ABSTRACT
The primary indication for the urgent percutaneous nephrostomy is to relieve an obstructed and infected renal collecting system (pyonephrosis). Percutaneous nephrostomy catheter placement is a safe procedure with a high technical success rate. This article will discuss all aspects regarding the emergent placement of nephrostomy catheters, including the indications, techniques, results, and complications. Differences between emergent and nonemergent placement of percutaneous nephrostomy catheters will also be addressed.
Keywords: Percutaneous nephrostomy, pyonephrosis, renal failure
Percutaneous nephrostomy (PCN) catheter insertion was first described by Goodwin et al in 19551 as an emergency procedure to relieve urinary obstruction.2 Subsequently, the safety and efficacy of this procedure has been established using a variety of different imaging modalities including various combinations of computed tomography (CT), fluoroscopy, and ultrasound. The scope of PCN catheter placement has been expanded and currently nonemergent clinical scenarios including relieving urinary obstructions, urinary diversion from a leak, and accessing the collecting system for diagnostic and therapeutic procedures outnumber urgent indictions.
Emergent nephrostomy is most common in the clinical setting of pyonephrosis, which can be defined as the presence of pus in an obstructed renal collecting system.3 Pyonephrosis may present with a classic triad of fever, flank pain, and hydronephrosis or simply hydronephrosis and sepsis. Pyonephrosis may also occur when imaging shows a complex appearance to a dilated collecting system. Due to the high risk of sepsis from pyonephrosis and the associated morbidity and mortality, the early recognition and treatment of this condition is of paramount importance. Even if sepsis is able to be treated medically, kidney function may be destroyed due to the formation of xanthogranulomatous pyelonephritis.3
Urinary obstruction may occur from a variety of etiologies in any age group. Any patient with obstruction may develop a superimposed infection and pyonephrosis. In children, urinary obstruction commonly is due to a ureteropelvic junction obstruction. In adults, urinary tract stones cause more than 50% of cases4 of urinary obstruction. An intraluminal or extraluminal tumor is another important cause of urinary obstruction with an associated high morbidity. When pyonephrosis is present, antibiotics with gram-negative coverage are usually the first line of treatment. Escherichia coli is the most common pathogen that is isolated. Other infectious agents, including fungus and tuberculosis, can also be present. The general rule is that prompt drainage is needed to prevent the risk of sepsis when “pus is under pressure.”3
Both PCN catheters and retrograde nephroureteral internal stents have been shown to be equally effective in relieving an obstructed renal collecting system, with similar complication rates.5 The desired method for drainage is often based on the experience of the urologist and/or interventional radiologist at the hospital. In our hospital, drainage by PCN catheter placement is the initial invasive treatment of choice for patients with pyonephrosis.
There are many arguments that are used to support the use of PCN catheters. A wide variety of catheter sizes can be placed (8 French to 12 French) depending on the characteristics of the fluid being drained. The catheters can be irrigated when the drainage is purulent or bloody, which will help avoid clogging. The urine output of the kidney can be measured. Excessive ureteral manipulation can be avoided, decreasing the risk for sepsis or rupture.5 The procedure can also be done with local anesthesia and under conscious sedation, which eliminates the need for an anesthesiologist and risks associated with general anesthesia.3 PCN catheter placement is often easier to schedule in the interventional radiology department, which can have flexibility in the daily schedule when compared with difficulties that arise with scheduling cases in busy operating rooms.
Some institutions attempt to perform retrograde ureteral stenting via cystoscopy as the primary invasive therapeutic procedure. Proponents of the placement of internal double-J stents via cystoscopy note that the patients are more comfortable without the burden of a catheter exiting the flank. There is also a lower potential for bleeding complications as the vascular kidney capsule is not punctured.5 Even though retrograde stenting by cystoscopy is attempted initially at many hospitals, if this procedure fails to alleviate symptoms, PCN insertion is typically pursued.
INDICATIONS
There are many indications for PCN placement, which include relief of obstruction, urinary diversion, and accessing the collecting system for diagnostic and therapeutic procedures. These indications will encompass more than 95% of the cases that are performed.4 Of these broad groups of indications, emergent PCN catheters are usually performed in a subset of this patient population.
As noted above, the main indication for emergent PCN placement is to relieve an obstructed and infected collecting system, also known as pyonephrosis. The emergent treatment of pyonephrosis is considered an acceptable emergent indication because of the risk of developing sepsis very rapidly. In our hospital, pyonephrosis is treated urgently regardless of the time of day or night. The second most common reason for emergent PCN placement is acute renal failure secondary to obstructive uropathy, leading to life-threatening biochemical abnormalities. The most worrisome biochemical abnormality is acute hyperkalemia.6 Although this has been described as an emergent indication,6 in our hospital, hyperkalemia is corrected either medically or by emergent dialysis before a PCN is considered. In general, we will insert nephrostomy catheters in patients with urinary obstruction and without infection during the daytime hours on weekends but rarely at night. In general, if either of these two emergent clinical situations are not present, a PCN is performed electively. In our hospital, certain exceptions are considered, such as if the patient has a renal transplant or a solitary kidney.
PATIENT PREPARATION
Patients requiring emergent PCN placement are either inpatients or are referred from the emergency room. Informed consent is obtained. Some patients in need of emergent PCN insertion are unable to give consent due to mental status changes associated with sepsis. In these situations, informed consent should be obtained from an eligible medical decision maker. If none is available, the procedure is performed as a medical necessity as determined by the primary attending physician caring for the patient.
There are several laboratory values that should be obtained prior to the procedure. A coagulation profile is obtained, which includes a prothrombin time, activated partial thromboplastin time, and an internal normalized ratio (INR). A platelet count is also needed. Our department considers an acceptable INR to be less than 1.5 and an adequate platelet count to be greater than 75,000/mm3. Although Farrell et al noted that a platelet count of less than 100,000/mm3 was significantly predictive for hemorrhage requiring blood transfusion, a similar trend has not been observed in our hospital.7,8 Funaki and Vatakencherry reported a series of 140 PCN placements in our institution and found the risk of bleeding to be between 3.7 and 4.7%, which is near the threshold limits described by the Society of Cardiovascular and Interventional Radiology.4,8 If needed, the patient can be transfused with fresh frozen plasma or with platelets before, during, or after the procedure. Blood cultures, complete blood counts, urinalysis, and urine cultures are usually already obtained by the primary clinical service.
PCN catheters are placed using local anesthesia with 1% Xylocaine (lidocaine). Conscious sedation is achieved using a standard protocol of 25 to 50 μg aliquots of fentanyl and 0.5 to 1 mg aliquots of midazolam. If the patient is allergic to contrast, a premedication regimen of 32 mg of methylprednisolone orally 12 hours and 2 hours before the procedure and 50 mg of Benadryl orally or intravenously 1 hour before the procedure is administered.9 Although the goal of the procedure is to inject the contrast into the collecting system, intra-arterial and intravenous contamination does occur with a high frequency, which necessitates the need for proper prophylaxis in the presence of a contrast allergy. If the patient's medical condition requires treatment that cannot be delayed by the premedication regimen, the procedure can be performed with alternative contrast agents such as gadolinium or carbon dioxide.
Before choosing a preprocedural antibiotic, it is important to determine whether the patient is septic. As most patients who are referred to interventional radiology for emergent PCN placement are presenting with urosepsis, antibiotic therapy has already been initiated in most cases. The coverage typically includes broad coverage for both gram-negative and gram-positive organisms. If antibiotics have not yet been administered, a broad-spectrum antibiotic such as ampicillin/sulbactam (Unasyn) may be given. If the PCN is placed emergently in a patient who is not septic, our department policy is to administer a first-generation cephalosporin for prophylaxis, such as Cefazolin 1 g intravenously.10 If the patient is allergic to penicillin, a fluoroquinolone such as levofloxacin can be administered. It is important to remember that if a fungal infection is suspected, an antifungal medication can also be given as prophylaxis.6 Again, because patients usually present with a clinical picture of urosepsis, antibiotics are continued for many days to weeks after the procedure. The treatment regimen can eventually be tailored to culture-specific antibiotics based on the urine specimen obtained from the PCN placement.
ACCESS/TECHNIQUES
Relevant Anatomy
The ideal puncture entry site into the kidney is via a posterior calyx using a subcostal approach. The posterior calyx is desirable because entry does not violate Brodel's avascular plane, which should help minimize the risk of bleeding complications. A posterior calyx entry also helps for subsequent dilations and catheter placement because these can be performed in a straight line with the wire in place. If an anterior calyx is entered, a sharp angle is needed to enter the renal pelvis, which can make catheter placement more difficult. A subcostal entry is desirable to eliminate the risk of pleural complications, which can be encountered with supracostal access. A supracostal approach has been described to have a significantly higher intrathoracic complication rate, including pneumothorax and pleural effusion, and overall complication rate when compared with subcostal access11 in patients undergoing percutaneous renal surgery.
Existing imaging, such as CT or ultrasound, are reviewed preoperatively to avoid potential complications from inadvertent organ puncture.12 Injury to adjacent viscera near the kidneys has been reported rarely, with rates between 0 and 3.0%.13 The colon has been described as the organ that is most likely to be injured. Inadvertent puncture typically occurs due to a very lateral puncture when the colon is in its normal location or due to a puncture using standard anatomic landmarks when the colon is in an aberrant position such as in a congenital retrorenal location.13,14
Imaging Guidance
PCN placement needs imaging guidance to be successful and safe. Many different techniques have been described including using fluoroscopy, ultrasound, CT, and various combinations of these three modalities.15,16 The final decision on the imaging modality is determined by the physician performing the procedure. The ultimate goal is to identify a safe access site for the procedure and imaging with a combination of ultrasound and fluoroscopy is typically used in most interventional radiology departments.
Standard Procedure for Initial Entry
The patient is placed prone on the angiography table and prepped and draped in the usual sterile fashion. Fluoroscopy is used to localize an appropriate subcostal puncture site. Ultrasound is then used to localize the kidney and identify the degree of hydronephrosis and a posterior calyx. The skin is anesthetized with 1% lidocaine and a small skin nick is made. The technique of entering the collecting system is again based on physician preference. Many techniques have been described including a direct trocar access, the Seldinger technique, and using one needle (“single-stick”) or two needles (“double-stick”) techniques.8,17,18 In most cases I prefer a hybrid technique, which will be described below.
A posterior calyx is targeted with real-time ultrasound guidance. A 21-gauge needle (Accustick introducer set; Boston Scientific, Natick, MA) is advanced toward a posterior calyx. Urine is aspirated through the skinny needle. The volume of urine that is aspirated is always less than the volume of dilute contrast that is injected to opacify the collecting system to verify needle position. A small amount of air can be injected after the patient is elevated to ~30 to 40 degrees so that air rises to fill a posterior calyx. If the puncture site is deemed inappropriate, a second skinny needle can be advanced toward a posterior calyx using fluoroscopic guidance.
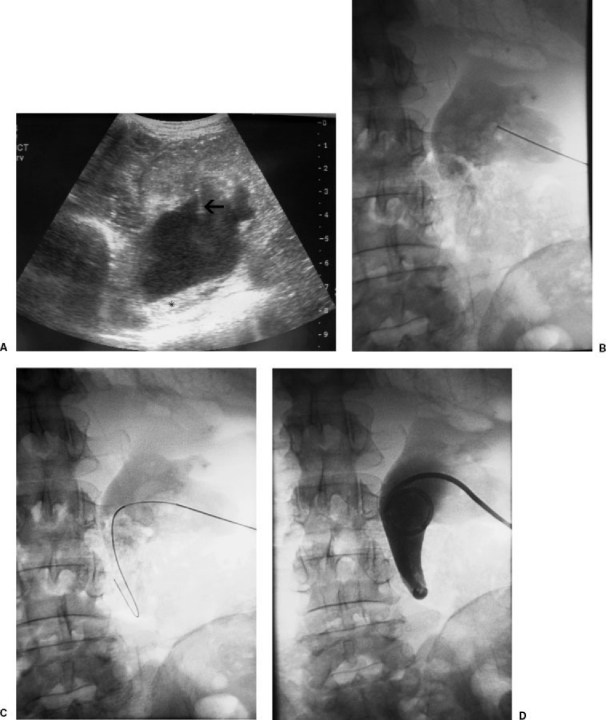
A 0.018-inch guide wire (V18 control wire; Boston Scientific) is advanced down the ureter. The needle is removed and coaxial 4-F and 6-F dilators with a metal stiffener are advanced over the guide wire. A stiff 0.035-inch wire (Amplatz superstiff guide wire; Boston Scientific) is advanced under fluoroscopic guidance through the outer 6-F dilator and down the ureter or coiled in the renal pelvis. The tract is dilated with 7-F and 9-F dilators and an 8.5-F all-purpose drainage pigtail catheter is coiled in the renal pelvis (Boston Scientific).8,18 Only a tiny amount of contrast is injected to confirm the catheter tip position (Fig. 1).
Figure 1.
(A) Transverse ultrasound of kidney with hydronephrosis. Arrow shows skinny needle accessing the kidney. Asterisk (*) indicates echogenic debris/fluid level. (B) Skinny needle access into posterior lower pole calyx. (C) Guide wire advanced into collecting system. (D) Final pigtail catheter placement.
If the patient has a fluoroscopically visualized target, such as a radiopaque kidney stone or an internal ureteral stent, the skinny needle can be advanced under fluoroscopic guidance using a “down the barrel” technique using ~20 to 30 degrees of fluoroscopic angulation. Once access into the collecting system is gained, the previously described steps above are performed.
A single-stick method has been described. After access into the collecting system is confirmed by the aspiration of urine, the nephrostomy is placed without the use of contrast to opacify the collecting system.8,15 The single-stick method is quicker and does not have the risks associated with injection of contrast. This aspect of the procedure may be desirable in this patient population with pyonephrosis.8,15
If emergent or urgent access is needed into a nondilated collecting system, such as in a patient with a urine leak, percutaneous puncture with ultrasound guidance is technically difficult.4,19 Administration of 50 to 100 mL of nonionic intravenous contrast will opacify the collecting system, enabling fluoroscopic puncture. Carbon dioxide or a small amount of air has been described as an alternative contrast agent in patients with allergies to iodinated contrast.19
ANTEGRADE PYELOGRAPHY
During routine nephrostomy catheter placement, antegrade pyleography is typically performed after pelvicalyceal access is achieved. Pyelography is helpful to evaluate both the anatomic and functional characteristics of the collecting system. Proper PCN catheter deployment is facilitated when the actual collecting system can be visualized. Underlying pathological lesions, such as an obstructive calculus or tumor, can be diagnosed during pyelography. Also, urodynamics of the collecting system can be evaluated.
During emergent PCN catheter placement, the goal is to inject as little contrast as possible to safely place the PCN catheter. Catheter placement is the primary objective and this is performed at the expense of interrogating the entire collecting system for underlying pathology or physiological information. Just enough of the collecting system is opacified to determine the adequacy of the puncture site and to safely deploy the catheter in the desired location. In the setting of pyonephrosis, the collecting system is usually already under pressure. If contrast is injected, the collecting system may become overdistended, which increases the risk of bacteremia and urosepsis. If urosepsis does occur, the patients can become clinically unstable very quickly. Contrast is injected only after an equal or larger volume of urine is aspirated to minimize the risk of bacteremia and urosepsis.
In a series described by Funaki and Vatakencherry, nephrostomy catheters can be successfully placed using a “single-stick” method without the injection of contrast.8 In the setting of pyonephrosis, this technique may be helpful to eliminate any injection of contrast into the collecting system, which may precipitate a bacteremic episode.
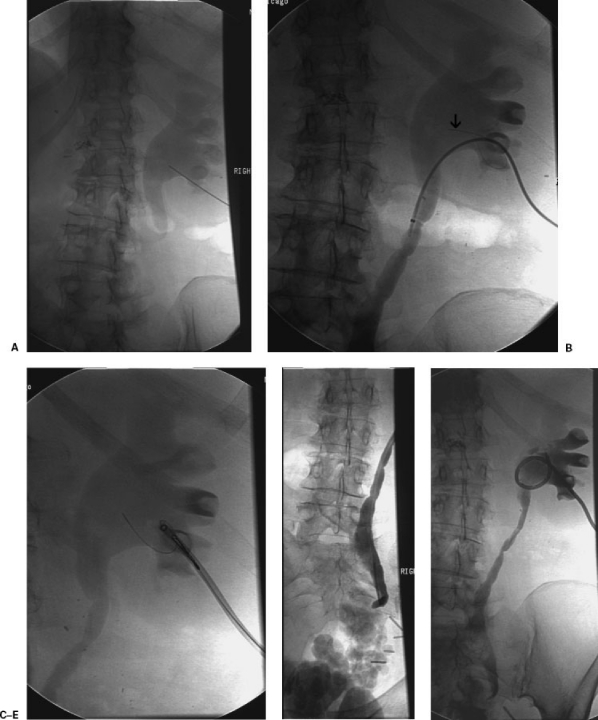
In the setting of pyonephrosis, additional diagnostic pyelography or further intervention, such as nephroureterostomy catheter placement during the initial procedure, should be avoided. Excessive manipulation of an infected collecting system can precipitate urosepsis.20 If further intervention is desired, the patient can return several days after decompression when the patient is stabilized (Fig. 2).
Figure 2.
(A) Skinny needle access into posterior lower pole calyx. (B) Arrow points to guide wire that was sheared off accidentally during wire manipulation. (C) Wire fragment was successfully snared. (D) Distal ureteral obstruction was not able to be crossed with a catheter and a guide wire. (E) A pigtail nephrostomy was successfully placed. The patient experienced a bacteremic episode after the procedure requiring an ICU admission, which was likely precipitated by excessive manipulation in an infected collecting system.
CATHETER PLACEMENT
Once pelvicalyceal access is achieved, a stiff 0.035-inch wire (Amplatz superstiff guide wire; Boston Scientific) is either coiled in the renal pelvis or is advanced down the ureter. Serial dilations are performed with sizes up to one French size larger than the catheter that will be placed. For instance, if an 8-F locking pigtail nephrostomy is desired, serial dilations are performed using 7-F and 9-F dilators. The 8-F self-retaining loop pigtail catheter is deployed over the wire and formed in the renal pelvis. A trace amount of contrast can be injected to confirm the final position. The catheter is either sutured to the skin or secured with an adhesive disc. The catheter is placed to gravity drainage.
AFTERCARE
Immediate
Catheters are placed to a gravity drainage bag. Routine flushing of the catheter is only performed if output drops or if output is very purulent or bloody. Urine has proteolytic enzymes that help break down blood clots.6 Patients who require emergent PCN catheter placement are sick enough to require admission to the hospital or are already admitted to the hospital. The patients are rounded on daily by the interventional radiology service. The amount and quality of urine output are noted. The skin at the puncture site is also evaluated.
Long-Term Care
Patients are schedule to return every 6 weeks for routine catheter changes. Patients can come back sooner after they are stabilized for diagnostic pyelography or for conversion to nephroureterostomy catheters or internal double-J stents as clinically indicated.
RESULTS
When PCN catheters are placed emergently, the initial technical success has been reported to be 98%.21 This is similar to the high technical success rate of 98 to 100% in a series reviewing both emergent and nonemergent PCN catheter placements.20 The risk of a technical failure for both emergent and routine procedures is higher in patients with nondilated collecting systems or who are obese.20 However, PCN catheter placement rates in nondilated collecting systems can be successful with a success rate of 92 to 96%.6,19 The presence of hydronephrosis, which is common in most cases of emergent PCN catheter placement, contributes to the high potential technical success for the procedure.
COMPLICATIONS
According to the Society of Interventional Radiology (SIR) standards, an upper threshold of complications should be set at 10%.4 Major complications include sepsis, hemorrhage, vascular injury, bowel transgression, and pleural complications4 and are categorized by outcome including those requiring therapy, minor hospitalization (< 48 hours), those requiring major therapy, unplanned increase in level of care and prolong hospitalization (> 48 hours), permanent adverse sequelae, and death. Minor complications require no therapy or have no consequence or nominal therapy of no consequence. This includes overnight admission for observation only.4
When PCN catheters are placed emergently, Lee et al described a major complication rate of 6% and a minor complication rate of 28%.21 This major complication rate is within the expected range for the guidelines set forth by the Standards of Practice by the SIR. As expected, more complications can occur when procedures are performed emergently after-hours. In the series by Lewis and Patel, a higher percentage of major complications during PCN catheter placement occurred after-hours (5.7%) versus during the working day hours (1.8%).22 The minor complication rate described by Lee et al of 28% is much higher than most series, which described minor complication rates to be lower, ranging from 15 to 25%.21 A very low mortality rate of 0.2% has been described with this procedure.6
The two most common and worrisome major complications include sepsis and hemorrhage, and the focus of the discussion will surround these two topics.
SEPSIS
One of the most feared complications of emergent PCN catheter placements is sepsis. Sepsis has a wide variety of presenting signs and symptoms. In emergent nephrostomy, many patients are already septic at the start of the procedure.20 Bacteria and endotoxins can be released from the urine and collecting system during the procedure with subsequent presenting symptoms of fever, chills, leukocytosis, rigors, and shock.
The SIR Standards of Practice describe the incidence of sepsis defined by fever and chills with hypotension, requiring a major increase in level of care at a reported rate of between 1 and 3%.4 The threshold for this complication is less than 4%. PCN catheter drainage of pyonephrosis has been shown to have higher complication rates.3 In the setting of pyonephrosis, septic shock is reported to occur in 7 to 9% of procedures. The threshold for expected sepsis is also higher but remains less than 10%.4 In a series reported by Lee et al on emergent PCN placement, a risk of sepsis was 3.6%20,21; however, 100% of the patients developed a transient increase in body temperature after the procedure.20,21 Higher rates of sepsis from 4.5% up to 21% have been reported; however, in many of these series, patients were already septic at the start of the procedure.20 As expected, in the emergency setting, the risk of complications increases. In reported series, this was attributed to a combination of the critical condition of the patients, less ancillary support, and/or the possibility of less experienced operators performing the procedure such as fellows or residents.
Bacteremia and sepsis can begin during or after the procedure. Careful monitoring is needed as patients can quickly become toxic and require emergent resuscitation and intubation. When this complication arises, intravenous fluids are aggressively administered. Rigors are controlled with 25 to 50 mg of Demerol (meperidine) intravenously. Another dose of broad-spectrum antibiotics such as Unasyn or Levaquin may also be given immediately.
HEMORRHAGE
The rate of hemorrhage requiring transfusion with PCN catheter placement is reported to be between 1 and 4% with a desired threshold of less than 4%.4 In a series of emergency PCN catheter placements, bleeding requiring transfusion occurred in 2.4%.20,21 Mild hematuria commonly occurs after PCN catheter placement and often resolves spontaneously after a few days. If bleeding is excessive, the catheter tip position is assessed to ensure the side holes have not been retracted into the parenchymal tract. In this situation, venous bleeding can be controlled by advancing the catheter more centrally. A larger-diameter catheter can be inserted to tamponade a bleeding source around the parenchymal tract. If the bleeding is heavy or pulsatile, an arterial injury should be considered, which may represent laceration of a renal artery branch, a bleeding pseudoaneurysm, or an arteriovenous fistula.20 These complications often require embolization using either coils, gelfoam, or polyvinyl alcohol particles.20
CONCLUSION
PCN catheter placement should be performed urgently, day or night, when obstruction is present and a clinical suspicion for pyonephrosis also exists. If hydronephrosis is present without infection, PCN catheter placement can be performed during the daylight hours on weekends or electively. In contrast to elective PCN insertion, the goal of urgent or emergent nephrostomy is to decompress the system as rapidly as possible with as little manipulation as necessary. Diagnostic antegrade pyelography should not be performed at the time of initial tube insertion. Instead, this procedure should be deferred for several days to enable the urinary system to decompress, which minimizes the risk of sepsis. In general, a slightly higher rate of complications is encountered during on-call nephrostomy insertion and may be attributed to the underlying comorbidities often present in this patient population.
REFERENCES
- Goodwin W E, Casey W C, Woolf W. Percutaneous trocar nephrostomy in hydronephrosis. J Am Med Assoc. 1955;157:891–894. doi: 10.1001/jama.1955.02950280015005. [DOI] [PubMed] [Google Scholar]
- Reznek R H, Talner L B. Percutaneous nephrostomy. Radiol Clin North Am. 1984;22:393–406. [PubMed] [Google Scholar]
- Yoder I C, Lindfors K K, Pfister R C. Diagnosis and treatment of pyonephrosis. Radiol Clin North Am. 1984;22:407–414. [PubMed] [Google Scholar]
- Ramchandani P, Cardella J F, Grassi C J, et al. Quality improvements guidelines for percutaneous nephrostomy. J Vasc Interv Radiol. 2001;12:1247–1251. doi: 10.1016/s1051-0443(07)61546-2. [DOI] [PubMed] [Google Scholar]
- Pearle M S, Pierce H L, Miller G L, et al. Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J Urol. 1998;160:1260–1264. [PubMed] [Google Scholar]
- Maher M M, Fotheringham T, Lee M J. Percutaneous nephrostomy. Semin Intervent Radiol. 2000;17:329–339. [Google Scholar]
- Farrell T A, Hicks M E. A review of radiologically guided percutaneous nephrostomies in 303 patients. J Vasc Interv Radiol. 1997;8:769–774. doi: 10.1016/s1051-0443(97)70658-4. [DOI] [PubMed] [Google Scholar]
- Funaki B, Vatakencherry G. Comparison of single-stick and double-stick techniques for percutaneous nephrostomy. Cardiovasc Intervent Radiol. 2004;27:35–37. doi: 10.1007/s00270-003-0088-8. [DOI] [PubMed] [Google Scholar]
- ACR American College of Radiology Manual on Contrast Media. 4th ed. Reston, VA: American College of Radiology; 1998.
- Zarrinpar A, Kerlan R K. A guide to antibiotics for the interventional radiologist. Semin Intervent Radiol. 2005;22:69–79. doi: 10.1055/s-2005-871861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munver R, Delvecchio F C, Newman G E, Preminger G M. Critical analysis of supracostal access for percutaneous renal surgery. J Urol. 2001;166:1242–1246. [PubMed] [Google Scholar]
- Dyer R B, Regan J D, Kavanagh P V, Khatod E G, Chen M Y, Zagoria R J. Percutaneous nephrostomy with extensions of the technique: step by step. Radiographics. 2002;22:503–525. doi: 10.1148/radiographics.22.3.g02ma19503. [DOI] [PubMed] [Google Scholar]
- Tuttle D N, Yeh B M, Meng M V, Breiman R S, Stoller M L, Coakley F V. Risk of injury to adjacent organs with lower-pole fluoroscopically guided percutaneous nephrostomy: evaluation with prone, supine, and multiplanar reformatted CT. J Vasc Interv Radiol. 2005;16:1489–1492. doi: 10.1097/01.RVI.0000175331.93499.44. [DOI] [PubMed] [Google Scholar]
- Zagoria R J, Dyer B B. Do's and don't's of percutaneous nephrostomy. Acad Radiol. 1999;6:370–377. doi: 10.1016/s1076-6332(99)80233-5. [DOI] [PubMed] [Google Scholar]
- Gupta S, Gulati M, Uday S K, Rungta U, Suri S. Percutaneous nephrostomy with real-time sonographic guidance. Acta Radiol. 1997;38:454–457. doi: 10.1080/02841859709172099. [DOI] [PubMed] [Google Scholar]
- Barbaric Z L, Hal T, Cochran S T, et al. Percutaneous nephrostomy: placement under CT and fluoroscopic guidance. AJR Am J Roentgenol. 1997;169:151–155. doi: 10.2214/ajr.169.1.9207516. [DOI] [PubMed] [Google Scholar]
- Agostini S, Dedola G L, Gabbrielli S, Masi A. A new percutaneous nephrostomy technique in the treatment of obstructive uropathy. Radiol Med (Torino) 2003;105:454–461. [PubMed] [Google Scholar]
- Funaki B, Tepper J A. Percutaneous nephrostomy. Semin Intervent Radiol. 2006;23:205–208. doi: 10.1055/s-2006-941451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel U, Hussain F F. Percutaneous nephrostomy of nondilated renal collecting system with fluoroscopic guidance: technique and results. Radiology. 2004;233:226–233. doi: 10.1148/radiol.2331031342. [DOI] [PubMed] [Google Scholar]
- Millward S F. Percutaneous nephrostomy: a practical approach. J Vasc Interv Radiol. 2000;11:955–964. doi: 10.1016/s1051-0443(07)61322-0. [DOI] [PubMed] [Google Scholar]
- Lee W J, Patel U, Patel S, Pillari G P. Emergency percutaneous nephrostomy: results and complications. J Vasc Interv Radiol. 1994;5:135–139. doi: 10.1016/s1051-0443(94)71470-6. [DOI] [PubMed] [Google Scholar]
- Lewis S, Patel U. Major complications after percutaneous nephrostomy: lessons from a department audit. Clin Radiol. 2004;59:171–179. doi: 10.1016/s0009-9260(03)00336-2. [DOI] [PubMed] [Google Scholar]