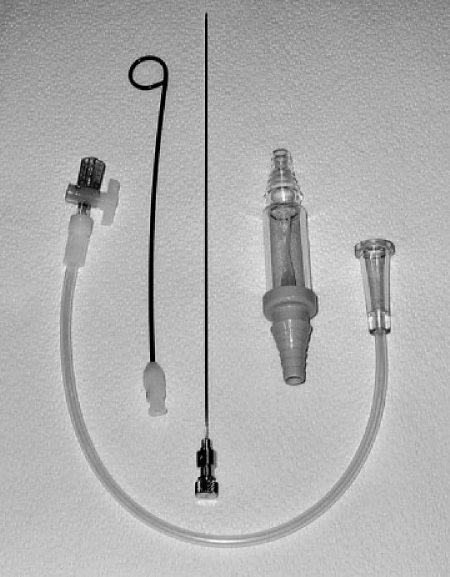
Figure 3.
The Turner kit for treatment of pneumothorax. A 6.3-French pigtail catheter with accompanying sharp stylet can be assembled and advanced into the pleural space under fluoroscopic or CT guidance. The accompanying tubing and one-way Heimlich valve can be assembled and attached.
Air leak persisting longer than 3 days is rare after iatrogenic lung injury. It most commonly arises in patients with severe emphysema, patients on positive pressure ventilation, and patients with substantial immunosuppression, particularly high-dose steroids. Thoracic surgical evaluation may be considered although continued chest drainage is usually the best course of action.
Air Embolism
Systemic air embolism is an extremely rare but potentially deadly complication of percutaneous lung biopsy, described in scattered case reports.5,6,7 Rapid death may result if embolism to the cerebral6 or coronary circulation5 occurs. Theoretically, air can enter the systemic arterial circulation through an open needle by direct puncture of a pulmonary venous branch, direct puncture of a pulmonary arterial branch (with passage of air across the capillary circulation or an arteriovenous malformation),8 or puncture of a cavitary lesion. Even a closed needle can cause a systemic air embolism by creation of a fistula between the bronchi and pulmonary circulation. Although all of these routes of embolism are possible during routine biopsies, precautions that reduce the risk include avoiding the central aspect of cavitary lesions, avoiding larger pulmonary vessels, and limiting the time the stylet is removed from the introducer needle to prevent the influx of air during negative intrathoracic pressure. Emergent treatments have included administration of pure oxygen through a nonrebreather mask, placement of the patient in the left lateral decubitus position,9 and the use of hyperbaric oxygen.
Seeding of the Tract
Another rare complication of percutaneous chest biopsy is seeding of the tract of the introducer needle resulting in pulmonary or chest wall metastasis, described in occasional case reports. Malignant seeding has been described months after biopsy of primary bronchogenic carcinoma as well as other primary chest tumors and metastases, including squamous cell carcinoma, malignant mesothelioma, and thymoma.10,11,12,13,14 Daily local radiotherapy following diagnostic procedures has been demonstrated to be beneficial in preventing seeding in patients with mesothelioma.11 Depending on the tumor cell type, when tract metastasis does occur, the treatment may include radiation therapy or radical full-thickness surgical resection with musculocutaneous flap.12,14
VASCULAR COMPLICATIONS
Bleeding complications from lung biopsy most often present as hemoptysis, in the case of arteriobronchial fistula formation and parenchymal hemorrhage, and less commonly as diminished hemodynamic and respiratory status in the case of hemothorax, mediastinal hemorrhage, or chest wall hemorrhage. Parenchymal hemorrhage is usually an incidental finding on follow-up computed tomography (CT) (Fig. 4) after percutaneous lung biopsy and is typically self-limited. This finding may present a technical problem during the procedure in that the lesion may become obscured by surrounding blood, limiting accuracy of needle placement and diminishing the diagnostic yield. Arteriobronchial fistula may lead to hemoptysis during and after the procedure, which typically resolves spontaneously or with supportive care (usually reassurance, supplemental oxygen, and suction).
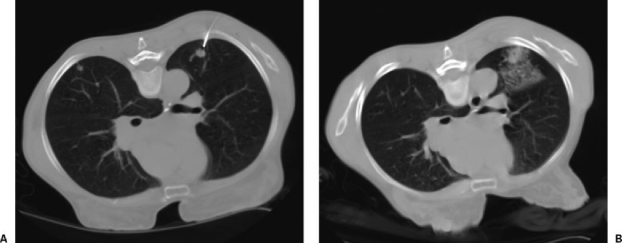
Figure 4.
Pulmonary hemorrhage after percutaneous lung biopsy. A 65-year-old woman with new 1 cm nodule in the left lung. (A) CT showing advancement of an introducer to a point just abutting the nodule. The patient experienced hemoptysis immediately after a core specimen was obtained. (B) Pulmonary hemorrhage after obtaining a single core biopsy specimen. The hemoptysis resolved, stability was verified by radiography, and the patient was discharged home the same day.
Chest wall hemorrhage, mediastinal hemorrhage, and hemothorax are extremely uncommon complications resulting from inadvertent passage of the introducer needle through a variety of structures, including an internal thoracic artery, an intercostal artery, or a chest wall artery. Hemothorax from chest biopsy may be well treated with chest tube drainage in the acute phase followed by lytic therapy in the subacute, organizing phase. After cessation of active bleeding, blood within the pleura may be effectively drained with even a small-bore chest tube placed in interventional radiology. Lytic therapy may be very successful at achieving complete pleural drainage and lung expansion in the subsequent weeks. Early thoracic surgical consultation should be obtained especially if there is any area of retained material or incomplete lung expansion. Early intervention by thoracoscopy may save a far more morbid thoracotomy and decortication in the event of retained material.
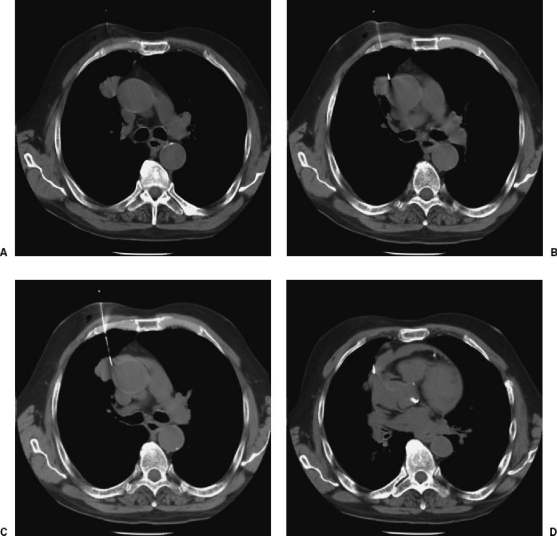
Lesions in close proximity to adjacent mediastinal structures increase the risk of inadvertent puncture of the heart or great vessels. This complication is very rare, most likely due to careful patient selection and procedural technique. However, rare examples have been reported associated with catastrophic outcomes (Fig. 5). Cases like these serve as reminders of the risks of chest biopsy, often perceived as “routine,” and the importance of a careful, methodical approach.
Figure 5.
Inadvertent advancement of an introducer needle into the wall of the aorta with pericardial hemorrhage and aortic dissection. (A) CT showing 2.5-cm nodule in the right upper lobe. (B) During needle advancement, the patient reportedly experienced a violent episode of coughing and sat up on the CT table. A CT scan obtained immediately after this episode showed inadvertent placement of the needle tip in the aortic wall. (C) Within 2 minutes, the patient experienced shortness of breath and oxygen desaturation, and a repeat scan showed extravasation into the pericardial space. (D) The patient expired after resuscitative efforts. Postmortem scan showed dissection of blood into the wall of the ascending aorta and complete effacement of the aortic lumen as evidenced by inward displacement of intimal calcifications.
REFERENCES
- Richardson C M, Pointon K S, Manhire A R, Macfarlane J T. Percutaneous lung biopsies: a survey of UK practice based on 5444 biopsies. Br J Radiol. 2002;75:731–735. doi: 10.1259/bjr.75.897.750731. [DOI] [PubMed] [Google Scholar]
- Ko J P, Shepard J O, Drucker E A, et al. Factors influencing pneumothorax rate at lung biopsy: are dwell time and angle of pleural puncture contributing factors? Radiology. 2001;218:491–496. doi: 10.1148/radiology.218.2.r01fe33491. [DOI] [PubMed] [Google Scholar]
- Saji H, Nakamura H, Tsuchida T, et al. The incidence and the risk of pneumothorax and chest tube placement after percutaneous CT-guided lung biopsy: the angle of the needle trajectory is a novel predictor. Chest. 2002;121:1521–1526. doi: 10.1378/chest.121.5.1521. [DOI] [PubMed] [Google Scholar]
- Poe R H, Kalley M C, Wicks C M, Odoroff C L. Predicting the risk of pneumothorax in needle biopsy of the lung. Chest. 1984;85:232–235. doi: 10.1378/chest.85.2.232. [DOI] [PubMed] [Google Scholar]
- Mokhlesi B, Ansaarie I, Bader M, Mona T, Boatman J. Coronary artery air embolism complicating a CT-guided transthoracic needle biopsy of the lung. Chest. 2002;121:993–996. doi: 10.1378/chest.121.3.993. [DOI] [PubMed] [Google Scholar]
- Kodama F, Ogawa T, Hashimoto M, et al. Fatal air embolism as a complication of CT-guided needle biopsy of the lung. J Comput Assist Tomogr. 1999;23:949–951. doi: 10.1097/00004728-199911000-00022. [DOI] [PubMed] [Google Scholar]
- Aberle D R, Gamsu G, Golden J A. Fatal systemic arterial air embolism following lung needle aspiration. Radiology. 1987;165:351–353. doi: 10.1148/radiology.165.2.3659355. [DOI] [PubMed] [Google Scholar]
- Westcott J L. Air embolism complicating percutaneous needle biopsy of the lung. Chest. 1973;63:108–110. doi: 10.1378/chest.63.1.108. [DOI] [PubMed] [Google Scholar]
- Muth C M, Shank E S. Gas embolism. N Engl J Med. 2000;342:476–482. doi: 10.1056/NEJM200002173420706. [DOI] [PubMed] [Google Scholar]
- Sing R F, Kefalides P T, Mette S A, Fallahnejad M. Chest wall metastasis after percutaneous fine-needle aspiration biopsy. J Am Osteopath Assoc. 1996;96:546–547. [PubMed] [Google Scholar]
- Boutin C, Rey F, Viallat J R. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma: a randomized trial of local radiotherapy. Chest. 1995;108:754–758. doi: 10.1378/chest.108.3.754. [DOI] [PubMed] [Google Scholar]
- Paik H C, Lee D Y, Lee H K, Kim S J, Lee K B. Chest wall implantation of carcinoma after fine needle aspiration biopsy. Yonsei Med J. 1994;35:349–354. doi: 10.3349/ymj.1994.35.3.349. [DOI] [PubMed] [Google Scholar]
- Nagasaka T, Nakashima N, Nunome H. Needle tract implantation of thymoma after transthoracic needle biopsy. J Clin Pathol. 1993;46:278–279. doi: 10.1136/jcp.46.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfer A E, Walsh D S, Graeber G M, Nuno I N, Eliasson A H. Chest wall implantation of lung cancer after thin-needle aspiration biopsy. Ann Thorac Surg. 1989;48:284–286. doi: 10.1016/0003-4975(89)90091-x. [DOI] [PubMed] [Google Scholar]