ABSTRACT
Acute limb ischemia is a potentially life-threatening clinical event. Thrombosis in situ, bypass graft thrombosis, and embolic occlusion are the three major precipitating events leading to acute limb ischemia. Management of acute ischemia depends on the clinical status of the affected limb and patient comorbidities. Catheter-directed thrombolysis (CDT) is the treatment of choice for patients with relatively mild acute limb ischemia (Rutherford categories I and IIa) with no contraindications to thrombolytic therapy. Patients with severe acute limb ischemia (Rutherford category IIb) need emergent revascularization. CDT should be considered, nonetheless, if the relative risks compared with primary operation are favorable. CDT is a life- and limb-saving treatment for many patients despite limitations of efficacy and associated complications. This article is a review of the etiology of acute arterial occlusion; clinical triage of patients presenting with acute limb ischemia; catheter guide wire techniques, pharmacological agents, and devices in current use for CDT; as well as the outcomes of CDT.
Keywords: Thrombolysis, acute limb ischemia, plasminogen activator, anticoagulation
Severe acute limb ischemia is a vascular emergency. Sudden loss or marked decrease in limb perfusion that threatens limb viability carries high risk of mortality and limb amputation despite current methods of treatment.1 Expedited clinical evaluation, diagnostic workup, and initiation of an appropriate treatment plan leads to improved outcomes.2 The involvement of interventional radiology in treatment of acute limb ischemia began with the pioneering work of Dotter et al,3 who were the first to report catheter-directed thrombolysis (CDT). The scope of percutaneous treatment has grown with the development of many pharmaceutical agents and endovascular devices. CDT is now accepted as the first-line treatment for properly selected patients with acute limb ischemia. In addition, there are many reports in the recent literature on using percutaneous mechanical thrombectomy (PMT) devices either as an adjunct to or in lieu of CDT.4
ETIOLOGY OF ACUTE LIMB ISCHEMIA
Thrombotic occlusion is the most common cause of acute limb ischemia.5 It occurs much more often in the lower than in the upper extremities. In the lower extremities, atherosclerotic peripheral arterial disease (PAD) is the usual primary cause. Plaque rupture and platelet adherence to the exposed surface coupled with sluggish flow and diseased vessel wall result in thrombosis. The thrombus usually extends proximal and distal from the inciting stenosis to the next significant branch point.5 Long-standing flow impairment from atherosclerotic stenosis leads to development of collateral vessels, and thus the severity of acute limb ischemia from sudden thrombosis of a stenotic vascular segment is variable and often less than seen with other causes. Thrombotic occlusions can occur in any segment of the lower extremities but most commonly involve the superficial femoral artery. Thrombosis in situ also occurs less commonly with cystic adventitial disease, popliteal entrapment and popliteal aneurysm in the lower extremities; vasculitis and repetitive trauma in the upper extremities; and trauma and dissection in both. In addition, procoagulative states such as hyperhomocysteinemia and diabetic ketoacidosis can produce thrombosis in situ in the absence of underlying occlusive disease.6,7
Infrainguinal bypass graft occlusion is common, with reported 5-year primary patency of autologous vein and synthetic above-the-knee bypass of 74 and 39%, respectively.8 The most common cause of synthetic arterial bypass graft occlusion is impaired native vessel outflow or poor arterial inflow. Autologous vein grafts develop anastamotic and midportion stenosis due to myointimal hyperplasia. Insufficient valve stripping can result in focal stenosis. Diffuse sclerosis can occur when the vaso vasorum is damaged.9
Nearly all emboli arise from the left heart, aorta, and iliac vessels. Atrial fibrillation, left ventricular aneurysm, penetrating ulcers, or aneurysms of the aorta and common iliac arteries are the common sources. A minority of emboli are paradoxical venous emboli that pass through an intracardiac shunt. Emboli typically lodge at branch points in the vascular tree and occlude both tributaries.5
CLINICAL EVALUATION
The clinical signs and symptoms of acute limb ischemia include the six “P's”: pain, paralysis, paresthesias, pulselessness, pallor, and polar (i.e., a cold extremity).5 The degree of symptoms suggests the underlying diagnosis. Patients with embolic occlusion are often the most symptomatic and have an abrupt onset because collateral vessels are not developed to perfuse the limb in the face of occlusion. Likewise, bypass surgery often results in reducing available collaterals and profound ischemia may occur with graft occlusion.
A thorough history is required, especially for patients previously unknown to the current treating vascular specialists. History that suggests underlying PAD and thrombosis in situ include (1) history of prior claudication or rest pain, (2) history of coronary artery or cerebrovascular arterial disease, or (3) risk factors for atherosclerotic disease.5 Prior open surgical or percutaneous revascularization renders the presence of PAD and thrombosis at the site of prior intervention likely. Embolic occlusion is suggested by history of a potential embolic source, lack of prior symptoms suggesting PAD, and an asymptomatic contralateral limb. A thorough pulse examination of the ischemic limb with Doppler, chest X-ray, and electrocardiogram and a chemistry panel, complete blood cell count, and prothrombin time/partial thromboplastin time (PTT) need to be obtained.
The medical status of the patient and the physical findings of the ischemic limb are the main guides for treatment. Rutherford et al10 proposed a classification scheme that, with subsequent modifications, was adopted by the Society of Vascular Surgery (SVS) and the International Society of Cardiovascular Surgery (ISCVS). It is the most commonly used method to describe acute limb ischemia (Table 1).
Table 1.
Severity of Acute Leg Ischemia
| Category | Description | Capillary Return | Muscle Paralysis | Sensory Loss | Doppler Signals |
||
|---|---|---|---|---|---|---|---|
| Arterial | Venous | ||||||
| Modified from Rutherford RB, Flanigan DP, Gupta SK, et al. Suggested standards for reports dealing with lower extremity ischaemia. J Vasc Surg 1986;4:80–94, with permission from The Society for Vascular Surgery. | |||||||
| I | Viable | Not immediately threatened | Intact | None | None | Audible | Audible |
| IIa | Threatened | Salvageable if treated | Intact/slow | None | Partial | Inaudible | Audible |
| IIb | Threatened | Salvageable if treated as emergency | Slow/absent | Partial | Partial | Inaudible | Audible |
| III | Irreversible | Primary amputation frequently required | Absent | Complete | Complete | Inaudible | Inaudible |
There are four immediate treatment options for acute limb ischemia depending on the severity: anticoagulation alone, percutaneous treatment, surgical revascularization, and amputation. Therapeutic anticoagulation halts propagation of arterial thrombosis and prevents further emboli. Continuous infusion with heparin sulfate to achieve a PTT 1.5 to 2.0 of control is typically used. Even when urgent surgical revascularization or percutaneous treatment is indicated, anticoagulation from the time of initial diagnosis until invasive therapy improves the outcome and is standard of care.11,12
In the mid 1990s, three randomized, multicenter trials were published comparing thrombolysis with operation: the Rochester study,13 the Surgery versus Thrombolysis for Ischemic Lower Extremity (STILE) trial,14 and the Thrombolysis Or Peripheral Arterial Surgery (TOPAS) trial.15 The consensus that emerged from these studies and others was that CDT should be considered as first-line treatment for acute limb ischemia under the following conditions: (1) symptoms of limb ischemia present for < 14 days, (2) no absolute contraindications to thrombolysis, and (3) the predicted time to reestablish antegrade flow is short enough to preserve limb viability.
Patients with viable limbs (category I) do not require immediate invasive treatment. It is safe to admit, place on anticoagulation, continue clinical workup, and proceed with treatment urgently but not emergently. Usually these patients are eventually treated with thrombolysis, but sometimes after a several hour or overnight delay if the clinical presentation is off-hours. Barring contraindications, CDT is indicated urgently for patients with marginal limb threat (category IIa). Patients with immediate limb threat (category IIb) often require emergent operation as thrombolysis will not reestablish perfusion quickly enough to salvage the limb. Nonetheless, surgery incurs the risk of systemic effects of rapid reperfusion with compromise of cardiac, pulmonary, and renal functions that may result in prolonged hospitalization or death.16,17 If the cardiovascular risks of general anesthesia due to comorbid disease and status of the affected limb outweigh the risk of a delay in reperfusion, then thrombolysis may be the best option. Primary amputation is the only option permitting survival for patients with irreversible ischemia (category III). High perioperative mortality is associated with this degree of ischemia.
CONTRAINDICATIONS
Many patients with acute limb ischemia are not candidates for thrombolysis because of excessive major bleeding risks. In 1998, the Working Party on Thrombolysis in the Management of Limb Ischemia18 divided contraindications into absolute, major, and minor (Table 2). All major and minor contraindications should be viewed in the context of the clinical circumstance. The increased risk of bleeding complications may be assumed if the alternative is likely limb loss or death. Note that other than case reports, there are no published data showing a risk of additional emboli when thrombolysis is used to treat embolic occlusions.19
Table 2.
Contraindications
| Absolute |
| 1. Established cerebrovascular event (including transient ischemic attacks within last 2 mo) |
| 2. Active bleeding diathesis |
| 3. Recent gastrointestinal bleeding (< 10 d) |
| 4. Neurosurgery (intracranial, spinal) within last 3 mo |
| 5. Intracranial trauma within last 3 mo |
| Relative major |
| 1. Cardiopulmonary resuscitation within last 10 d |
| 2. Major nonvascular surgery or trauma within last 10 d |
| 3. Uncontrolled hypertension: > 180 mm Hg systolic or > 110 mm Hg diastolic |
| 4. Puncture of noncompressible vessel |
| 5. Intracranial tumor |
| 6. Recent eye surgery |
| Minor |
| 1. Hepatic failure, particularly those with coagulopathy |
| 2. Bacterial endocarditis |
| 3. Pregnancy |
| 4. Diabetic hemorrhagic retinopathy |
Reprinted with permission from Working Party on Thrombolysis in the Management of Limb Ischemia. Thrombolysis in the management of lower limb peripheral arterial occlusion: a consensus document. J Vasc Interv Radiol 2003;14:S337–S349.
IMAGING STUDIES
Catheter angiography is indicated as the initial vascular imaging modality in most instances. Nonetheless, initial noninvasive vascular imaging is an option in some circumstances such as: (1) when it is likely the patient will need emergent primary operation (e.g., trauma, irreversible ischemia), (2) when category I ischemia is present, or (3) when the diagnosis of acute limb ischemia is in doubt. Both computed tomography angiography using a multidetector computed tomography scanner and magnetic resonance angiography have been shown to have equivalent accuracy to catheter angiography for peripheral arterial disease.20,21,22 Vascular ultrasound performed by a skilled sonographer can detect stenosis and occlusion accurately for infrainguinal disease.20 Diagnostic accuracy in the abdomen and pelvis is limited. The choice of noninvasive vascular imaging modality depends on the timeliness of and quality of hospital resources, renal function, allergy history, whether the suspected occlusion is above or below the inguinal ligament, and the urgency of obtaining the information.
PLASMINOGEN ACTIVATORS
All currently available thrombolytic agents are exogenous plasminogen activators. Plasminogen activators are enzymes that convert plasminogen into plasmin. Plasmin then digests fibrin polymers that along with aggregated platelets and red blood cells compose thrombus. Currently available agents include alteplase (recombinant tissue plasminogen activator [rtPA], Activase, Genentech), derived from cloned human tissue plasminogen activator (tPA), and two genetically engineered mutants of tPA, reteplase (rPA, Retevase, Centocor), a deletion mutant lacking two domains at the amino terminus, and tenecteplase (TNK, TNKase, Genentech), containing three point mutations. Streptokinase, the first-generation agent, is no longer used for CDT because of a high rate of bleeding complications and allergic reactions relative to later generation agents.23,24,25 Urokinase (UK) was the most widely used thrombolytic in the United States from ~1985 until the Food and Drug Administration removed it from the market in 1999. UK returned to the market in 2002 but failed to regain market dominance because the available alternatives cost less,26 and most interventional radiologists by then were comfortable with their use. It is no longer available. At least six other plasminogen activators and the direct fibrinolytic alfimeprase have been described.27 These also are not available for clinical use at the present time.
The commercially available exogenous plasminogen activators differ substantially in physiological and biochemical properties including plasma half-life, fibrin specificity (relative plasminogen activator activity in the presence of fibrin versus fibrinogen), and fibrin affinity (stability of fibrin binding). In CDT, these differences are muted by the complex interactions between the thrombolytic drugs and the components and products of the hemostasis system as well as the intrathrombus method of drug delivery. Reteplase differs from alteplase primarily by its lower fibrin affinity. Theoretically, rPA penetrates thrombus more effectively than rtPA, allowing for more rapid thrombolysis, shorter thrombolysis duration, and consequently a lower incidence of bleeding complications. There are no controlled studies comparing alteplase with reteplase for peripheral arterial CDT. Nonetheless, the efficacy and complications associated with these two agents in CDT have been equivalent.26,27,28,29 Tenecteplase has the highest fibrin specificity of all available thrombolytic agents.30 Fibrin affinity is equivalent to rtPA. The theoretical advantages of TNK versus rtPA and rPA include less activation of circulating plasminogen because of very high fibrin affinity and more rapid thrombolysis because of greater resistance to plasminogen activator inhibitor-1, a plasma component of the human hemostatic system.27 These differences should result in less distant bleeding. The few reports on TNK use in limb ischemia, all retrospective case series, suggest that using TNK may indeed result in a lower rate of bleeding complications with equivalent efficacy compared with historical data.31 There are, however, no reported controlled studies comparing TNK with either rtPA or rPA for peripheral arterial CDT, and thus the level of evidence is low.
ADJUNCTIVE PHARMACEUTICALS
It is thought that a local hypercoagulable state may be present in the vessel undergoing local thrombolysis. Heparin acts to prevent immediate rethrombosis of the treated vessel and to prevent pericatheter thrombosis.32 Systemic anticoagulation was standard with UK. Use of an equivalent heparin dose with tPA-derived thrombolytics increases bleeding complications but does not improve efficacy.33 It is now standard to use a “subtherapeutic” dose that produces only mild prolongation of the PTT. Alteplase and heparin mixed together will precipitate and each should be infused separately.34 The European distributor of reteplase reported several cases of precipitation and recommends infusing separate from heparin. Precipitation of tenecteplase with heparin has not been reported.
Warfarin (Coumadin) is an antagonist to vitamin K that suppresses the availability of several clotting factors. Bleeding complications during thrombolysis are very high when a patient is anticoagulated with Coumadin. The best approach if thrombolysis is indicated emergently is to reverse the effects of Coumadin with fresh frozen plasma prior to thrombolysis. For semielective thrombolysis, reversal with vitamin K and initiation of heparin infusion is another option.
Glycoprotein IIb/IIIa (GPIIb/IIIa) is a receptor on the surface of platelets that binds fibrinogen to form a platelet aggregate, the final step in platelet function.35 GPIIb/IIIa binds fibrin as well and renders bound fibrin resistant to thrombolysis. Several pharmaceuticals are in clinical use that bind GPIIb/IIIa, reduce platelet aggregation, and reduce thrombolytic resistance of platelet-bound fibrin. These include abxicimab (Reopro, Centocor), a monoclonal antibody fragment; and eptifibatide (Integrillin, Schering-Plough and COR Therapeutics) and tirofiban (Aggrastat, Merck), both small peptides. Of the three, abxicimab has the longest effect clinically with antiplatelet activity lasting up to 48 hours. The effect of eptifibatide and tirofiban lasts 2 to 4 hours and 3 to 8 hours, respectively.35 Studies comparing adjunctive use of abxicimab during peripheral arterial catheter-directed thrombolysis to thrombolytic alone have shown no difference in technical success. Infusion time was either shorter or equivalent. The frequency of bleeding complications was either equivalent or higher.29,31,36,37,38,39 Experience with tirofiban and eptifibatide is more limited.40 Use of these agents in peripheral arterial CDT has not been widely adopted given the results so far.
CATHETER TECHNIQUE OF PERIPHERAL ARTERIAL THROMBOLYSIS
The usual puncture site for lower extremity thrombolysis procedures is the common femoral artery (CFA). A contralateral retrograde approach has several advantages over an ipsilateral antegrade approach. Accurate puncture of the CFA anterior to the femoral head is easier to achieve from a contralateral retrograde approach, especially with obese patients. With either approach, placing a sheath at the puncture site helps limit puncture site bleeding; and it is convenient to inject contrast or infuse medications through a 5.5F or 6F sheath during catheter manipulations or during thrombolytic infusion. With the contralateral approach, however, a 35- to 45-cm up-and-over sheath with the tip in the CFA of the affected limb is more difficult to dislodge during patient transfer or by a delirious patient than 11-cm sheaths used for an ipsilateral approach. One popular up-and-over sheath is the Balkin (Cook Inc., Bloomington, IN). Advantages of the antegrade ipsilateral approach include more mechanical advantage to traverse an occlusion and avoidance of a sometimes difficult placement of an up-and-over sheath across an acutely angled aortic bifurcation. A popliteal or direct graft puncture may be the best option in certain cases such as an occluded graft lacking a proximal patent stump. The CFA is the usual puncture site for upper extremity thrombolysis as well. Placing a long sheath from the groin to the chest is usually unnecessary (Fig. 1). Alternative puncture sites include axillary and distal brachial. The main advantage of an upper extremity approach is to avoid leaving a catheter across the arch vessels with the incurrent possibility of embolic stroke from pericatheter thrombus. The disadvantages of an upper extremity approach are vasospasm at the puncture site and the greater risk of compartment syndrome from a puncture site hematoma.
Figure 1.
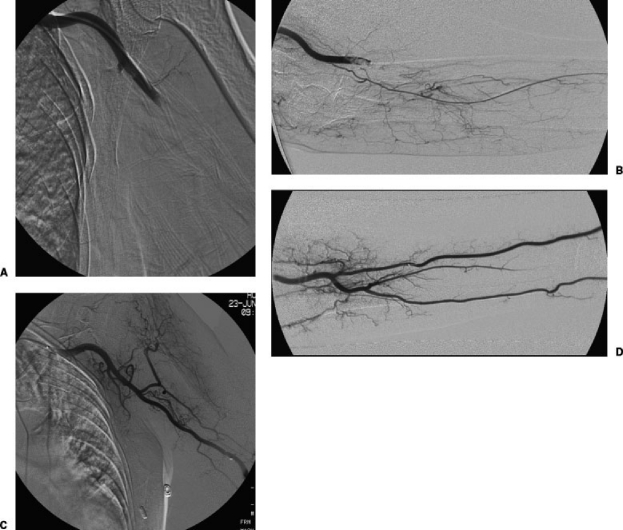
An elderly woman with history of atrial fibrillation presented with painful and cold left hand and absent brachial and wrist pulses. Initial angiography from a left CFA approach showed tandem emboli in the (A) proximal and (B) distal left brachial artery. Following 36-hour infusion of alteplase at 1.0 mg/h divided between a 5F multi-side hole infusion catheter and a 3F microcatheter, with heparin at 300 U/h, there was no residual embolus in the (C) left upper arm or (D) the left forearm.
A skin incision, if used at all, should be no larger than the sheath to minimize surface oozing. Single wall puncture technique is essential. Puncture of the posterior wall of the CFA increases the chance of groin and retroperitoneal hematoma. A 4F or 5F micropuncture access set (Cook Inc.) further increases safety as the initial puncture is smaller and the hole produced by inadvertent puncture of the common femoral vein or an unusable glancing arterial puncture will be smaller. Ultrasound guidance should be considered (Siemens Medical Solutions USA, Inc., Malvern, PA) if the artery is difficult to palpate. Angiographic confirmation of the arterial puncture site can reveal external iliac or superficial femoral artery puncture and give an assessment of the suitability of the puncture for closure device placement. If the external iliac artery was punctured, abandoning thrombolysis should be seriously considered. Once the arterial system has been entered with an 18-gauge needle or a micropuncture cannula, this access needs to be maintained throughout thrombolysis, even if another more suitable puncture is performed subsequently.
If diagnostic-quality noninvasive imaging had been obtained prior to intervention, then contrast injections can be reserved to guide catheter manipulations. If not, then complete diagnostic angiography of the affected limb is indicated. Delayed imaging up to 30 seconds and large contrast volumes may be needed to opacify the tibial runoff vessels. Bilateral lower extremity angiography should always be considered in cases of embolic occlusion because there may be clinically silent emboli in the “asymptomatic” limb that will need treatment to prevent thrombotic propagation. Note that the presence or absence of intact runoff is one predictor of favorable clinical outcome of CDT. Also note that the length of the occlusion does not affect clinical outcome.41
Once the occlusion is delineated, probing it with a guide wire is the next step. The technique depends on whether an artery or a graft is occluded and whether or not an arterial occlusion involves underlying arterial disease. For thrombotic occlusion of diseased arteries, soft Teflon-coated braided steel guide wires such as the Bentson (Cook Inc.) are best to use first because these are least likely to dissect and such guide wires give information as to the consistency of the clot. If a soft guide wire easily traverses the occluded segment, then the clot is likely to respond well to thrombolysis.32 If a soft guide wire will not penetrate the clot one needs to decide how aggressively to pursue guide wire traversal. The guide wires that should be considered are somewhat stiff J-tipped wires such as the Rosen (Cook Inc.). The J-tip reduces the probability of dissection and the stiffness of the wire gives greater mechanical advantage. The last set of guide wires that one should consider are hydrophilic polymer-coated, nitinol core wires such at the Glidewire (Terumo, Tokyo, Japan) or the Roadrunner wire (Cook Inc.). These wires have the highest propensity for arterial dissection especially when one is working blind in an occluded diseased arterial segment. One should carefully weigh the risks when deciding to proceed. Synthetic grafts can be approached aggressively as there is no risk of dissection. Some caution is needed with autologous vein grafts. Embolic occlusions are usually relatively simple to traverse because of the lack of a hard platelet-rich fibrin plug at the proximal end that is present in thrombosed arteries and grafts. Vertebral-type catheters such as the Berenstein (Angiodynamics, Queensbury, NY) provide both stiffness and sufficient maneuverability to negotiate infrainguinal occlusions. Reverse curve catheters such as the Simmons-1 (Angiodynamics) are best suited for common iliac artery occlusions via a contralateral approach.
If the occlusion cannot be traversed, then an end-hole catheter can be placed at the proximal end of the occlusion and thrombolysis initiated. Often, after several hours of thrombolysis, the proximal fibrin plug will soften and a guide wire can be advanced through the occlusion. If on this return to the angiography suite a guide wire cannot be advanced, one should consider abandoning thrombolysis. Persistence, however, does pay off at times and if the patient is stable and low risk for hemorrhage, then an overnight continuance of the infusion may be contemplated.
If a guide wire can be advanced through the occlusion and into a runoff vessel then a multi-side hole infusion catheter should be placed that spans the length of the occlusion. The two most popular are the Cragg-McNamara (Micro Therapeutics Inc., Irvine, CA) and the Unifuse (Angiodynamics). Both are available in different catheter and infusion segment lengths. Both have an end-hole occlusion device that forces the infused solution to exit through the numerous side holes. These devices differ in that the side holes of the Unifuse are in fact slits that act as one-way valves instead of holes. Theoretically, thrombolytic delivery is more uniform across the infusion segment and this leads to more rapid thrombolysis, but no difference has been reported. A microcatheter or infusion wire can be placed through a 4F or 5F infusion catheter to directly infuse small distal arteries in the lower leg and forearm.
INFUSION METHODS AND DOSAGES
There are several recognized methods for thrombolytic delivery: continuous infusion, bolusing (lacing), pulse spray, graded infusion, and stepwise infusion.18 The simplest and most often employed today is continuous infusion. Thrombolytic infusion is initiated and the patient returns periodically for follow-up angiography until a procedure end point is reached. The bolus method is delivery of a single highly concentrated dose of thrombolytic agent throughout the occlusion and then initiation of a continuous infusion. Pulse spray refers to repeated forceful injection of small aliquots of thrombolytic, thus distributing the agent rapidly throughout the thrombus as well as mechanically disrupting the thrombus. Pulse spray is initiated and continued in the angiography suite until antegrade flow is reestablished. In graded infusion, the rate of drug infusion is diminished over time. Stepwise infusion differs from the other methods in that it employs an end-hole catheter that is imbedded in the proximal portion of the thrombus. After a short infusion, a contrast injection is used to confirm lysis of the proximal portion, the catheter is repositioned, and the steps are repeated until completion.
The choice of infusion method depends on the severity of limb ischemia and the time of day of initiating therapy. There is no evidence of significant differences in amputation-free survival between these methods.42 Continuous infusion requires the least procedure time and is an appropriate choice for relatively mild acute limb ischemia. If an infusion is begun at the end of the day, the infusion rate can be set relatively low for a 12- to 15-hour overnight infusion. There are reports showing thrombolysis acceleration with either the bolus or pulse-spray methods, and thus these methods are more attractive when time is limited to reestablish antegrade flow. Some reports, however, show an increased rate of bleeding complications compared with continuous infusion,43 whereas others do not.44,45,46 There is concern about distal embolization with either method due to clot fragmentation and close monitoring is needed.47 The graded infusion technique was popularized with publication of the high-dose UK infusion method of McNamara and Fischer,32 but it has not been applied widely using tPA derivatives because of bleeding risk concerns. The stepwise infusion method is the most labor-intensive and requires that the patient remain in the angiography suite during the procedure. The advantage is very close monitoring of the course of treatment and prompt recognition of complications, but the method has few advocates today.
Both weight-based and non–weight-based dosing regimens for continuous infusion of alteplase have been used. There is no evidence that either is more advantageous.27 In Semba et al,48 a Society of Interventional Radiologists (SIR) advisory panel recommended a maximum dose of 2 mg/h for a maximum total infusion of 40 mg. Since then, it has been shown that a dose > 1 mg/h dose not improve efficacy but does increase bleeding complications.27,28 Similarly, a reteplase dose > 0.5 U/h28 and a tenecteplase dose > 0.25 mg/h31 do not improve efficacy but increase bleeding complications. The following summarizes commonly used doses today:
Alteplase: continuous, 0.5 to 1.0 mg/kg/h (40 mg maximum); bolus, 2 to 5 mg bolus, then continuous infusion; pulse spray, 0.5 mg/mL at 0.2 mL every 30 to 60 seconds49
Reteplase: continuous, 0.25 to 0.5 U/h (20 units maximum); bolus, 2 to 5 U bolus, then continuous infusion
Tenecteplase: continuous, 0.125 to 0.25 mg/h; bolus, 1 to 5 mg, then continuous infusion
Heparin can be infused intravenously or intra-arterially through the sheath. A bolus at the time of thrombolysis initiation is not recommended. An infusion rate to raise PTT to only 1.25 to 1.5 of control is recommended. Most practitioners use between 200 and 500 U per hour. Some do not monitor PTT values, whereas others do because of the unpredictable dose response of heparin. For patients who arrive at angiography with heparin anticoagulation and PTT ≥ 1.5 of control, one convenient method to lower the PTT is to stop heparin as soon as the patient arrives and resume at a lower “subtherapeutic” dose at the conclusion of the procedure.
The doses for GPIIb/IIIa inhibitors used for peripheral arterial CDT are the same as the doses derived for coronary interventions. There is no data today to customize the doses for treatment of acute limb ischemia. The doses are as follows35:
Abxicimab: 0.25 μg/kg intravenous (IV) bolus, then 0.125 μg/kg/min IV for 12 hours maximum
Eptifibatide: 180 μg/kg IV bolus, then 2 μg/kg/min
Tirofiban: 0.4 μg/kg/min IV for 30 minutes, then 0.1 μg/kg/min
Some practitioners monitor fibrinogen levels and consider halting thrombolysis or giving fresh frozen plasma if the value drops below 100 mg/dL. There was a correlation between fibrinogen level and bleeding complications in the STILE trial,14 but there is no prospective evidence that the above practice lowers bleeding risk, and consequently, many other practitioners do not monitor fibrinogen. Fibrinogen level can be artificially high or normal in rtPA infusion due to accumulation of fragment X, one of several fibrin degradation products.50,51
All patients treated with continuous infusions should be admitted to an intensive care unit or step-down unit bed for intensive monitoring. Infusion catheters and sheaths should be secured with skin suture and adhesive bandages. One convenient method of securing the catheter is to loop the external portion at the groin and cover with Tegaderm (3M, St. Paul, MN). If there is sudden tension on the infusion tubing, the redundant loop at the skin safeguards against accidental removal. Also, the puncture site is easily monitored for hematoma, and oozing is contained by the adhesive seal. Patients return to the interventional radiology suite every 4 to 6 hours or first case in the morning.
END POINTS AND POSTPROCEDURAL CARE
There are several possible procedural end points of thrombolysis including complete lysis with restoration of flow, complete lysis of all amenable thrombus with persistent occlusion from organized thrombus or embolized plaque, failure of thrombolysis progression and aborting thrombolysis either due to a major complication, exceeding the accepted time or dosage limits (see above), or progression to irreversible (category III) ischemia. Complete thrombolysis reveals the underlying lesions responsible for arterial or graft thrombosis (Fig. 2). These should be corrected by either percutaneous or open surgical methods as evidence shows that correction is crucial for long-term benefits from thrombolysis.52,53,54 Residual thrombolysis-resistant material may be removed by aspiration thrombectomy55,56 or balloon embolectomy may be needed. Failure of guide wire traversal despite a trial end-hole catheter infusion at the point of occlusion is an appropriate time to stop treatment. Alternative treatments including subintimal recannalization, balloon embolectomy, and bypass grafting should be considered.
Figure 2.
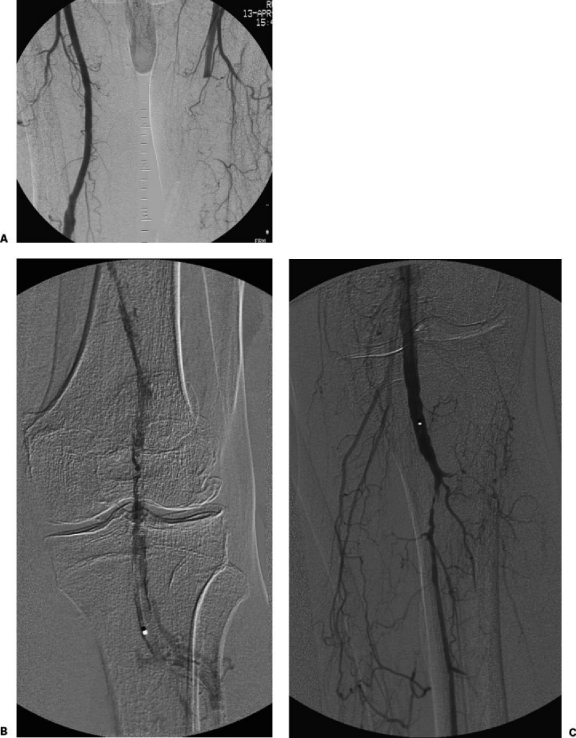
An 80-year-old man with history of hypertension presented with a painful and cold left foot and absent popliteal and pedal pulses. (A) Initial angiography from a right CFA approach showed acute occlusion of the left superficial femoral artery. (B) After placing a 6F up-and-over sheath, a 5F multi-side hole infusion catheter was placed with the tip in the distal left popliteal artery. Alteplase (1.0 mg/h via the catheter) and heparin (200 U/h via the sheath) infusions were initiated. (C) Follow-up angiography the next day showed no residual thrombus, a high-grade stenosis at the origin of the left peroneal artery, and proximal occlusion of the left posterior and anterior tibial arteries.
Closure devices have not been shown to decrease the risk of groin hematoma.57 These devices add cost and add risk of device-related complications. Nonetheless, use of a closure device should be considered at completion of a thrombolytic infusion, especially if a heparin or GPIIB/IIIa inhibitor bolus was given before postthrombolysis angioplasty or stent implantation. An alternate approach is to maintain sheath access and allow time for clearance of the thrombolytic and adjunctive agents, then remove the sheath and use manual compression for hemostasis. Postangioplasty prophylaxis with aspirin (81 to 325 mg daily indefinitely), clopidogrel (75 mg daily for 6 weeks), or both is commonly used to prevent recurrent thromboses.35 For patients with embolic sources, therapeutic heparin infusion is maintained until warfarin anticoagulation is therapeutic. All patients treated for arterial occlusion due to aneurismal or occlusive peripheral vascular disease should be followed in clinic for life at annual or shorter intervals depending on disease severity.5
RESULTS
Restoration of antegrade flow and dissolution of at least 95% of the occlusion is the consensus definition of technical success.58 Published reports differ widely in the reported technical success, and this reflects differences in patient factors as well as the nature and age of the occlusions. These vary from 38 to 91% for occlusions of less than 3-month duration.15,33,59,60,61,62 Clinical success is defined as amputation-free survival.58 In the Rochester, STILE, and TOPAS trials, the amputation rate ranged from 5 to 18% and the death rate ranged from 4 to 20%. Surgical outcome was equivalent to CDT.13,14,15 More recent studies show similar results despite changes in thrombolytic agents and methods.63
COMPLICATIONS
The reported complication rate of CDT is dependent on patient factors, thrombolytic method, and the doses of thrombolytic and adjunctive agents. The CIRSE and SIR Standards of Practice Committees recently reviewed the published literature of CDT for acute limb ischemia, listed the reported major complication rates, and recommended complication-specific thresholds for quality improvement program purposes.58
Intracranial hemorrhage: 0 to 2.5% (2% threshold)
Major bleeding requiring transfusion or surgery: 1 to 20% (10% threshold)
Compartment syndrome: 1 to 10% (4% threshold)
Distal embolization: 1 to 5% (5% threshold)
Compartment syndrome and major bleeding complications in the brain, peritoneal cavity, extraperitoneal spaces, gastrointestinal tract, urinary tract, and extremities necessitate immediate halting of thrombolytic agent and heparin infusions (Fig. 3). Fresh frozen plasma should be administered to restore fibrinogen and clotting factors. Appropriate specialists should be consulted emergently. Distal embolization (i.e., “trash foot”) can be managed by continuing thrombolytic infusion with a microcatheter in an occluded distal tibial or pedal artery or by microtibial balloon embolectomy.64
Figure 3.
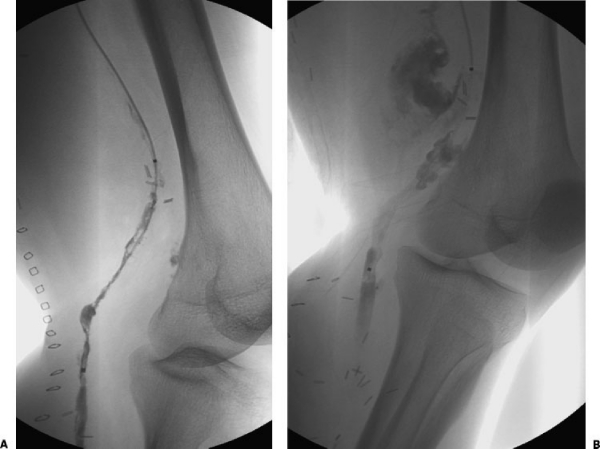
A 67-year-old man 1 week after left popliteal artery reconstruction using autologous vein bypass graft presented with acute ischemia in the left foot and calf. The patient was referred for CDT because of risks involved in reoperation and despite warning of significant bleeding risks with CDT therapy. (A) A 5F multi-side hole catheter was placed within the graft and alteplase (0.5 mg/h via the catheter) and heparin (200 U/h via an up-and-over sheath) were initiated. (B) Follow-up angiography the next day showed a large pseudoaneurysm at the proximal anastamosis and persistent occlusion of the distal anastamosis. The patient was then taken to the operating room emergently for hematoma evacuation and graft repair.
Most bleeding during CDT occurs at the site of arterial puncture. Pericatheter bleeding is usually minor and can be controlled by direct pressure or upsizing the sheath. In rare circumstances, a suture in the artery or graft may be needed. Also, one may discontinue the thrombolytic infusion until the next planned return to the angiography suite.
CONCLUSION
Being informed of “a patient in the emergency room with a cold leg” is often the initial step in the involvement of interventional radiologists in the diagnosis and treatment of acute limb ischemia. These patients remain a significant portion of those needing urgent or emergent vascular interventional treatment. Because a significant percentage of patients with acute limb ischemia are best served by primary open surgical treatment, evaluation and management ought to be a collaborative effort by an interventional radiologist and a vascular surgeon. The SVS/ISCVS classification of acute limb ischemia should be used as the main instrument for triage. Patients with marginal limb threat (category IIa) are most likely to benefit from emergent catheter-directed thrombolysis, and barring contraindications, therapy should commence as soon as possible. Patients with viable limbs (category I) can be safely anticoagulated with heparin and referred for noninvasive vascular imaging prior to intervention. Conservative technique using continuous infusion with a relatively low dose of thrombolytic agent is usually sufficient and does not incur excess bleeding risk. It is the best choice for patients with categories I and IIa ischemia. For patients with immediate limb threat (category IIb), the risks and benefits of primary surgery versus CDT must be weighed; and if CDT is the best option, then more aggressive technique such as pulse-spray thrombolysis or adjunctive use of PMT or both may be to rapidly restore flow. None of the three commercially available thrombolytic agents has demonstrated superiority over the other two in peripheral arterial CDT. One should be familiar with dosing for all three agents as hospital formularies vary. Heparin infusion should be maintained at “subtherapeutic” levels. Evidence for adjunctive use of GPIIb/IIIa inhibitors remains lacking, but these agents may have a role in special circumstances such as rethrombosis during postthrombolysis percutaneous revascularization.
REFERENCES
- Kasirajan K, Ouriel K. Management of acute lower extremity ischemia: treatment strategies and outcome. Curr Interv Cardiol Rep. 2000;2:119–129. [PubMed] [Google Scholar]
- Camerta A, White J V. In: Camerta A, editor. Thrombolytic Therapy for Peripheral Vascular Disease. Philadelphia: Lippincott-Ravenm; 1995. Overview of catheter directed thrombolytic therapy for arterial and graft occlusion. pp. 249–252.
- Dotter C T, Rosch J, Seaman A J. Selective clot lysis with low-dose streptokinase. Radiology. 1974;111:31–37. doi: 10.1148/111.1.31. [DOI] [PubMed] [Google Scholar]
- Kasirajan K, Haskal Z J, Ouriel K. The use of mechanical thrombectomy devices in the management of acute peripheral arterial occlusive disease. J Vasc Interv Radiol. 2001;12:405–411. doi: 10.1016/s1051-0443(07)61877-6. [DOI] [PubMed] [Google Scholar]
- Hirsch A T, Haskal Z J, Hertzer N R, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine and Biology, and the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease). American College of Cardiology Web Site. Available at: http://www.acc.orglclinicaJ/guidelines/padlindex.pdf. Accessed March 16, 2006. Available at: http://www.acc.orglclinicaJ/guidelines/padlindex.pdf [DOI] [PubMed]
- Makris M. Hyperhomocysteinemia and thrombosis. Clin Lab Haematol. 2000;22:133–143. doi: 10.1046/j.1365-2257.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- Zipser S, Kirsch C M, Lien C, et al. Acute aortoiliac and femoral artery thrombosis complicating diabetic ketoacidosis. J Vasc Interv Radiol. 2005;16:1737–1739. doi: 10.1097/01.RVI.0000175321.56202.8D. [DOI] [PubMed] [Google Scholar]
- Klinkert P, Post P N, Breslau P J, et al. Saphenous vein versus PTFE for above-knee femoropopliteal bypass: a review of the literature. Eur J Vasc Endovasc Surg. 2004;27:357–362. doi: 10.1016/j.ejvs.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Sanchez L A, Suggs W D, Veith F J, et al. Is surveillance to detect failing polytetrafluoroethylene bypasses worthwhile? Twelve-year experience with ninety-one grafts. J Vasc Surg. 1993;18:981–989. doi: 10.1067/mva.1993.51251. [DOI] [PubMed] [Google Scholar]
- Rutherford R B, Flanigan D P, Gupta S K, et al. Suggested standards for reports dealing with lower extremity ischaemia. J Vasc Surg. 1986;4:80–94. [PubMed] [Google Scholar]
- Blaisdell F W, Steele M, Allen R E. Management of acute lower extremity ischemia due to embolism and thrombosis. Surgery. 1978;84:822–834. [PubMed] [Google Scholar]
- Jivegard L, Holm J, Schersten T. The outcome of arterial embolism. Acta Chir Scand. 1986;152:251–256. [PubMed] [Google Scholar]
- Ouriel K, Shortell C K, De Weese J A, et al. A comparison of thrombolytic therapy with operative vascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg. 1994;19:1021–1070. doi: 10.1016/s0741-5214(94)70214-4. [DOI] [PubMed] [Google Scholar]
- The STILE Investigators Results of a prospective randomised trial evaluating surgery versus thrombolysis for ischaemia of the lower extremity. The STILE trial. Ann Surg. 1994;220:251–268. doi: 10.1097/00000658-199409000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouriel K, Veith F J, Sasahara A A, for the TOPAS Investigators Thrombolysis or peripheral arterial surgery (TOPAS): phase I results. J Vasc Surg. 1996;23:64–75. doi: 10.1016/s0741-5214(05)80036-9. [DOI] [PubMed] [Google Scholar]
- Diffin D C, Kandarpa K. Assessment of peripheral intraarterial thrombolysis versus surgical revascularization in acute lower-limb ischemia: a review of limb-salvage and mortality statistics. J Vasc Interv Radiol. 1996;7:57–63. doi: 10.1016/s1051-0443(96)70734-0. [DOI] [PubMed] [Google Scholar]
- Beyersdorf F, Matheis G, Kruger S, et al. Avoiding reperfusion injury after limb revascularization: experimental observations and recommendations for clinical application. J Vasc Surg. 1989;9:757–766. [PubMed] [Google Scholar]
- Working Party on Thrombolysis in the Management of Limb Ischemia Thrombolysis in the management of lower limb peripheral arterial occlusion: a consensus document. J Vasc Interv Radiol. 2003;14:S337–S349. doi: 10.1016/s1051-0443(07)61244-5. [DOI] [PubMed] [Google Scholar]
- Lonsdale R J, Berridge D C, Makin E S, et al. Role of echocardiography prior to peripheral arterial thrombolysis. Br J Surg. 1990;77:1429–1430 (Abstract). [Google Scholar]
- Willmann J K, Baumert B, Schertler T, et al. Aortoiliac and lower extremity arteries assessed with 16-detector row CT angiography: prospective comparison with digital subtraction angiography. Radiology. 2005;236:1083–1093. doi: 10.1148/radiol.2362040895. [DOI] [PubMed] [Google Scholar]
- Martin M L, Tay K H, Flak B, et al. Multidetector CT angiography of the aortoiliac system and lower extremities: a prospective comparison with digital subtraction angiography. AJR Am J Roentgenol. 2003;180:1085–1091. doi: 10.2214/ajr.180.4.1801085. [DOI] [PubMed] [Google Scholar]
- Quinn S F, Sheley R C, Semonsen K G, et al. Aortic and lower-extremity arterial disease: evaluation with MR angiography versus conventional angiography. Radiology. 1998;206:693–701. doi: 10.1148/radiology.206.3.9494487. [DOI] [PubMed] [Google Scholar]
- Breda A van, Katzen B T, Deutsch A S. Urokinase versus streptokinase in local thrombolysis. Radiology. 1987;165:109–111. doi: 10.1148/radiology.165.1.3628756. [DOI] [PubMed] [Google Scholar]
- Lonsdale R J, Berridge D C, Earnshaw J J, et al. Recombinant tissue-type plasminogen activator is superior to streptokinase for local intra-arterial thrombolysis. Br J Surg. 1992;79:272–275. doi: 10.1002/bjs.1800790330. [DOI] [PubMed] [Google Scholar]
- Berridge D C, Gregson R H, Hopkinson B R, et al. Randomized trial of intra-arterial recombinant tissue plasminogen activator, intravenous recombinant tissue plasminogen activator and intra-arterial streptokinase in peripheral arterial thrombolysis. Br J Surg. 1991;78:988–995. doi: 10.1002/bjs.1800780831. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Hofmann L V, Razavi M K, et al. The safety, efficacy, and pharmacoeconomics of low-dose alteplase compared with urokinase for catheter-directed thrombolysis of arterial and venous occlusions. J Vasc Surg. 2003;37:512–517. doi: 10.1067/mva.2003.41. [DOI] [PubMed] [Google Scholar]
- Razavi M K, Lee D S, Hofmann L V. Catheter-directed thrombolytic therapy for limb ischemia: current status and controversies. J Vasc Interv Radiol. 2004;15:13–23. doi: 10.1097/01.rvi.0000112621.22203.12. [DOI] [PubMed] [Google Scholar]
- Castaneda F, Swischuk J L, Li R, et al. Declining-dose study of reteplase treatment for lower extremity arterial occlusions. J Vasc Interv Radiol. 2002;13:1093–1098. doi: 10.1016/s1051-0443(07)61949-6. [DOI] [PubMed] [Google Scholar]
- Drescher P, Crain M R, Rilling W S. Initial experience with the combination of reteplase and abciximab for thrombolytic therapy in peripheral arterial occlusive disease: a pilot study. J Vasc Interv Radiol. 2002;13:37–43. doi: 10.1016/s1051-0443(07)60006-2. [DOI] [PubMed] [Google Scholar]
- Stewart R J, Fredenburgh J C, Leslie B A, et al. Identification of the mechanism responsible for the increased fibrin specificity of TNK-tissue plasminogen activator relative to tissue plasminogen activator. J Biol Chem. 2000;275:10112–10120. doi: 10.1074/jbc.275.14.10112. [DOI] [PubMed] [Google Scholar]
- Hull J E, Hull M K, Urso J A, et al. Tenecteplase in acute lower-leg ischemia: efficacy, dose, and adverse events. J Vasc Interv Radiol. 2006;17:629–636. doi: 10.1097/01.RVI.0000202751.74625.79. [DOI] [PubMed] [Google Scholar]
- McNamara T O, Fischer J R. Thrombolysis of peripheral arterial and graft occlusions: improved results using high-dose urokinase. AJR Am J Roentgenol. 1985;144:769–775. doi: 10.2214/ajr.144.4.769. [DOI] [PubMed] [Google Scholar]
- Ouriel K, Gray B, Clair D G, et al. Complications associated with the use of urokinase and recombinant tissue plasminogen activator for catheter-directed peripheral arterial and venous thrombolysis. J Vasc Interv Radiol. 2000;11:295–298. doi: 10.1016/s1051-0443(07)61420-1. [DOI] [PubMed] [Google Scholar]
- Semba C P, Bakal C W, Calis K A, et al. Alteplase as an alternative to urokinase. Advisory Panel on Catheter-Directed Thrombolytic Therapy. J Vasc Interv Radiol. 2000;11:279–287. doi: 10.1016/s1051-0443(07)61418-3. [DOI] [PubMed] [Google Scholar]
- Mehta R P, Johnson M S. Update on anticoagulant medications for the interventional radiologist. J Vasc Interv Radiol. 2006;17:597–612. doi: 10.1097/01.RVI.0000209226.54671.42. [DOI] [PubMed] [Google Scholar]
- Ouriel K, Castaneda F, McNamara T, et al. Reteplase monotherapy and reteplase/abciximab combination therapy in peripheral arterial occlusive disease: results from the RELAX trial. J Vasc Interv Radiol. 2004;15:229–238. doi: 10.1097/01.rvi.0000116193.44877.0f. [DOI] [PubMed] [Google Scholar]
- Hull J E, Hull M K, Urso J A. Reteplase with or without Abciximab for peripheral arterial occlusions: efficacy and adverse events. J Vasc Interv Radiol. 2004;15:557–564. doi: 10.1097/01.rvi.0000127891.54811.02. [DOI] [PubMed] [Google Scholar]
- Davidian M M, Powell A, Benenati J F, et al. Initial results of reteplase in the treatment of acute lower extremity arterial occlusions. J Vasc Interv Radiol. 2000;11:289–294. doi: 10.1016/s1051-0443(07)61419-5. [DOI] [PubMed] [Google Scholar]
- Duda S H, Tepe G, Luz O, et al. Peripheral artery occlusion: treatment with abciximab plus urokinase versus with urokinase alone: a randomized pilot trial (the PROMPT Study). Platelet Receptor Antibodies in Order to Manage Peripheral Artery Thrombosis. Radiology. 2001;221:689–696. doi: 10.1148/radiol.2213010400. [DOI] [PubMed] [Google Scholar]
- Allie D E, Hebert C J, Lirtzman M D, et al. Continuous tenecteplase infusion combined with peri/postprocedural platelet glycoprotein IIb/IIIa inhibition in peripheral arterial thrombolysis: initial safety and feasibility experience. J Endovasc Ther. 2004;11:427–435. doi: 10.1583/03-1170.1. [DOI] [PubMed] [Google Scholar]
- Clouse M E, Stokes K R, Perry L J, et al. Percutaneous intraarterial thrombolysis: analysis of factors affecting outcome. J Vasc Interv Radiol. 1994;5:93–100. doi: 10.1016/s1051-0443(94)71461-5. [DOI] [PubMed] [Google Scholar]
- Kessel D O, Berridge D C, Robertson I. Infusion techniques for peripheral arterial thrombolysis. Cochrane Database Syst Rev. 2004;(1):CD000985. doi: 10.1002/14651858.CD000985.pub2. [DOI] [PubMed] [Google Scholar]
- Ward A S, Andaz S K, Bygrave S. Thrombolysis with tissue-plasminogen activator: results with a high-dose transthrombus technique. J Vasc Surg. 1994;19:503–508. doi: 10.1016/s0741-5214(94)70078-8. [DOI] [PubMed] [Google Scholar]
- Braithwaite B D, Buckenham T M, Galland R B, et al. Prospective randomized trial of high-dose bolus versus low-dose tissue plasminogen activator infusion in the management of acute limb ischaemia. Thrombolysis Study Group. Br J Surg. 1997;84:646–650. [PubMed] [Google Scholar]
- Juhan C, Haupert S, Miltgen G, et al. A new intra arterial rt-PA dosage regimen in peripheral arterial occlusion: bolus followed by continuous infusion. Thromb Haemost. 1991;65:635. [PubMed] [Google Scholar]
- Kandarpa K, Chopra P S, Aruny J E, et al. Intraarterial thrombolysis of lower extremity occlusions: prospective, randomized comparison of forced periodic infusion and conventional slow continuous infusion. Radiology. 1993;188:861–867. doi: 10.1148/radiology.188.3.8351363. [DOI] [PubMed] [Google Scholar]
- Valji K, Bookstein J J, Roberts A C, et al. Occluded peripheral arteries and bypass grafts: lytic stagnation as an end point for pulse-spray pharmacomechanical thrombolysis. Radiology. 1993;188:389–394. doi: 10.1148/radiology.188.2.8327683. [DOI] [PubMed] [Google Scholar]
- Semba C P, Murphy T P, Bakal C W, et al. Thrombolytic therapy with use of alteplase (rt-PA) in peripheral arterial occlusive disease: review of the clinical literature. J Vasc Interv Radiol. 2000;11:149–161. doi: 10.1016/s1051-0443(07)61459-6. [DOI] [PubMed] [Google Scholar]
- Yusuf S W, Whitaker S C, Gregson R H, et al. Immediate and early follow-up results of pulse spray thrombolysis in patients with peripheral ischaemia. Br J Surg. 1995;82:338–340. doi: 10.1002/bjs.1800820318. [DOI] [PubMed] [Google Scholar]
- Suenson E, Bjerrum P, Holm A, et al. The role of fragment X polymers in the fibrin enhancement of tissue plasminogen activator-catalyzed plasmin formation. J Biol Chem. 1990;265:22228–22237. [PubMed] [Google Scholar]
- Sakharov D V, Rijken D C. Superficial accumulation of plasminogen during plasma clot lysis. Circulation. 1995;92:1883–1890. doi: 10.1161/01.cir.92.7.1883. [DOI] [PubMed] [Google Scholar]
- McNamara T O, Bomberger R A. Factors affecting initial and six month patency rates after intra-arterial thrombolysis with high dose urokinase. Am J Surg. 1986;152:709–712. doi: 10.1016/0002-9610(86)90454-x. [DOI] [PubMed] [Google Scholar]
- Gardiner G A, Jr, Harrington D P, Koltun W, Whittemore A, Mannick J A, Levin D C. Salvage of occluded bypass grafts by means of thrombolysis. J Vasc Surg. 1989;9:426–431. doi: 10.1067/mva.1989.vs0090426. [DOI] [PubMed] [Google Scholar]
- Durham J D, Rutherford R B. Assessment of long-term efficacy of fibrinolytic therapy in the ischemic extremity. Semin Intervent Radiol. 1992;9:166–173. [Google Scholar]
- Sniderman K W, Bodner L, Saddekni S, et al. Percutaneous embolectomy by transcatheter aspiration. Work in progress. Radiology. 1984;150:357–361. doi: 10.1148/radiology.150.2.6228952. [DOI] [PubMed] [Google Scholar]
- Wagner H J, Starck E E. Acute embolic occlusions of the infrainguinal arteries: percutaneous aspiration embolectomy in 102 patients. Radiology. 1992;182:403–407. doi: 10.1148/radiology.182.2.1732957. [DOI] [PubMed] [Google Scholar]
- Lasic Z, Nikolsky E, Kesanakurthy S, et al. Vascular closure devices: a review of their use after invasive procedures. Am J Cardiovasc Drugs. 2005;5:185–200. doi: 10.2165/00129784-200505030-00005. [DOI] [PubMed] [Google Scholar]
- Rajan D K, Patel N H, Valji K, et al. Quality improvement guidelines for percutaneous management of acute limb ischemia. J Vasc Interv Radiol. 2005;16:585–595. doi: 10.1097/01.RVI.0000156191.83408.B4. [DOI] [PubMed] [Google Scholar]
- Schweizer J, Altmann E, Stosslein F, et al. Comparison of tissue plasminogen activator and urokinase in the local infiltration thrombolysis of peripheral arterial occlusions. Eur J Radiol. 1996;22:129–132. doi: 10.1016/0720-048x(96)00742-5. [DOI] [PubMed] [Google Scholar]
- Meyerovitz M F, Goldhaber S Z, Reagan K, et al. Recombinant tissue-type plasminogen activator versus urokinase in peripheral arterial and graft occlusions: a randomized trial. Radiology. 1990;175:75–78. doi: 10.1148/radiology.175.1.2107563. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Hofmann L V, Razavi M K, et al. The safety, efficacy, and pharmacoeconomics of low-dose alteplase compared with urokinase for catheter-directed thrombolysis of arterial and venous occlusions. J Vasc Surg. 2003;37:512–517. doi: 10.1067/mva.2003.41. [DOI] [PubMed] [Google Scholar]
- Cina C S, Goh R H, Chan J, et al. Intraarterial catheter-directed thrombolysis: urokinase versus tissue plasminogen activator. Ann Vasc Surg. 1999;13:571–575. doi: 10.1007/s100169900300. [DOI] [PubMed] [Google Scholar]
- Hanover T M, Kalbaugh C A, Gray B H, et al. Safety and efficacy of reteplase for the treatment of acute arterial occlusion: complexity of underlying lesion predicts outcome. Ann Vasc Surg. 2005;19:817–822. doi: 10.1007/s10016-005-8047-2. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Hardy R, Garnham A, et al. Microtibial embolectomy. Eur J Vasc Endovasc Surg. 2003;25:35–39. doi: 10.1053/ejvs.2002.1768. [DOI] [PubMed] [Google Scholar]