ABSTRACT
Embolization is a remarkably versatile procedure used in nearly all vascular and nonvascular systems to treat a wide range of pathology. The published literature is rich with studies demonstrating the enormous therapeutic potential offered by embolization procedures, and the possibilities continue to expand with the advent of new embolization agents and techniques. Unfortunately, with this variety and innovation comes a wide spectrum of potential complications, not always easy to classify and summarize, associated with embolization. This article reviews the procedures and associated complications of arterial and venous embolization procedures, organized by vascular distribution.
Keywords: Embolization, complications
Embolization can be defined as any endoluminal procedure, vascular or nonvascular, to occlude a vessel to obtain therapeutic benefit. The first embolizations date from the 1960s and 1970s, and articles by Baum, Dotter, Rösch and Tadavarthy belong to the history of medicine.1 Many series demonstrate the enormous therapeutic potential offered by the various procedures that belong under the heading of embolizations. A wide variety of materials and techniques have been used, and new possibilities and fields have opened up regarding the therapeutic benefits of occluding a vessel. Because these techniques result in a variety of possible complications, it is not easy to classify, summarize, or explain the reason for each of the possible complications of embolization. What follows is a description of the complications that can arise when different vascular areas are embolized.
HEAD AND NECK
Embolization procedures for extracranial disease in the head and neck region are mostly performed for intractable epistaxis or in the presence of a hypervascular tumor either prior to surgical removal or as a palliative treatment. Since the first treatment performed by Sokoloff et al in 1974,2 arterial embolization is nowadays widely recommended as the method of choice for the treatment of posterior intractable epistaxis. The majority of cases (70%) are idiopathic, but epistaxis can occur secondary to trauma, tumor, radiotherapy, coagulopathy, or vascular malformations or diseases such as Osler-Webber-Rendu. As a result, follow-up radiological and endoscopic evaluations are essential to avoid misdiagnosis.3
The source of bleeding is usually the septal branch and less commonly the posterolateral branch of the sphenopalatine artery, which arise from the distal internal maxillary artery. There is also rich collateral supply from the ophthalmic artery via the anterior and posterior ethmoidal arteries, the descending palatine artery, and the facial artery via the superior labial artery.4 Nowadays, with the development of new materials, it is possible to selectively catheterize and embolize these branches with the aid of a microcatheter.
The embolic materials most commonly employed are particles, either of gelatin sponge or polyvinyl alcohol (PVA), and more recently trys-acril gelatin microspheres.5,6 The use of coils is not recommended because proximal occlusion may not only induce the development of collateral circulation but also close the door to repeat access in case of recurrence. The cure rate achieved in most reported series is 80% or higher, particularly when the ipsilateral facial artery and contralateral sphenopalatine artery are simultaneously embolized.7,8,9,10,11,12 Some have even used a mixture of hystoacril with Lipiodol (Guerbet, Paris, France).13
The reported complication rates are very variable. Minor complication rates range between 2.2 and 25%,7,8,9,11,12,14 although Oguni et al have reported a rate of 45%, attributing this to the difference in definition of minor complication.15 Serious complication rates vary between 0 and 6% when looking at large series.7,9,14
Generally considered minor, the most common complication encountered is headache or temporofacial pain, more common in cases where two or more arteries are embolized with gelatin sponge.15 In fact, Siniluoto et al reported that 96.8% of their patients experienced mild to moderate pain in the temporal area during the first 24 hours after embolization.16
Soft tissue necrosis has also been described as a complication, particularly when more than one vessel is occluded (contralateral sphenopalatine artery or facial artery) or when liquid embolic agents are used.10,15 Small particles tend to produce a more distal occlusion and may also result in local ischemia and even nasal septal perforation.17 At times, it is difficult to discern if the necrosis is secondary to the embolization procedure or to prolonged packing. Ischemic injury may not only affect the mucosa but also the cranial nerves, possibly resulting in temporary or permanent cranial nerve palsy.18,19 Depending on the cranial nerve affected, the presenting symptoms will be very variable, including diplopia, dysphagia, and numbness.
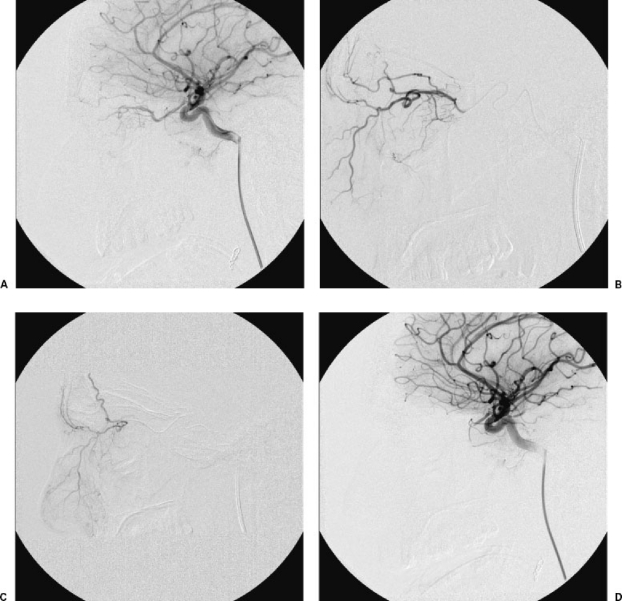
The most feared complications, however, are those in connection with the passage of embolic material into the intracranial arteries, which may result in stroke9 or blindness.20 This may occur due to reflux from the external carotid artery into the internal carotid artery or through passage of embolic material through extracranial-intracranial anastomoses. At times, it may become necessary to perform selective embolization of branches originating from the ophthalmic artery. In this situation, the microcatheter should be advanced beyond the second portion of the ophthalmic artery to prevent embolic material from entering the central artery of the retina21 (Fig. 1).
Figure 1.
(A–D) Patient with massive epistaxis who has had three embolizations of the internal maxillary artery branches on both sides. The arteriography of the internal carotid artery shows the presence of a thick infraorbital artery that reaches the nostril and depends on the ophthalmic artery. After the catheterization of the ophthalmic artery, a microcatheter is inserted distally to the origin of the retinal branches; 150 to 300 microparticles were used. During the embolization a retrograde opacification of the whole ophthalmic artery was observed. An arteriography was performed from the internal carotid artery, which showed complete occlusion of the ophthalmic artery. The patient lost the vision in his left eye. There were no further episodes of epistaxis.
In the presence of ipsilateral internal carotid artery stenosis or occlusion, the use of particles may be particularly dangerous due to the development of external carotid to internal carotid anastomoses. In this situation, endovascular therapy may still be safely and effectively performed with coils in the pterygopalatine segment of the internal maxillary artery.22
PULMONARY AND BRONCHIAL ARTERIES
Pulmonary arteriovenous malformations (PAVMs) consist of abnormal direct communications between the pulmonary artery and the pulmonary vein. Most commonly they are congenital, and often they are associated with hereditary hemorrhagic telangiectasia, also known as Rendu-Osler-Weber disease. The hemodynamic result is a right-to-left shunt, which may cause paradoxical embolism and systemic infection. Hemothorax and hemoptysis are also potential complications.23,24,25 Traditionally, surgery was the only curative treatment available, but nowadays transcatheter embolization (TCE) is widely accepted as the procedure of choice26,27 since the first report by Porstmann in 1977.28 The overall rate of complications during TCE is estimated to range between 10 and 15% when performed by experienced hands, with no reported mortality.29,30,31 Given the low complication rate of TCE and its efficacy with a success rate approaching 99%, the indication for treating a PAVM is established in the presence of related symptoms or when the feeding vessels are 3 mm in diameter or larger.29,32,33
The most commonly used devices for closing PAVMs are detachable balloons and coils. Whatever device is chosen, the aim of the procedure is to permanently occlude the abnormal communication, preserving the blood flow in any proximal vessel supplying normal lung parenchyma. Ideally, the catheter tip should be placed as close to the neck of the PAVM as possible or even on the venous sac when there is a short arterial feeder.34,35 An occlusion that is very proximal may result in early recanalization by collateral perfusion from bronchial arteries or lung infarction, although due to the dual blood supply to the lung, the latter will only occur when the bronchial arteries are also occluded.36 On the other hand, if the closing device is too small, the coil or balloon may migrate into the systemic circulation. Traditionally, balloons had the advantage over coils in that they could be retrieved before detachment if they were found to be of incorrect size or improperly positioned. Nowadays, with the introduction of detachable coils, this is no longer an issue.23,32,36
Systemic migration of the embolic device, which has been reported to occur in up to 3% of cases, is probably the most feared complication as it may result in stroke.30,33,38 Retrieval of a migrated coil might be possible with devices such as a gooseneck snare. When a balloon migrates, attempts can be made to deflate it by puncturing it with a needle inserted percutaneously, depending on its location.39
Pleuritic chest pain is the most commonly reported complication following TCE.29,30,36 It usually appears within the first 48 hours and responds well to analgesics. The incidence of this complication appears to be related to the size of the PAVM, being present in up to 31% of patients in whom the occluded PAVM has feeding vessels larger than 8 mm in diameter.33 Delayed pleurisy (4 to 6 weeks after TCE) in association with high fever has also been reported to occur, particularly following coil embolization.32
It has been reported that ~2% of patients might experience air embolism during the procedure, which clinically manifests as angina and bradycardia.27,29 A further possible complication is the development or aggravation of preexisting pulmonary hypertension presumably in connection with the closure of the PAVM that acts as a low-resistance vascular circuit.37,38,40
To minimize the chances of balloons deflating spontaneously, it is important that they are filled with contrast isotonic to blood. In this respect, silicone balloons remain inflated during a longer period than latex balloons although both are equally effective in occluding the PAVM.41 Tight coil packing is also advised to prevent recanalization. In a recently published study there was no statistically significant difference between platinum and stainless steel coils in terms of achieving a successful sustained occlusion.31
Bronchial artery embolization (BAE) is a well-established procedure in the management of massive and recurrent hemoptysis.42,43,44,45,46,47 Embolization in this territory is most commonly performed with particles such as PVA or more recently tris-acryl gelatin microspheres.48,49 The use of thrombin as an embolic agent has also been recently described.50 A thorough knowledge of normal and variant bronchial arterial anatomy as well as collateral supply is necessary prior to embarking on any embolization procedure.51,52 The most dreaded and serious complication of this procedure is spinal cord ischemia secondary to inadvertent embolization of the anterior or posterior spinal arteries via radiculomedullary arteries, which may arise from the bronchial arteries. This complication has been described to occur in 1.4 to 6.5% of BAE according to different published series.44,53,54 The use of a microcatheter may be indicated in this situation so that BAE may be performed from a more distal position.55
One must also be aware of the presence of any bronchopulmonary anastomoses to either the pulmonary arteries or veins. It has been experimentally demonstrated that these anastomoses measure just over 300 μm,56 and because of this it is recommended that any particle employed as embolic agent should be larger than this.57 We had in fact one case in which passage of embolic trys-acril gelatin microspheres through tumoral bronchopulmonary anastomoses resulted in cerebral embolization, which fortunately resolved, leaving no sequelae. Furthermore, the use of smaller particles will also result in a overly distal occlusion that may impede the normal blood supply to other anatomical structures, such as the esophagus, and lead to necrosis. This is also why liquid embolic agents are no longer recommended.57 In fact, dysphagia has been described to develop following BAE in up to 18% of the cases,53,58 and the occurrence of a bronchoesophageal fistula has also been reported.59 Equally, aortic and bronchial necrosis, although rare, may also occur.60 However, the most commonly reported complication following BAE is chest pain, which has been described to occur in 24 to 91% of BAE procedures.53,58,61
Coils may be employed when an aneurysm is present or also when there is a need to protect a normal vascular territory. Otherwise, the use of coils for bronchial embolization is not recommended as they may produce an overly proximal occlusion, preventing the performance of a repeat procedure should the hemoptysis recur.57,62 Sometimes, the recurrence is due to the presence of nonbronchial systemic feeder vessels that must be searched for.54,57,62
LIVER
Trauma
Embolization is a useful technique in the therapeutic management of patients with vascular injuries caused by trauma, liver surgery, or percutaneous procedures. Although such injuries are unusual, the presence of hemobilia or hemoperitoneum resulting from pseudoaneurysm or laceration of a blood vessel may put the patient critically at risk. Selective embolization of the injury provides excellent results as far as hemostasis is concerned (86%),63 although an associated morbidity of 58% has been described,64 which is basically connected with bile leakage, liver abscesses, and/or necrosis. Such lesions should therefore be treated on an individual basis,65 with full awareness of the possible consequences of a technique that is not risk-free.
Hepatocarcinoma
In patients with liver cancer that cannot be treated surgically, but who have good liver function, embolization only with particles, or associated with the introduction of lipiodol and drugs (chemoembolization), is a palliative therapeutic measure that is widely accepted.66
Although controversy remains as to the criteria for inclusion and the technique that should be used (coaxial catheters, selection of particles, etc.), many series have been published, and as a result, there is a large amount of information available regarding possible complications and steps to prevent them. The most common complication after embolization, the cause of which is still not properly understood, is the occurrence of fever, pain in the right hypochondrium, and a rise in transaminases. High temperature (< 38.5°C) may occur between 41 and 74% of cases67,68 and seems to be related to the presence of necrosis of the tumor and the healthy tissue. Cytolysis (increase in transaminases), which is present in up to 93% of cases, is related to damage to healthy (noncancerous) hepatocytes. Contrary to what was previously thought, it seems that the presence of fever and cytolysis is not associated with a better response or with an increase in survival.67
The possibility of acute hepatic decompensation has been described in 20% of cases, although it is irreversible in only 2%.68 The possible appearance of hepatic insufficiency is related both with the dose of cisplatin and the appropriate selection of patients. It occurs more frequently in cases of advanced cirrhosis with hyperbilirubinemia and abnormalities of the prothrombin time68,69 and is practically absent in patients with Child stages A and B.70
Hepatocarcinoma may manifest itself clinically in the form of hemoperitoneum because of spontaneous rupture; in such cases, embolization is a good therapeutic option.71 Although this is infrequent, cases of tumor rupture after arteriography have been described,72 after lipiodol injection for tumor staging,73 and after therapeutic embolization.74,75
While Lipoidol is being introduced, in addition to tumor uptake of the agent, it is common to observe that small nontumor vascular branches that belong to portal vessels become opacified and are occluded. This occlusion is temporary, but it causes an increase in presinusoidal portal pressure, which can bring about a pressure increase in the esophageal varices,76 possibly leading to rupture and gastrointestinal hemorrhage.
The biliary system is fed exclusively through the hepatic artery, and as a result, arterial ischemia can result in a biliary lesion, a well-known outcome in liver transplant patients. Kim et al presented their experience with 807 patients with liver cancer treated by embolization; they observed a 2% incidence of biliary complications.77 Among these, the most frequent was the presence of subcapsular bilomas (71%), but there was also focal stenosis of the common hepatic duct or of small intrahepatic radicals.77 It would seem that biliary complications are more frequent in patients with prior dilatation of the bile ducts, small tumors, treatment that is repeated within a short period of time, or when drugs are associated with embolization.78 In exceptional cases, a direct arteriobiliary connection has been described as a result of biliary ischemia.79 Ischemia of the gallbladder is worthy of a special mention. In nonselective embolizations performed from the right hepatic artery, the embolizing agent can occlude the cystic branches, and although complications due to this occlusion are infrequent, cases of gallbladder necrosis have been described.80
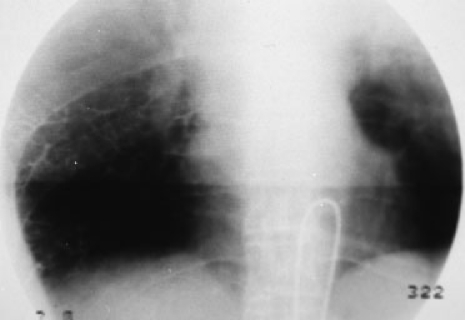
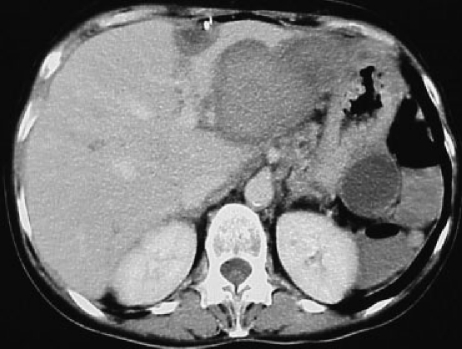
Hepatocarcinoma tends to produce infiltration of the adjacent portal and hepatic veins. In fact, portal infiltration is one of the angiographic features of hepatocarcinoma. Less frequently, early opacification of the hepatic veins is observed, and there may even be high-flow channels that pass undetected because they do not become opacified. The use of small particles (< 100 μm) may promote flow through the shunts mentioned toward the heart, and therefore toward the pulmonary circulation, resulting in massive pulmonary infarction with disastrous consequences.81 It would seem that these undesired embolizations are more frequent if the embolization is performed through nonhepatic vessels that vascularize the tumor (for example, the phrenic arteries)82 (Fig. 2).
Figure 2.
Image taken after the embolization of a hepatocarcinoma through the right inferior phrenic artery. Lipiodol (10 mL) was used. Massive opacification of pulmonary vascularization can be seen.
Hepatic Metastases
In symptomatic patients with hepatic metastases of neuroendocrine tumors, embolization seems to be an appropriate treatment and has an excellent response and low morbidity.83,84 One complication that is feared in these cases is the massive release of serotonin caused by ischemia. Various methods of preventing this have been described, such as infusion of a somatostatin (octeotride) during the procedure. However, recent studies85 seem to show that this treatment does not prevent serotonin release during the procedure.
In patients with liver metastases of gastrointestinal tumors, various chemotherapy protocols have been applied both intravenously and intra-arterially, with results that are positive in both cases, though perhaps slightly better in the case of intra-arterial administration. For this reason, series have been described with repeated insertion of catheters in the hepatic artery via femoral catheterization or with insertion of a permanent catheter fixed to a subcutaneous reservoir. In both cases, the most frequent complications were hepatobiliary toxicity with possible associated biliary lesion, occlusion of the hepatic artery, infection of the catheter, or extrahepatic drug infusion86 (Fig. 3). To prevent this last complication, all extrahepatic vessels that originate from the hepatic artery must be embolized (gastroduodenal, pancreatic, right gastric, etc.).87 A special mention must be made of the right gastric artery, the origin of which lies between the proper hepatic and the left hepatic artery, although it may also originate from a small segmentary artery. It is important to detect and embolize it to prevent chemical injuries of the stomach, which may occasionally be severe (Fig. 4). There is a debate as to whether this artery ought to be embolized from its hepatic origin86 or by entering from the left gastric artery87; it appears that the second possibility is safer, as unsuitable manipulations of the hepatic arteries, which might result in spasm or dissection, are avoided.
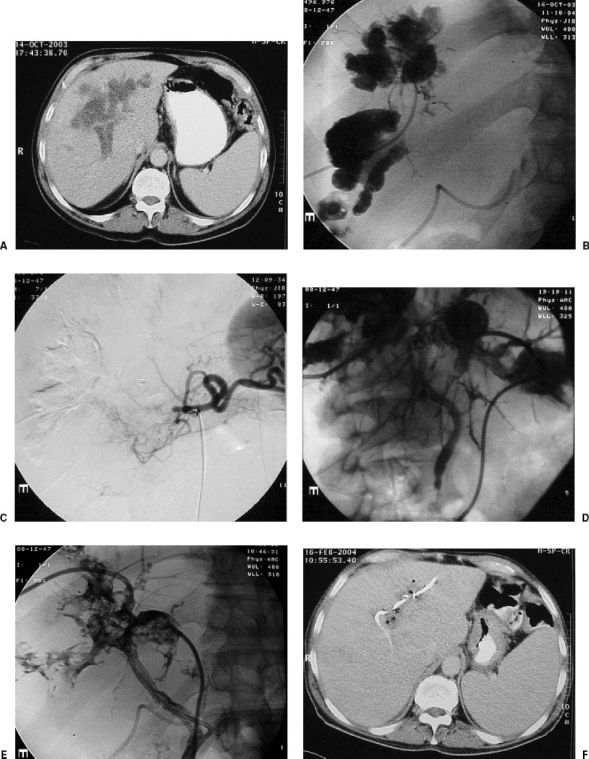
Figure 3.
Patient with hepatic metastasis from a colonic adenocarcinoma consulted us because of ictericia following intra-arterial hepatic treatment performed by means of a reservoir implanted surgically in the proper hepatic artery. (A) The computed tomography (CT) scan showed a liquid collection surrounding the portal branches. The suspected diagnosis was biliary necrosis due to ischemia and chemotoxicity. (B) Image taken after the percutaneous implantation of a catheter in the cavity. The contrast surrounds all the right portal branches and corresponds to a large periportal “biloma.” A catheter can be seen inserted surgically into the hepatic artery. (C) Obstruction of the common hepatic artery with little collateral arterial vascularization to the liver. (D) Two weeks after the placement of two external catheter drainages in both lobes, opacification of small interhepatic biliary branches and of the coledocus can be seen. (E) After the punction of peripheral biliary branches, external-internal catheter drainages are introduced, through the “biloma,” connecting with the coledocus and the duodenum. (F) After great clinical improvement, the CT taken 3 months later shows reduction of the size of the intrahepatic collection and no biliary dilatation.
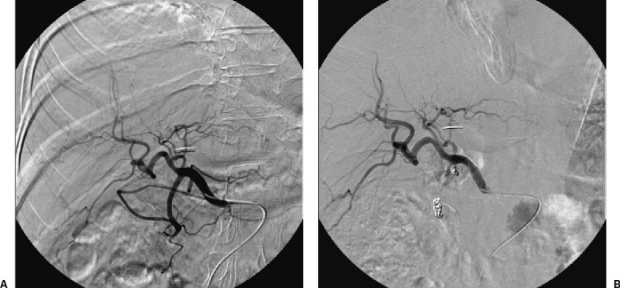
Figure 4.
Patient with adenocarcinoma of the kidney who was treated by nephrectomy and who now shows hepatic metastasis. The arteriography was taken to plan the treatment with embolization using yttrium radiospheres. (A) Arteriography from the common hepatic artery, which, apart from the gastroduodenal artery, shows a thick right gastric artery. (B) To avoid infusion of particles into extrahepatic areas, both arteries are embolized by means of coils.
Portal Vein Embolization
The complications of portal embolization can be divided into several groups. First, the effect that ischemia has on the hepatocytes is “apoptosis” and not “cell necrosis,” and so the postembolization syndrome and cytolysis are much less severe than in the case of arterial embolization and may be absent altogether. Second, there are the complications deriving from the transhepatic puncture that are similar to those described in other transparietohepatic techniques such as biliary drainage; there are reports of pneumothorax, subcapsular hematoma, arterial pseudoaneurysm, or hemobilia88 (Fig. 5). A third group of complications are those caused by portal management itself. If the puncture is insufficient or the manipulation excessive, or if the embolization has affected large areas of the porta, complete thrombosis of the porta may occur. This is a severe complication that requires immediate direct thrombolytic treatment. Therefore, there is a debate as to whether the approach should be ipsilateral (entering the segmentary veins that are to be embolized) or contralateral (through the veins that are to be left). Some specialists consider that in view of the large number of anatomical variants in the right portal vein, the ipsilateral approach may involve technical difficulties, with more manipulation and a greater risk of thrombosis; others believe that the contralateral approach puts an area at risk that should be left untouched.88
Figure 5.
CT performed 3 days after a presurgical portal embolization. The portal punction was done, accidentally, through a metastasic lesion placed in segment II, which had previously received radiofrequency treatment. A subhepatic hematoma, which did not need treatment, was observed. The nonembolized liver grew correctly, in spite of the compression caused by the hematoma.
The rise in portal pressure is moderate (generally 5 to 7 mm Hg), and this rise does not usually have clinical consequences. However, in cirrhotic patients with portal hypertension, this hemodynamic alteration can result in rupture of gastroesophageal varices, and in one case it was reported that it was necessary to perform an emergency transjugular intrahepatic portosystemic shunt straight after portal embolization. One complication that should be borne in mind is that the tumor may remain untreated during the waiting time, leading to the growth of nonembolized liver tissue, and it may happen that resectable tumors have ceased to be resectable by the time that surgery is finally performed.89
SPLEEN
The present indications for performing partial occlusion of the splenic flow and therefore a partial reduction in its function have widened. The improvement of hypersplenism is still a classic indication, being a frequent complication in cirrhotic patients with portal hypertension. The embolization must preserve some flow to the spleen to maintain partial splenic function. In addition, if there is no flow in the splenic artery, the inferior mesenteric vein may drain bacteria (physiological mesenteric bacteremia) toward the devascularized splenic parenchyma rather than the liver.90 This phenomenon may lead to splenic abscesses, bacterial peritonitis, and sepsis,91 although these severe complications are infrequent (1 to 2%). Vascular necrosis more commonly results in high temperature (94%) and abdominal pain (82%). The occurrence of a left pleural effusion (10%) or basal atelectasis (30%) is not uncommon, related to the reduced mobility of the diaphragm due to inflammation of the spleen.91 A further serious but infrequent complication is portal thrombosis, which can occur in cases in which devascularization is complete, and the patient presents with portal hypertension.
DIGESTIVE TRACT
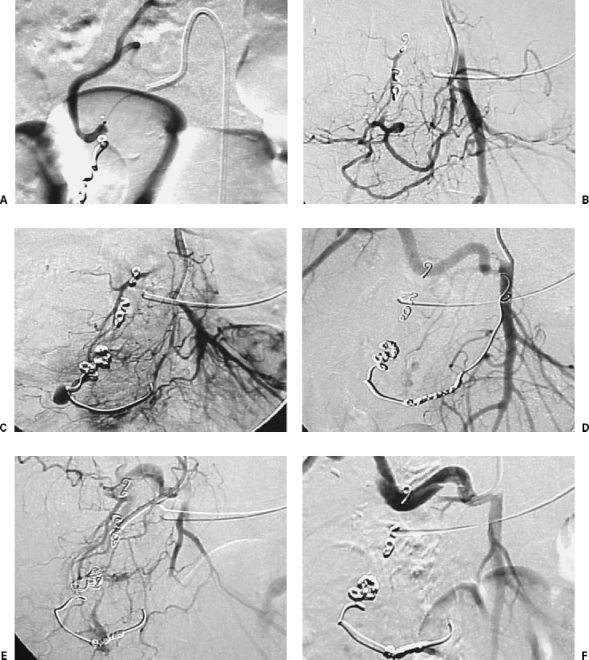
The particular embolization technique used in patients with gastrointestinal hemorrhage depends on various factors. First, recognizing the differences between the organs to be embolized is critical. For example, the stomach has multiple afferent pedicles and an excellent supply of collateral circulation. On the contrary, the colon has abundant marginal vessels but of fine caliber with little collateral supply. Second, cases that have already undergone surgery require highly individual assessment, as the ligature of pedicles means that many vessels may be terminal, and the possibility of necrosis resulting from ischemia exists. Third, attention should be paid to the cause of hemorrhage because in some cases it is enough to provoke hemostasis by occluding the vessel temporarily, while in others, for example in the case of neoplasm, careful embolization of all tumor vessels is required (Fig. 6). For these three reasons, and for others that are equally important, such as the state of coagulation, the complications resulting from an embolization may vary considerably.92
Figure 6.
Patient with pancreatic head carcinoma treated with radiotherapy and surgery. He presents severe digestive hemorrhage. (A) Pseudoaneurysm in right hepatic artery. (B) Indirect portography from splenic artery. Portal thrombosis. (C) Selective catheterization of pseudoaneurysm. (D) Rupture of pseudoaneurysm during the endocavitary manipulation of the coaxial catheter. (E) Arteriography after the selective embolization of the lesion. The flow of the right hepatic artery, through arterial intrahepatic collateras, is preserved. (F) Four months later the patient had another episode of digestive bleeding. In the arteriography a large pseudoaneurysm was observed, placed proximal to the previous one. (G) Further rupture of the pseudoaneurysm during the catheterization. Major digestive bleeding. (H) Arteriography after the complete occlusion of the vascular lesion and the hepatic artery distal and proximal to the rupture. The arterial flow is partially maintained through collaterals of the lesser epiploon. The patient had no further episodes of bleeding or hepatic ischemia.
Stomach
In patients with no history of gastric surgery, embolization of a bleeding ulcer is simple, and ischemic complications are rare. Special mention must be made of the case of patients with diffuse hemorrhage that is not controlled by endoscopic techniques. Occlusion of several main gastric pedicles such as the left gastric and right gastroepiploic arteries with gelatin sponge particles of 1 to 3 mm has been described, and is a common practice in many hospitals.93 This “blind embolization” is thought to reduce the blood supply temporarily, given that the characteristics of the embolizing material and the rich collateral arterial supply mean that the ischemia will be of short duration. Cases of gastric ischemia have been described when this technique is used, and so although it is effective, it is not totally free of complications.94
Duodenum
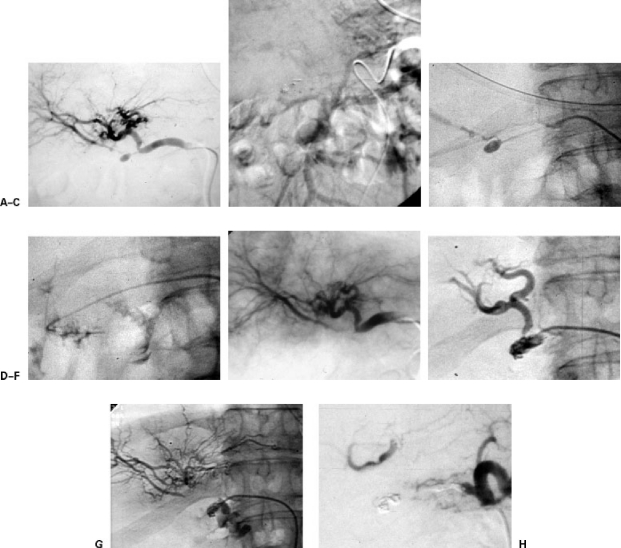
The arterial vascularization of this area is an “arcade” that connects the anterior and posterior superior branches, which depend on the gastroduodenal artery, with the inferior ones, which depend on the superior mesenteric artery. It is useful to know that this area can also receive supply from accessory duodenal-pancreatic branches, which may have their origin in possible aberrant right hepatic arteries (Fig. 7).
Figure 7.
Digestive hemorrhage after endoscopic papillotomy. The patient was treated by embolization of the gastroduodenal artery with coils placed distally and proximally to the source of bleeding. A week later she had a new episode of hemorrhage. (A) Arteriography from the proper hepatic artery shows good occlusion of the gastroduodenal artery. (B) Catheterization of the posteroinferior pancreatoduodenal branch. Return of the bleeding. (C) Arteriography taken during the embolization of both arteries, persistent hemorrhage. (D) Arteriography taken after the embolization of the pancreatoduodenal arch. Persistent hemorrhage. Coil in superior mesenteric artery, which was extracted with a gooseneck. (E) Selective catheterization of an accessory pancreatoduodenal branch, which comes from the origin of the aberrant right hepatic artery. (F) Arteriography taken after the embolization, with segments of gelfoam, of the aberrant artery. The bleeding has stopped. The patient has had no further episodes of hemorrhage.
When attention is paid to the anatomy and an appropriate technique is employed, the technical success rate of embolization is very high (98%) and clinical success may reach 80%.95 Therapeutic failures are more frequent in cirrhotic patients and those with coagulation disorders.95,96 This is even more likely when microcoils alone are used in patients of this kind,95 and so on some occasions it is necessary to use particles (gelfoam or PVA) as well. Nonetheless, when particles, and above all glues, are used, the possibility of ischemia is greater,95,97 and the presence of duodenal stenosis (40% of cases) has been reported in studies with sufficient follow-up time.98
Small and Large Intestine
In the 1980s, embolization in this area was found to be associated with unacceptably high rates of intestinal ischemia and necrosis. For this reason, the use of vasopressin infusions became widespread to encourage lasting vasoconstriction of the bleeding area. However, it became apparent that this technique was also not free of complications such as myocardial infarction, hypertension, and arrhythmia.99 As new materials for catheterization and embolization were developed, it was found that they provided greater vascular occlusion with regard to clinical performance and that the complication rates were lower.99,100 At present, the use of vasopressin and similar agents is generally not advisable. Even if the area is of high risk for embolization and temporary vasoconstriction is required, it is preferable to achieve this end by triggering vasospasm through intravascular manipulations of catheters and guide wires.101
In digestive hemorrhage of intestinal origin, the current recommendation is to embolize, using a coaxial technique, the vasa recta or the marginal arteries closest to the bleeding point.102,103 If the occlusion is more distal, the risk of necrosis rises, and if it is excessively proximal, depending on the site, both ischemia and rebleeding from collaterals may come about. If the material is appropriately selected and the anatomy is studied carefully, major complications will not occur and minor ones (abdominal pain) should happen in only 10% of cases.103
KIDNEY
The indications for performing renal embolization have been considerably reduced.104 In the 1980s, this technique was performed preoperatively, and many series were published concerning this indication.105 The rate of major and minor complications was around 10%, the most frequent being renal insufficiency and unintentional embolization of nontarget areas.105 Infectious complications were much less frequent, although some retroperitoneal abscesses did occur.106 The use of ethanol as an embolic agent carries a greater possibility of ischemia and necrosis than the use of coils, particles, or even arterial occlusion balloons. The most frequently described complication is colonic infarct caused by ethanol passing through the ventral area of the aorta to the inferior mesenteric artery,107,108 but cases of skin necrosis110 and testicular infarction have also been reported (due to the aberrant origin of the left testicular artery).109
In the case of nonfunctioning kidneys, ablation with ethanol is an effective alternative to nephrectomy.111 When this technique has been used, postembolization syndrome has been described as occurring in 62% of cases.111 Other occasional complications include renal abscess112 and emphysematous pyelonephritis.113
Cases of renal arteriovenous malformations are increasingly being treated with embolization. In comparison with surgery, the therapeutic benefit is high, as the parenchyma can be preserved, thereby maintaining kidney function. Complications are rare: one case of massive hematuria caused by an arteriopelvic fistula has been reported after embolization with gelfoam and microcoils,114 and another has been described resulting from a fistula to the descending colon caused by coils inside the lesion.115
PELVIC EMBOLIZATION
As well as those secondary complications due to the puncture (hematoma, pseudoaneurysm, arteriovenous fístula, etc.), catheterization (dissection, perforation, thrombosis, embolia, etc.), use of coaxial systems (intraluminal thrombosis, embolus, etc.), and use of microcatheters and microguides (arterial perforation, dissection, etc.) and those specifically due to the use of different embolization materials (coils, particles, polymerizing materials, etc.), there are other complications specific to the arterial system being embolized.
Uterine Myoma
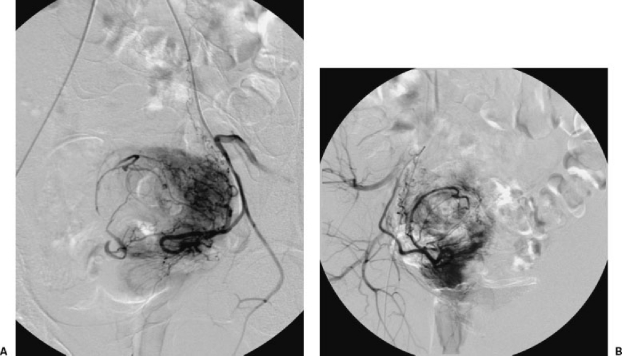
According to Spies et al,116 in the embolization of uterine myomas the most frequent complications are: allergic reactions (4%), fever (2%), hemorrhage (0.75%), and vaginal bleeding (0.5%). Other complications include , which appears in almost all cases in the first 12 hours and frequently persists for several days (it is usually controlled by the use of analgesics such as paracetamol), and infection, which may become pyometra and be the cause of a hysterectomy. Some extremely rare complications have been described, such as septicemia and thrombophlebitis of the pelvic veins, sometimes associated with a pulmonary embolism that can even result in death. This type of complication is more common when the myomas are large.117,118,119 Other studies have reported complications such as uterine prolapse and loss of ovarian function, appearing as transitory or permanent amenorrhea. This latter complication is more frequent in women over 50120 (Fig. 8). There are also descriptions of secondary amenorrhea associated with endometrial atrophy despite normal ovarian function.121 Similar complications are possible in the embolization of the uterine arteries to control postpartum hemorrhage, hemorrhage associated with spontaneous or induced abortion,122,123,124 or hemorrhage associated with gestational trophoblastic disease, sometimes treated by embolization of the internal iliac arteries.125 To avoid the complications described in this section it is preferable to selectively embolize the uterine arteries.
Figure 8.
Embolization of a uterine myoma after bilateral catheterization (A, B) of the uterine arteries. Both arteries can be seen; 500 to 700 microparticles were used. After embolization, the patient had permanent amenorrhea.
Embolization of the hypogastric arteries is frequently used as a therapeutic measure in cases of uncontrollable hematuria of vesicular or prostatic origin, arterial lacerations, and multifocal pelvic trauma. In these cases, unilateral or bilateral embolization of the hypogastric arteries is typically performed. For example, although aneurysms that affect the common or internal iliac arteries are treated by implanting percutaneous prostheses, aneurysms associated with retrograde flow from an aortobifemoral bypass are often treated by embolization of the hypogastric arteries. In all these cases, the most frequent complications are fever, gluteus ischemia with or without claudication, infection, and vascular perforation.126,127 There are also descriptions of necrosis of the skin, sexual dysfunction such as vaginal dryness and impotence, and muscular atrophy, although these problems may be due to the underlying disease and have no relationship with the embolization.128,129 When the origins of the superior and inferior mesenteric arteries are abnormal, intestinal vascularization may depend on the hypogastric arteries. In such situations, intestinal vascularization may depend on the hypogastric arteries, and the embolization of these arteries may result in intestinal ischemia. Distal internal iliac artery embolization may also result in colon ischemia. As a result, in these situations embolization of the hypogastric arteries must be as proximal as possible.130 Finally, when treating aneurysms of the common and internal iliac arteries, careful technique is necessary to prevent rupture.
Other Pelvic Embolizations
Neurological damage (paraparesis, neuropathy) may result from several pelvic embolization procedures, including the treatment of vascular malformations with bucrylate or the treatment of trauma to the sacral plexus or pelvic ring using particles that are too small. Necrosis of the skin, bladder,131 or other intra-abdominal organs has also been described, and even ischemia of the spinal medulla may occur, most commonly when embolization of the arteries of the lumbar, sacral, or vertebral areas is necessary.
In the treatment of priapism by means of embolization, impotence is described as a secondary complication. Occlusion of the arteries of the pudenda should be avoided132 whenever possible during pelvic embolization to avoid this complication.
Finally, embolization plays an important role in the treatment of retroperitoneal hematomas to achieve the hemodynamic stability of the patient. Complications vary depending on the vascular distribution that must be embolized, but in every case, the most feared complication is infection of the hematoma. Therefore aseptic technique and correct antibiotic prophylaxis is of extreme importance.
VASCULAR MALFORMATIONS
Vascular malformations may be classified according to flow characteristics, including low flow (capillary, lymphatic, and venous) and high flow (arteriovenous).133,134 Low-flow malformations are mainly treated by sclerosant injection, and high-flow malformations are treated by embolization.135,136,137,138
Among the sclerosant agents employed in the treatment of venous malformations, the most commonly used are absolute ethanol139,140 and the detergent sclerosants, including sodium tetradecyl sulfate, polydocanol, sodium morrhuate, and ethanolamine.141,142,143,144,145 All have in common the ability to produce endothelial damage, which ultimately results in thrombosis and fibrosis.
Absolute ethanol is the most potent of the sclerosant drugs and hence the most difficult to use, potentially causing serious complications and side effects. The maximum dose of 1 mL/kg weight should not be exceeded in any single session because of its toxic effect.135 Pain and substantial swelling should be considered expected side effects rather than complications.146,147 The use of systemic steroids and analgesics may minimize these side effects. Depending on the location of the lesion, the swelling may obstruct vital structures such as the airway or the alimentary canal135 and thereby lead to additional problems. Administration should be performed under general anesthesia with close monitoring, even with a Swan Ganz catheter in the pulmonary artery as pulmonary vasospasm, cardiac arrhythmias, and cardiopulmonary collapse are known complications following its use.146,148 Skin necrosis, blistering, and even more serious ischemic damage may occur, particularly when inadvertently injected into a nontarget artery with resultant embolization of normal capillary beds.135,146,149 Peripheral nerve deficits may develop not only because of ischemic damage to the vasa nervorum but also due to sustained compression by the swelling.138
The detergent sclerosant drugs rarely cause adverse effects, although there has been at least one report of a reversible cardiac arrest following the use of polydocanol.150 Allergic reactions have also been described.151 When used as a foam or microfoam, it is possible that small air emboli may reach the pulmonary circulation but usually without any clinical significance in our personal experience. Hemoglobinuria may develop as a consequence of the hemolysis that follows any sclerosant injection; therefore, good hydration and monitoring of urine output and alkalinization are strongly recommended.135
Among the sclerosant agents employed in the treatment of lymphatic malformations, some (e.g., OK-432) have virtually no side effects and others (e.g., bleomycin), when used in large doses, may produce toxic effects such as pulmonary fibrosis and alopecia.152 Ethibloc virtually always causes the skin to split as it is extruded and this should not be considered a complication unless infection develops. Prophylactic antibiotics are advised when treating lymphatic malformations as they tend to become infected quite frequently, even spontaneously.135
What has been said so far in connection with the use of absolute ethanol in low-flow malformations applies as well for arteriovenous malformations. Embolization with particles or liquid agents may be performed, most commonly preoperatively with the intention of reducing the size of the nidus and minimizing the amount of blood loss during surgery.153 This practice is particularly helpful for those malformations that are not situated in the limbs and therefore cannot be excised with the aid of tourniquets.
REFERENCES
- Di Segni R, Young A T, Qian Z, Castañeda-Zùñiga W R. In: Castañeda-Zúñiga WR, editor. Interventional Radiology. Baltimore: Williams and Wilkins; 1992. Embolotherapy: agents, equipments and techniques. pp. 29–103.
- Sokoloff J, Wickbom I, McDonald D, et al. Therapeutic percutaneous embolization in intractable epistaxis. Radiology. 1974;111:285–287. doi: 10.1148/111.2.285. [DOI] [PubMed] [Google Scholar]
- Ortiz J M, Bhattacharyya N. Management pitfalls in the use of embolization for the treatment of severe epistaxis. Ear Nose Throat J. 2002;81:178–183. [PubMed] [Google Scholar]
- Koh E, Frazzini V I, Kagetsu N J. Epistaxis: vascular anatomy, origins, and endovascular treatment. AJR Am J Roentgenol. 2000;174:845–851. doi: 10.2214/ajr.174.3.1740845. [DOI] [PubMed] [Google Scholar]
- Remonda L, Schroth G, Caversaccio M, et al. Endovascular treatment of acute and subacute hemorrhage in the head and neck. Arch Otolaryngol Head Neck Surg. 2000;126:1255–1262. doi: 10.1001/archotol.126.10.1255. [DOI] [PubMed] [Google Scholar]
- Koebbe C J, Horowitz M, Levy E I, Adelson D, Jungries C. Endovascular particulate and alcohol embolization for near-fatal epistaxis from a skull base vascular malformation. Pediatr Neurosurg. 2001;35:257–261. doi: 10.1159/000050432. [DOI] [PubMed] [Google Scholar]
- Vitek J. Idiopathic intractable epistaxis: endovascular therapy. Radiology. 1991;181:113–116. doi: 10.1148/radiology.181.1.1887018. [DOI] [PubMed] [Google Scholar]
- Tseng E Y, Narducci C A, Willing S J, Sillers M J. Angiographic embolization for epistaxis: a review of 114 cases. Laryngoscope. 1998;108:615–619. doi: 10.1097/00005537-199804000-00028. [DOI] [PubMed] [Google Scholar]
- Elden L, Montanera W, Terbrugge K, Willinsky R, Lasjaunias P, Charles D. Angiographic embolization for the treatment of epistaxis: a review of 108 cases. Otolaryngol Head Neck Surg. 1994;111:44–50. doi: 10.1177/019459989411100110. [DOI] [PubMed] [Google Scholar]
- Wehrli M, Lieberherr U, Valavanis A. Superselective embolization for intractable epistaxis: experiences with 19 patients. Clin Otolaryngol. 1988;13:415–420. doi: 10.1111/j.1365-2273.1988.tb00314.x. [DOI] [PubMed] [Google Scholar]
- Moreau S, De Rugy M G, Babin E, Courtheoux P, Valdazo A. Supraselective embolization in intractable epistaxis: review of 45 cases. Laryngoscope. 1998;108:887–888. doi: 10.1097/00005537-199806000-00018. [DOI] [PubMed] [Google Scholar]
- Cullen M M, Tami T A. Comparison of internal maxillary artery ligation versus embolization for refractory posterior epistaxis. Otolaryngol Head Neck Surg. 1998;118:636–642. doi: 10.1177/019459989811800512. [DOI] [PubMed] [Google Scholar]
- della Faille D, Schmelzer B, Vidts G, et al. Posterior epistaxis: our experience with transantral ligation and embolization. Acta Otorhinolaryngol Belg. 1997;51:167–171. [PubMed] [Google Scholar]
- Elahi M M, Parnes L S, Fox A J, et al. Therapeutic embolization in the treatment of intractable epistaxis. Arch Otolaryngol Head Neck Surg. 1995;121:65–69. doi: 10.1001/archotol.1995.01890010051009. [DOI] [PubMed] [Google Scholar]
- Oguni T, Korogi Y, Yasunaga T, et al. Superselective embolization for intractable idiopathic epistaxis. Br J Radiol. 2000;73:1148–1153. doi: 10.1259/bjr.73.875.11144790. [DOI] [PubMed] [Google Scholar]
- Siniluoto T MJ, Leinonen A S, Karttunen A I, et al. Embolization for the treatment of posterior epistaxis. Arch Otolaryngol Head Neck Surg. 1993;119:837–841. doi: 10.1001/archotol.1993.01880200037005. [DOI] [PubMed] [Google Scholar]
- Bent J P, III, Wood B P. Complications resulting from treatment of severe posterior epistaxis. J Laryngol Otol. 1999;113:252–254. doi: 10.1017/s0022215100143701. [DOI] [PubMed] [Google Scholar]
- de Vries N, Versluis R J, Valk J, Snow G B. Facial nerve paralysis following embolization for severe epistaxis (case report and review of the literature) J Laryngol Otol. 1986;100:207–210. doi: 10.1017/s0022215100098996. [DOI] [PubMed] [Google Scholar]
- Low Y M, Goh Y H. Endovascular treatment of epistaxis in patients irradiated for nasopharyngeal carcinoma. Clin Otolaryngol. 2003;28:244–247. doi: 10.1046/j.1365-2273.2003.00699.x. [DOI] [PubMed] [Google Scholar]
- Mames R N, Snady-McCoy L, Guy J. Central retinal and posterior ciliary artery occlusion after particle embolization of the external carotid artery system. Ophthalmology. 1991;98:527–531. doi: 10.1016/s0161-6420(91)32261-9. [DOI] [PubMed] [Google Scholar]
- Sedat J, Dib M, Odin J, Pedulla F, Aboulker C, Santini J. Endovascular embolization of ophthalmic artery branches for control of refractory epistaxis: report of one case. J Radiol. 2001;82:670–672. [PubMed] [Google Scholar]
- Ernst R J, Bulas R V, Gaskill-Shipley M, Tomsick T A. Endovascular therapy of intractable epistaxis complicated by carotid artery occlusive disease. AJNR Am J Neuroradiol. 1995;16:1463–1468. [PMC free article] [PubMed] [Google Scholar]
- Coley S C, Jackson J E. Pulmonary arteriovenous malformations. Clin Radiol. 1998;53:396–404. doi: 10.1016/s0009-9260(98)80267-5. [DOI] [PubMed] [Google Scholar]
- Iqbal M, Rossoff L J, Steinberg H N, Marzouk K A, Siegel D N. Pulmonary arteriovenous malformations: a clinical review. Postgrad Med J. 2000;76:390–394. doi: 10.1136/pmj.76.897.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossage J R, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med. 1998;158:643–661. doi: 10.1164/ajrccm.158.2.9711041. [DOI] [PubMed] [Google Scholar]
- Swanson K L, Prakash U B, Stanson A W. Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982–1997. Mayo Clin Proc. 1999;74:671–680. doi: 10.4065/74.7.671. [DOI] [PubMed] [Google Scholar]
- Saluja S, Henderson K J, White R I., Jr Embolotherapy in the bronchial and pulmonary circulations. Radiol Clin North Am. 2000;38:425–448. doi: 10.1016/s0033-8389(05)70172-x. [DOI] [PubMed] [Google Scholar]
- Porstmann W. In: Kelop O, editor. Current Concepts in Pediatric Radiology. Berlin: Springer; 1977. Therapeutic embolization of arteriovenous pulmonary fistula by catheter technique. pp. 23–31.
- White R I, Jr, Lynch-Nyhan A, Terry P, et al. Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology. 1988;169:663–669. doi: 10.1148/radiology.169.3.3186989. [DOI] [PubMed] [Google Scholar]
- Dutton J A, Jackson J E, Hughes J M, et al. Pulmonary arteriovenous malformations: results of treatment with coil embolization in 53 patients. AJR Am J Roentgenol. 1995;165:1119–1125. doi: 10.2214/ajr.165.5.7572487. [DOI] [PubMed] [Google Scholar]
- Prasad V, Chan R P, Faughnan M E. Embolotherapy of pulmonary arteriovenous malformations: efficacy of platinum versus stainless steel coils. J Vasc Interv Radiol. 2004;15:153–160. doi: 10.1097/01.rvi.0000106390.63463.05. [DOI] [PubMed] [Google Scholar]
- White R I, Jr, Pollak J S, Wirth J A. Pulmonary arteriovenous malformations: diagnosis and transcatheter embolotherapy. J Vasc Interv Radiol. 1996;7:787–804. doi: 10.1016/s1051-0443(96)70851-5. [DOI] [PubMed] [Google Scholar]
- Lee D W, White R I, Jr, Egglin T K, et al. Embolotherapy of large pulmonary arteriovenous malformations: long-term results. Ann Thorac Surg. 1997;64:930–939. doi: 10.1016/s0003-4975(97)00815-1. discussion 939–40. [DOI] [PubMed] [Google Scholar]
- Tal M G, Saluja S, Henderson K J, White R I., Jr Vein of Galen technique for occluding the aneurysmal sac of pulmonary arteriovenous malformations. J Vasc Interv Radiol. 2002;13:1261–1264. doi: 10.1016/s1051-0443(07)61975-7. [DOI] [PubMed] [Google Scholar]
- Coley S C, Jackson J E. Venous sac embolization of pulmonary arteriovenous malformations in two patients. AJR Am J Roentgenol. 1996;167:452–454. doi: 10.2214/ajr.167.2.8686624. [DOI] [PubMed] [Google Scholar]
- Remy-Jardin M, Wattinne L, Remy J. Transcatheter occlusion of pulmonary arterial circulation and collateral supply: failures, incidents, and complications. Radiology. 1991;180:699–705. doi: 10.1148/radiology.180.3.1871280. [DOI] [PubMed] [Google Scholar]
- Dinkel H P, Triller J. Pulmonary arteriovenous malformations: embolotherapy with superselective coaxial catheter placement and filling of venous sac with Guglielmi detachable coils. Radiology. 2002;223:709–714. doi: 10.1148/radiol.2233010953. [DOI] [PubMed] [Google Scholar]
- Haitjema T, ten Berg J M, Overtoom T T, Ernst J M, Westermann C J. Unusual complications after embolization of a pulmonary arteriovenous malformation. Chest. 1996;109:1401–1404. doi: 10.1378/chest.109.5.1401. [DOI] [PubMed] [Google Scholar]
- Pollak J S, Egglin T K, Rosenblatt M M, Dickey K W, White R I., Jr Clinical results of transvenous systemic embolotherapy with a neuroradiologic detachable balloon. Radiology. 1994;191:477–482. doi: 10.1148/radiology.191.2.8153325. [DOI] [PubMed] [Google Scholar]
- Pennington D W, Gold W M, Gordon R L, Steiger D, Ring E J, Golden J A. Treatment of pulmonary arteriovenous malformations by therapeutic embolization. Rest and exercise physiology in eight patients. Am Rev Respir Dis. 1992;145:1047–1051. doi: 10.1164/ajrccm/145.5.1047. [DOI] [PubMed] [Google Scholar]
- Saluja S, Sitko I, Lee D W, Pollak J, White R I., Jr Embolotherapy of pulmonary arteriovenous malformations with detachable balloons: long-term durability and efficacy. J Vasc Interv Radiol. 1999;10:883–889. doi: 10.1016/s1051-0443(99)70132-6. [DOI] [PubMed] [Google Scholar]
- Fernando H C, Stein M, Benfield J R, Link D P. Role of bronchial artery embolization in the management of hemoptysis. Arch Surg. 1998;133:862–866. doi: 10.1001/archsurg.133.8.862. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Tanaka F, Torizuka T, et al. Bronchial artery embolization for hemoptysis: immediate and long-term results. Cardiovasc Intervent Radiol. 1992;15:154–158. doi: 10.1007/BF02735578. [DOI] [PubMed] [Google Scholar]
- Mal H, Rullon I, Mellot F, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest. 1999;115:996–1001. doi: 10.1378/chest.115.4.996. [DOI] [PubMed] [Google Scholar]
- Najarian K E, Morris C S. Arterial embolization in the chest. J Thorac Imaging. 1998;13:93–104. doi: 10.1097/00005382-199804000-00004. [DOI] [PubMed] [Google Scholar]
- Kato A, Kudo S, Matsumoto K, et al. Bronchial artery embolization for hemoptysis due to benign diseases: immediate and long-term results. Cardiovasc Intervent Radiol. 2000;23:351–357. doi: 10.1007/s002700010062. [DOI] [PubMed] [Google Scholar]
- Uflacker R, Kaemmerer A, Picon P D, et al. Bronchial artery embolization in the management of hemoptysis: technical aspects and long-term results. Radiology. 1985;157:637–644. doi: 10.1148/radiology.157.3.4059552. [DOI] [PubMed] [Google Scholar]
- Yoon W. Embolic agents used for bronchial artery embolization in massive haemoptysis. Expert Opin Pharmacother. 2004;5:361–367. doi: 10.1517/14656566.5.2.361. [DOI] [PubMed] [Google Scholar]
- White R I., Jr Bronchial artery embolotherapy for control of acute hemoptysis: analysis of outcome. Chest. 1999;115:912–915. doi: 10.1378/chest.115.4.912. [DOI] [PubMed] [Google Scholar]
- Vrachliotis T, Sheiman R G. Treatment of massive hemoptysis with intraarterial thrombin injection of a bronchial artery. AJR Am J Roentgenol. 2002;179:113–114. doi: 10.2214/ajr.179.1.1790113. [DOI] [PubMed] [Google Scholar]
- McPherson S, Routh W D, Nath H, Keller F S. Anomalous origin of bronchial arteries: potential pitfall of embolotherapy for hemoptysis. J Vasc Interv Radiol. 1990;1:86–88. doi: 10.1016/s1051-0443(90)72509-2. [DOI] [PubMed] [Google Scholar]
- Sancho C, Escalante E, Dominguez J, et al. Embolization of bronchial arteries of anomalous origin. Cardiovasc Intervent Radiol. 1998;21:300–304. doi: 10.1007/s002709900265. [DOI] [PubMed] [Google Scholar]
- Ramakantan R, Bandekar V G, Gandhi M S, Aulakh B G, Deshmukh H L. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology. 1996;200:691–694. doi: 10.1148/radiology.200.3.8756916. [DOI] [PubMed] [Google Scholar]
- Wong M L, Szkup P, Hopley M J. Percutaneous embolotherapy for life-threatening hemoptysis. Chest. 2002;121:95–102. doi: 10.1378/chest.121.1.95. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Yamakado K, Murashima S, et al. Superselective bronchial artery embolization for hemoptysis with a coaxial microcatheter system. J Vasc Interv Radiol. 1997;8:65–70. doi: 10.1016/s1051-0443(97)70517-7. [DOI] [PubMed] [Google Scholar]
- Pump K K. Distribution of bronchial arteries in the human lung. Chest. 1972;62:447–451. doi: 10.1378/chest.62.4.447. [DOI] [PubMed] [Google Scholar]
- Yoon W, Kim J K, Kim Y H, Chung T W, Kang H K. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002;22:1395–1409. doi: 10.1148/rg.226015180. [DOI] [PubMed] [Google Scholar]
- Tonkin I L, Hanissian A S, Boulden T F, et al. Bronchial arteriography and embolotherapy for hemoptysis in patients with cystic fibrosis. Cardiovasc Intervent Radiol. 1991;14:241–246. doi: 10.1007/BF02578470. [DOI] [PubMed] [Google Scholar]
- Munk P L, Morris D C, Nelems B. Left main bronchial-esophageal fistula: a complication of bronchial artery embolization. Cardiovasc Intervent Radiol. 1990;13:95–97. doi: 10.1007/BF02577360. [DOI] [PubMed] [Google Scholar]
- Girard P, Baldeyrou P, Lemoine G, Grunewald D. Left main-stem bronchial stenosis complicating bronchial artery embolization. Chest. 1990;97:1246–1248. doi: 10.1378/chest.97.5.1246. [DOI] [PubMed] [Google Scholar]
- Cohen A M, Doershuk C F, Stern R C. Bronchial artery embolization to control hemoptysis in cystic fibrosis. Radiology. 1990;175:401–405. doi: 10.1148/radiology.175.2.2326467. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Heianna J, Okane K, Izumi J, Watarai J. Internal mammary artery embolization for hemoptysis. Acta Radiol. 1999;40:187–190. doi: 10.3109/02841859909177736. [DOI] [PubMed] [Google Scholar]
- Tessier D J, Fowl R J, Stone W M, et al. Iatrogenic hepatic artery pseudoaneurysms: an uncommon complication after hepatic, biliary and pancreatic procedures. Ann Vasc Surg. 2003;17:663–669. doi: 10.1007/s10016-003-0075-1. [DOI] [PubMed] [Google Scholar]
- Mohr A M, Lavery R F, Barone A, et al. Angiographic embolization for liver injuries: low mortality, high morbidity. J Trauma. 2003;55:1077–1081. doi: 10.1097/01.TA.0000100219.02085.AB. [DOI] [PubMed] [Google Scholar]
- Bilbao J I, Torres E, Martìnez-Cuesta A. nontraumatic abdominal emergencies: imaging and intervention in gastrointestinal hemorrhage and ischemia. Eur Radiol. 2002;12:2161–2171. doi: 10.1007/s00330-002-1568-y. [DOI] [PubMed] [Google Scholar]
- Llovet J M, Real M I, Montañà X, et al. Arterial embolization and chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- Wigmore S J, Redhead D N, Thomson B N, et al. Postchemoembolization syndrome. Tumor necrosis or hepatic injury? Br J Cancer. 2003;89:1423–1427. doi: 10.1038/sj.bjc.6601329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A O, Yuen M F, Hui C K, Tso W K, Lai C L. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747–1752. doi: 10.1002/cncr.10407. [DOI] [PubMed] [Google Scholar]
- Carr B I. Hepatic artery chemoembolization for advance stage HCC: experience of 650 patients. Hepatogastroenterology. 2002;49:79–86. [PubMed] [Google Scholar]
- Caturelli E, Siena D A, Fusilli S, et al. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of damage to nontumorous liver tissue. Long-term prospective study. Radiology. 2000;215:123–128. doi: 10.1148/radiology.215.1.r00ap21123. [DOI] [PubMed] [Google Scholar]
- Lau K Y, Wong T P, Wong W W, Tan L T, Chan J K, Lee A S. Emergency embolization of spontaneous ruptured hepatocellular carcinoma: correlation between survival and Child-Pugh classification. Australas Radiol. 2003;47:231–235. doi: 10.1046/j.1440-1673.2003.01168.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Sato S, Hashimoto M, et al. A case of hepatocellular carcinoma rupturing after angiography. Kurume Med J. 2001;48:241–245. doi: 10.2739/kurumemedj.48.241. [DOI] [PubMed] [Google Scholar]
- Bilbao J I, Ruza M, Longo J M, Lecumberri F J. Intraperitoneal hemorrhage due to rupture of hepatocellular carcinoma after transcatheter arterial embolization with Lipiodol. A case report. Eur J Radiol. 1992;15:68–70. doi: 10.1016/0720-048x(92)90208-q. [DOI] [PubMed] [Google Scholar]
- Yeh C N, Chen H M, Chen M F, Chao T C. Peritoneal implanted hepatocellular carcinoma with rupture after TACE presented as acute appendicitis. Hepatogastroenterology. 2002;49:938–940. [PubMed] [Google Scholar]
- Dozio B, Scanzani R, Rovere G, Sangalli L, Sacerdoti S, Surian M. Hemoperitoneum in a continuous ambulatory peritoneal dialysis patient caused by a hepatocarcinoma treated with percutaneous embolization. Am J Kidney Dis. 2001;38:E11. doi: 10.1053/ajkd.2001.26915. [DOI] [PubMed] [Google Scholar]
- Okada K, Koda M, Murawaki Y, Kawasaki H. Changes in esophageal variceal pressure after transcatheter arterial embolization for hepatocellular carcinoma. Endoscopy. 2001;33:595–600. doi: 10.1055/s-2001-15310. [DOI] [PubMed] [Google Scholar]
- Kim H K, Chung Y H, Song B C, et al. Ischemic bile duct injury as a serious complication after transarterial chemoembolization in patients with hepatocellular carcinoma. J Clin Gastroenterol. 2001;32:423–427. doi: 10.1097/00004836-200105000-00013. [DOI] [PubMed] [Google Scholar]
- Sakamoto I, Iwanaga S, Nagaoki K, et al. Intrahepatic biloma formation (bile duct necrosis) after transcatheter arterial chemoembolization. AJR Am J Roentgenol. 2003;181:79–87. doi: 10.2214/ajr.181.1.1810079. [DOI] [PubMed] [Google Scholar]
- Chen J H, Ho Y J, Shen W C. Asymptomatic arterio-biliary fistula after transarterial chemoembolization of metastatic liver tumors. Hepatogastroenterology. 2001;48:842–843. [PubMed] [Google Scholar]
- Takayasu K, Moriyama N, Muramatsu Y, et al. Gallbladder infarction alter hepatic artery embolization. AJR Am J Roentgenol. 1985;144:135–138. doi: 10.2214/ajr.144.1.135. [DOI] [PubMed] [Google Scholar]
- Brown K T. Fatal pulmonary complications alter arterial embolization with 40–120 μ Tris-acril gelatine microspheres. J Vasc Interv Radiol. 2004;15:197–200. doi: 10.1097/01.rvi.0000109400.52762.1f. [DOI] [PubMed] [Google Scholar]
- Tajima T, Honda H, Kuroiwa T, et al. Pulmonary complications after hepatic artery chemoembolization or infusion via the inferior phrenic artery for primary liver cancer. J Vasc Interv Radiol. 2002;13:893–900. doi: 10.1016/s1051-0443(07)61772-2. [DOI] [PubMed] [Google Scholar]
- Gupta S, Yao J C, Ahrar K, et al. Hepatic artery embolization and chemoembolization for treatment of patients with metastatic carcinoid tumors: the M.D. Anderson experience. Cancer J. 2003;9:261–267. doi: 10.1097/00130404-200307000-00008. [DOI] [PubMed] [Google Scholar]
- Roche A, Girish B V, de Baere T, et al. Trans-catheter arterial chemoembolization as first-line treatment for hepatic metastases from endocrine tumors. Eur Radiol. 2003;13:136–140. doi: 10.1007/s00330-002-1558-0. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Kienbaum P, Wiesemes R, Peters J. Somatostatin does not prevent serotonin release and flushing during chemoembolization of carcinoid liver metastases. Anesthesiology. 2003;98:1007–1011. doi: 10.1097/00000542-200304000-00030. [DOI] [PubMed] [Google Scholar]
- Inaba Y, Arai Y, Matsueda K, Takeuchi Y, Aramaki T. Right gastric artery embolization to prevent acute gastric mucosal lesions in patients undergoing repeat hepatic arterial infusion chemotherapy. J Vasc Interv Radiol. 2001;12:957–963. doi: 10.1016/s1051-0443(07)61576-0. [DOI] [PubMed] [Google Scholar]
- Yamagami T, Nakamura T, Iida S, Kato T, Nishimura T. Embolization of the right gastric artery before hepatic arterial infusion chemotherapy to prevent gastric mucosal lesions: approach through the hepatic artery versus the left gastric artery. AJR Am J Roentgenol. 2002;179:1605–1610. doi: 10.2214/ajr.179.6.1791605. [DOI] [PubMed] [Google Scholar]
- Kodama Y, Shimizu T, Endo H, Miyamoto N, Miyasaka K. Complications of percutaneous transhepatic portal vein embolization. J Vasc Interv Radiol. 2002;13:1233–1237. doi: 10.1016/s1051-0443(07)61970-8. [DOI] [PubMed] [Google Scholar]
- Jaeck D, Bachellier P, Nakano H, et al. One or two-stage hepatectomy combined with portal vein embolization for initially nonresectable colorectal liver metastases. Am J Surg. 2003;185:221–229. doi: 10.1016/s0002-9610(02)01373-9. [DOI] [PubMed] [Google Scholar]
- Sangro B, Bilbao J I, Herrero I, et al. Partial splenic embolization for the treatment of hypersplenism in cirrhosis. Hepatology. 1993;18:309–314. [PubMed] [Google Scholar]
- Sakai T, Shiraki K, Inoue H, et al. Complications of partial splenic embolization in cirrhotic patients. Dig Dis Sci. 2002;47:388–391. doi: 10.1023/a:1013786509418. [DOI] [PubMed] [Google Scholar]
- Funaki B. Superselective embolization of lower gastrointestinal hemorrhage: a new paradigm. Abdom Imaging. 2004;29:434–438. doi: 10.1007/s00261-003-0150-7. [DOI] [PubMed] [Google Scholar]
- Morris D C, Nichols D M, Connell D G, Burhenne H J. Embolization of the left gastric artery in the absence of angiographic extravasation. Cardiovasc Intervent Radiol. 1986;9:195–198. doi: 10.1007/BF02577940. [DOI] [PubMed] [Google Scholar]
- Dempsey D T, Burke D R, Reilly R S, McLean G K, Rosato E F. Angiography in poor-risk patients with massive nonvariceal upper gastrointestinal bleeding. Am J Surg. 1990;159:282–286. doi: 10.1016/s0002-9610(05)81218-8. [DOI] [PubMed] [Google Scholar]
- Aina R, Oliva V L, Therasse E, et al. Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol. 2001;12:195–200. doi: 10.1016/s1051-0443(07)61825-9. [DOI] [PubMed] [Google Scholar]
- Encarnacion C E, Kadir S, Beam C A, Payne C S. Gastrointestinal bleeding: treatment with gastrointestinal arterial embolization. Radiology. 1992;183:505–508. doi: 10.1148/radiology.183.2.1561358. [DOI] [PubMed] [Google Scholar]
- Shapiro N, Brandt L, Sprayregen S, Mitsudo S, Glotzer P. Duodenal infarction after therapeutic Gelfoam embolization of a bleeding duodenal ulcer. Gastroenterology. 1981;80:176–180. [PubMed] [Google Scholar]
- Lang E V, Picus D, Marx M V, Hicks M E, Friedland G W. Massive upper gastrointestinal hemorrhage with normal findings on arteriography: value of prophylactic embolization of the left gastric artery. AJR Am J Roentgenol. 1992;158:547–549. doi: 10.2214/ajr.158.3.1738991. [DOI] [PubMed] [Google Scholar]
- Darcy M. Treatment of lower gastrointestinal bleeding: vasopressin infusion versus embolization. J Vasc Interv Radiol. 2003;14:535–543. doi: 10.1097/01.rvi.0000064862.65229.8a. [DOI] [PubMed] [Google Scholar]
- Gomes A S, Lois J F, McCoy R D. Angiographic treatment of gastrointestinal hemorrhage: comparison of vasopressin infusion and embolization. AJR Am J Roentgenol. 1986;146:1031–1037. doi: 10.2214/ajr.146.5.1031. [DOI] [PubMed] [Google Scholar]
- Cynamon J, Atar E, Steiner A, et al. Catheter-induced vasospasm in the treatment of acute lower gastrointestinal bleeding. J Vasc Interv Radiol. 2003;14:211–216. doi: 10.1097/01.rvi.0000058323.82956.e4. [DOI] [PubMed] [Google Scholar]
- Ledermann H P, Schoch E, Jost R, Decurtins M, Zollikofer C L. Superselective coil embolization in acute gastrointestinal hemorrhage: personal experience in 10 patients and review of the literature. J Vasc Interv Radiol. 1998;9:753–760. doi: 10.1016/s1051-0443(98)70387-2. [DOI] [PubMed] [Google Scholar]
- Kuo W T, Lee D E, Saad W E, Patel N, Sahler L G, Waldman D L. Superselective microcoil embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2003;14:1503–1509. doi: 10.1097/01.rvi.0000099780.23569.e6. [DOI] [PubMed] [Google Scholar]
- Munro N P, Woodhams S, Nawrocki J D, Fletcher M S, Thomas P J. The role of transarterial embolization in the treatment of renal cell carcinoma. BJU Int. 2003;92:240–244. doi: 10.1046/j.1464-410x.2003.04314.x. [DOI] [PubMed] [Google Scholar]
- Lammer J, Justich E, Schreyer H, Pettek R. Complications of renal tumor embolization. Cardiovasc Intervent Radiol. 1985;8:31–35. doi: 10.1007/BF02552637. [DOI] [PubMed] [Google Scholar]
- Koga F, Goto S, Suzuki S. Retroperitoneal abscess formation accompanied by intraabdominal free air, a rare complication of transcatheter arterial embolization of renal tumor: a case report. Hinyokika Kiyo. 1996;42:443–446. [PubMed] [Google Scholar]
- Zambelli S, Vergara E, Piccardo M, Zai G. Colonic infarct as a complication of renal embolization with absolute ethanol. Radiol Med (Torino) 1986;72:586–589. [PubMed] [Google Scholar]
- Mulligan B D, Espinosa G A. Bowel infarction: complication of ethanol ablation of a renal tumor. Cardiovasc Intervent Radiol. 1983;6:55–57. doi: 10.1007/BF02552794. [DOI] [PubMed] [Google Scholar]
- Siniluoto T M, Hellstrom P A, Paivansalo M J, Leinonen A S. Testicular infarction following ethanol embolization of a renal neoplasm. Cardiovasc Intervent Radiol. 1988;11:162–164. doi: 10.1007/BF02577110. [DOI] [PubMed] [Google Scholar]
- Twomey B P, Wilkins R A, Mee A D. Skin necrosis: a complication of alcohol infarction of a hypernephroma. Cardiovasc Intervent Radiol. 1985;8:202–203. doi: 10.1007/BF02552899. [DOI] [PubMed] [Google Scholar]
- Delgado P, Diaz F, Gonzalez A, et al. Transvascular ethanol embolization: first option for the management of symptomatic nonfunctioning renal allograft in situ. Transplant Proc. 2003;35:1684–1685. doi: 10.1016/s0041-1345(03)00624-9. [DOI] [PubMed] [Google Scholar]
- Magner P, Bear R A. Renal abscess: complication of ethanol renal devitalization for hypertension in chronic renal failure. CMAJ. 1987;136:1063–1064. [PMC free article] [PubMed] [Google Scholar]
- Atar E, Belenky A, Neuman-Levin M, Yussim A, Bar-Nathan N, Bachar G N. Nonfunctioning renal allograft embolization as alternative to graft nephrectomy: report on seven years' experience. Cardiovasc Intervent Radiol. 2003;26:37–39. doi: 10.1007/s00270-002-1976-z. [DOI] [PubMed] [Google Scholar]
- Kamai T, Saito K, Hirokawa M, Tukamoto H, Ashida H. A case of gross hematuria arising during embolization of renal arteriovenous malformation. Urol Int. 1997;58:55–57. doi: 10.1159/000282948. [DOI] [PubMed] [Google Scholar]
- Yoon J W, Koo J R, Baik G H, Kim J B, Kim D J, Kim H K. Erosion of embolization coils and guidewires from the kidney to the colon: delayed complication from coil and guidewire occlusion of renal arteriovenous malformation. Am J Kidney Dis. 2004;43:1109–1112. doi: 10.1053/j.ajkd.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Spies J B, Spector A, Roth A R, et al. Complications after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:873–880. doi: 10.1016/s0029-7844(02)02341-4. [DOI] [PubMed] [Google Scholar]
- Goodwin S C, Walker W J. Uterine artery embolization for treatment of uterine fibroids. Curr Opin Obstet Gynecol. 1998;10:315–320. doi: 10.1097/00001703-199808000-00006. [DOI] [PubMed] [Google Scholar]
- Vashisht A, Studd J, Carey A, Burn P. Fatal septicemia after fibroid embolization. Lancet. 1999;354:307–308. doi: 10.1016/S0140-6736(99)02987-6. [DOI] [PubMed] [Google Scholar]
- Payne J F, Haney A F. Serious complications of uterine artery embolization for conservative treatment of fibroids. Fertil Steril. 2003;79:128–131. doi: 10.1016/s0015-0282(02)04398-4. [DOI] [PubMed] [Google Scholar]
- Pelage J P, Le Dref O, Soyer P, et al. Fibroid-related menorrhagia: treatment with superselective embolization of uterine arteries and midterm follow-up. Radiology. 2000;215:428–431. doi: 10.1148/radiology.215.2.r00ma11428. [DOI] [PubMed] [Google Scholar]
- Tropeano G, Litiwcka K, Di Stasi C, et al. Permanent amenorrhea associated with endometrial atrophy after uterine artery embolization for symptomatic uterine fibroids. Fertil Steril. 2003;79:132–135. doi: 10.1016/s0015-0282(02)04400-x. [DOI] [PubMed] [Google Scholar]
- Hong T M, Tseng H S, Lee R C, Wang J H, Chang C Y. Uterine artery embolization: an effective treatment for intractable obstetric haemorrhage. Clin Radiol. 2004;59:96–101. doi: 10.1016/j.crad.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Ornan D, White R, Pollak J, Tal M. Pelvic embolization for intractable postpartum hemorrhage: long-term follow-up and implications for fertility. Obstet Gynecol. 2003;102:904–910. doi: 10.1016/s0029-7844(03)00769-5. [DOI] [PubMed] [Google Scholar]
- Deffieux X, Berkane N, Uzan S. Pelvic embolization for treatment of hemorrhage related to spontaneous and induced abortion. Am J Obstet Gynecol. 2002;187:819–820. doi: 10.1067/mob.126627. [DOI] [PubMed] [Google Scholar]
- Moodley M, Moodley J. Transcatheter angiographic embolization for the control of massive pelvic hemorrhage due to gestational trophoblastic disease: a case series and review of the literature. Int J Gynecol Cancer. 2003;13:94–97. doi: 10.1046/j.1525-1438.2003.13016.x. [DOI] [PubMed] [Google Scholar]
- Alvarez M, Lockwood C J, Ghidini A, et al. Prophylactic and emergent arterial catheterization for selective embolization in obstetric hemorrhage. Am J Perinatol. 1992;9:441–444. doi: 10.1055/s-2007-999284. [DOI] [PubMed] [Google Scholar]
- Nabi G, Sheikh N, Greene D, Marsh R. Therapeutic transcatheter arterial embolization in the management of intractable haemorrhage from pelvic urological malignancies: preliminary experience and long-term follow-up. BJU Int. 2003;92:245–247. doi: 10.1046/j.1464-410x.2003.04328.x. [DOI] [PubMed] [Google Scholar]
- Ramirez J I, Velmahos G C, Best C R, Chan L S, Demetriades D. Male sexual function after bilateral internal iliac artery embolization for pelvic fracture. J Trauma. 2004;56:734–741. doi: 10.1097/01.ta.0000120287.04574.78. [DOI] [PubMed] [Google Scholar]
- Lin P H, Bush R L, Chaikof E L, et al. A prospective evaluation of hypogastric artery embolization in endovascular aortoiliac aneurysm repair. J Vasc Surg. 2002;36:500–506. doi: 10.1067/mva.2002.127350. [DOI] [PubMed] [Google Scholar]
- Kritpracha B, Pigott J P, Price C I, Russell T E, Corbey M J, Beebe H G. Distal internal iliac artery embolization: a procedure to avoid. J Vasc Surg. 2003;37:943–948. doi: 10.1067/mva.2003.251. [DOI] [PubMed] [Google Scholar]
- Kassardjian Z, Lebret T, Mellot F, et al. Major complex pelvic arteriovenous malformation in a patient with Down syndrome. Urol Int. 2002;69:145–149. doi: 10.1159/000065565. [DOI] [PubMed] [Google Scholar]
- Kolbenstvedt A, Jenssen G, Hedlund H. Priapism of the glans and corpus spongiosum. Report of two cases with angiography. Acta Radiol. 2003;44:456–459. doi: 10.1080/j.1600-0455.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Mulliken J B, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- Mulliken J B. Cutaneous vascular anomalies. Semin Vasc Surg. 1993;6:204–218. [PubMed] [Google Scholar]
- Burrows P E, Mason K P. Percutaneous treatment of low flow vascular malformations. J Vasc Interv Radiol. 2004;15:431–445. doi: 10.1097/01.rvi.0000124949.24134.cf. [DOI] [PubMed] [Google Scholar]
- Lookstein R A, Guller J. Embolization of complex vascular lesions. Mt Sinai J Med. 2004;71:17–28. [PubMed] [Google Scholar]
- Widlus D M, Murray R R, White R I, Jr, et al. Congenital arteriovenous malformations: tailored embolotherapy. Radiology. 1988;169:511–516. doi: 10.1148/radiology.169.2.3175000. [DOI] [PubMed] [Google Scholar]
- Lee B B, Do Y S, Yakes W, Kim D I, Mattassi R, Hyon W S. Management of arteriovenous malformations: a multidisciplinary approach. J Vasc Surg. 2004;39:590–600. doi: 10.1016/j.jvs.2003.10.048. [DOI] [PubMed] [Google Scholar]
- Berenguer B, Burrows P E, Zurakowski D, Mulliken J B. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104:1–11. discussion 12–5. [PubMed] [Google Scholar]
- Lee B B, Do Y S, Byun H S, Choo I W, Kim D I, Huh S H. Advanced management of venous malformation with ethanol sclerotherapy: mid-term results. J Vasc Surg. 2003;37:533–538. doi: 10.1067/mva.2003.91. [DOI] [PubMed] [Google Scholar]
- de Lorimier A A. Sclerotherapy for venous malformations. J Pediatr Surg. 1995;30:188–193. doi: 10.1016/0022-3468(95)90558-8. [DOI] [PubMed] [Google Scholar]
- Gelbert F, Enjolras O, Deffrenne D, Aymard A, Mounayer C, Merland J J. Percutaneous sclerotherapy for venous malformation of the lips: a retrospective study of 23 patients. Neuroradiology. 2000;42:692–696. doi: 10.1007/s002340000364. [DOI] [PubMed] [Google Scholar]
- Siniluoto T M, Svendsen P A, Wikholm G M, Fogdestam I, Edstrom S. Percutaneous sclerotherapy of venous malformations of the head and neck using sodium tetradecyl sulphate (sotradecol) Scand J Plast Reconstr Surg Hand Surg. 1997;31:145–150. doi: 10.3109/02844319709085481. [DOI] [PubMed] [Google Scholar]
- Yao Y, Lomis N N, Scott S M, Yoon H C, Miller F J. Percutaneous sclerotherapy for congenital venous malformations in the extremities. Orthopedics. 2001;24:45–51. doi: 10.3928/0147-7447-20010101-18. [DOI] [PubMed] [Google Scholar]
- Cabrera J, Cabrera J, Jr, Garcia-Olmedo M A, Redondo P. Treatment of venous malformations with sclerosant in microfoam form. Arch Dermatol. 2003;139:1409–1416. doi: 10.1001/archderm.139.11.1409. [DOI] [PubMed] [Google Scholar]
- Yakes W F, Rossi P, Odink H. Arteriovenous malformation management. How I do it. Cardiovasc Intervent Radiol. 1996;19:65–71. doi: 10.1007/BF02563895. [DOI] [PubMed] [Google Scholar]
- Donnelly L F, Bisset G S, III, Adams D M. Marked acute tissue swelling following percutaneous sclerosis of low-flow vascular malformations: a predictor of both prolonged recovery and therapeutic effect. Pediatr Radiol. 2000;30:415–419. doi: 10.1007/s002470050775. [DOI] [PubMed] [Google Scholar]
- Anadon M J, Almendral J, Gonzalez P, et al. Alcohol concentration determines the type of atrial arrhythmia induced in a porcine model of acute alcoholic intoxication. Pacing Clin Electrophysiol. 1996;19:1962–1967. doi: 10.1111/j.1540-8159.1996.tb03262.x. [DOI] [PubMed] [Google Scholar]
- Yakes W F, Luethke J M, Merland J J, et al. Ethanol embolization of arteriovenous fistulas: a primary mode of therapy. J Vasc Interv Radiol. 1990;1:89–96. doi: 10.1016/s1051-0443(90)72510-9. [DOI] [PubMed] [Google Scholar]
- Marrocco-Trischitta M M, Guerrini P, Abeni D, Stillo F. Reversible cardiac arrest after polidocanol sclerotherapy of peripheral venous malformation. Dermatol Surg. 2002;28:153–155. doi: 10.1046/j.1524-4725.2002.00344.x. [DOI] [PubMed] [Google Scholar]
- Ouvry P, Chaudet A, Guillerot E. Aetoxisclerol: 1st impressions. Phlebologie. 1978;31:75–77. [PubMed] [Google Scholar]
- Orford J, Barker A, Thonell S, King P, Murphy J. Bleomycin therapy for cystic hygroma. J Pediatr Surg. 1995;30:1282–1287. doi: 10.1016/0022-3468(95)90485-9. [DOI] [PubMed] [Google Scholar]
- Mulliken J B, Fishman S J, Burrows P E. Vascular anomalies. Curr Probl Surg. 2000;37:517–584. doi: 10.1016/s0011-3840(00)80013-1. [DOI] [PubMed] [Google Scholar]