Abstract
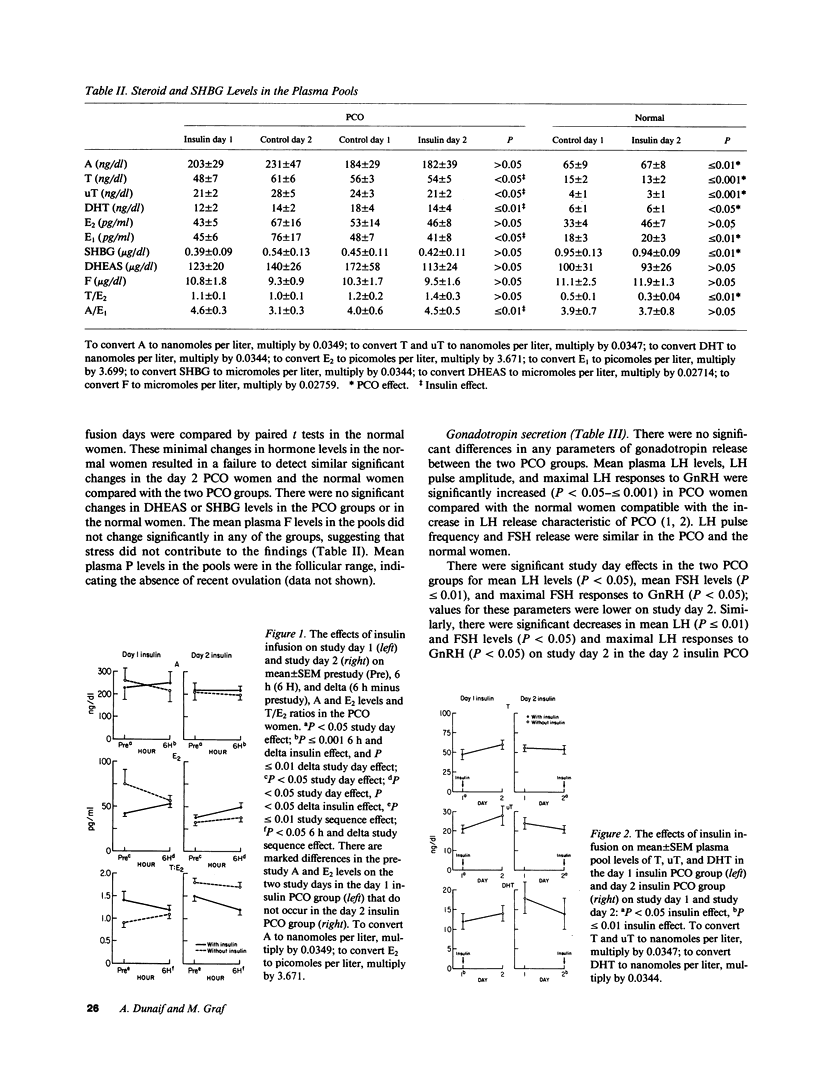
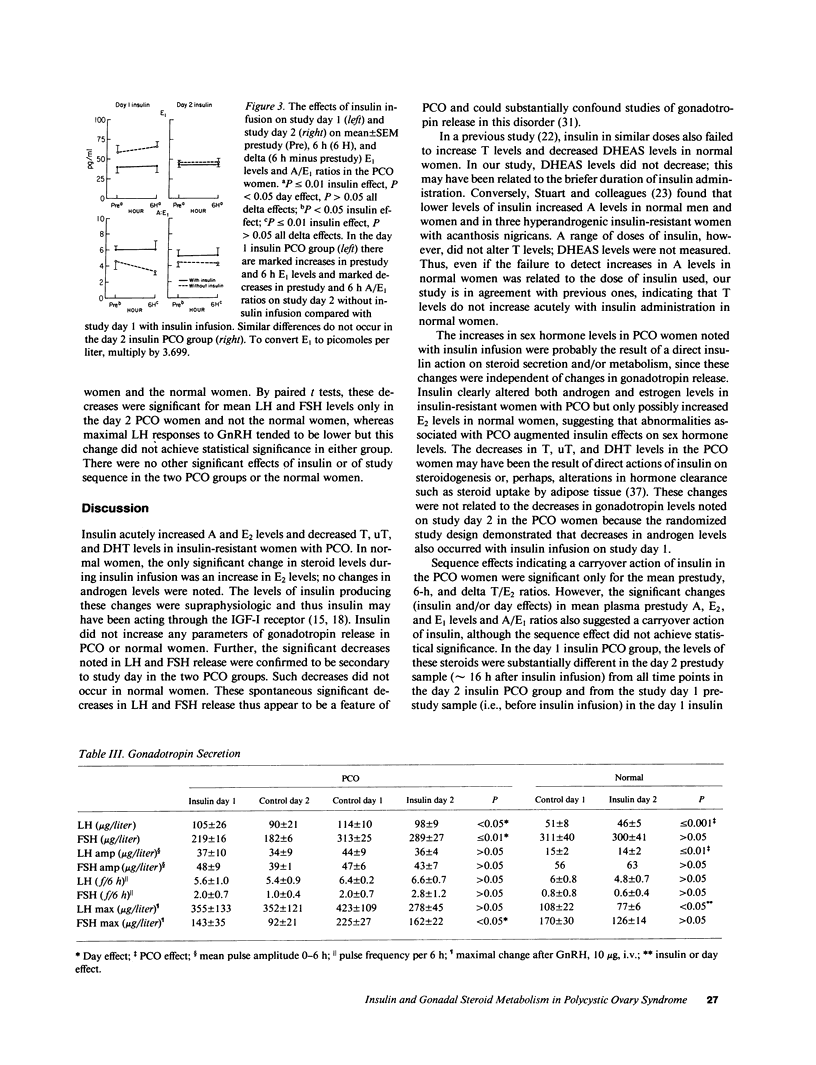
We have investigated the hypothesis that hyperinsulinemia may cause the polycystic ovary syndrome (PCO) by directly stimulating gonadal steroidogenesis and/or gonadotropin secretion. 10 insulin-resistant women with PCO and 5 age- and weight-matched ovulatory normal women had pulsatile gonadotropin release, gonadotrope sensitivity to gonadotropin-releasing hormone, and sex hormone levels studied on two consecutive study days, basally and during the infusion of insulin (mean +/- SEM steady state insulin levels, 1,254 +/- 63 microU/ml PCO vs. 907 +/- 92 microU/ml normal, P less than or equal to 0.01). Insulin acutely increased mean delta (6 h minus prestudy) levels of androstenedione (A) (P less than or equal to 0.001) and estradiol (E2) (P less than or equal to 0.05) and decreased mean plasma pool (0-6 h) levels of testosterone (T) (P less than 0.05), nonsex hormone binding globulin-bound T (P less than 0.05), and dihydrotestosterone (P less than or equal to 0.01) in the PCO women. Insulin also decreased mean plasma 6 h A to estrone (E1) ratios and increased 6 h E1 levels (both P less than or equal to 0.05) in the PCO women. There were significant sequence effects (insulin + day) in the PCO women on T/E2 ratios, indicating a carryover action of insulin. Insulin had no effects on gonadotropin release in the PCO women. In the normal women, the only significant change was an insulin or study day effect that increased mean 6 h E2 levels (P less than or equal to 0.01). There were significant spontaneous decreases in mean luteinizing hormone (p less than 0.05) and follicle-stimulating hormone levels (p less than or equal to 0.01) in the PCO but not the normal women on the second day of study. This study indicates that insulin can directly alter peripheral sex hormone levels independent of changes in gonadotropin release in insulin-resistent PCO women. Insulin decreased the levels of potent androgens in PCO women and did not increase androgen levels in normal women, arguing against a simple, direct causal relationship between hyperinsulinemia and hyperandrogenism in PCO.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adashi E. Y., Hsueh A. J., Yen S. S. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology. 1981 Apr;108(4):1441–1449. doi: 10.1210/endo-108-4-1441. [DOI] [PubMed] [Google Scholar]
- Adashi E. Y., Resnick C. E., D'Ercole A. J., Svoboda M. E., Van Wyk J. J. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr Rev. 1985 Summer;6(3):400–420. doi: 10.1210/edrv-6-3-400. [DOI] [PubMed] [Google Scholar]
- Barbieri R. L., Makris A., Randall R. W., Daniels G., Kistner R. W., Ryan K. J. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab. 1986 May;62(5):904–910. doi: 10.1210/jcem-62-5-904. [DOI] [PubMed] [Google Scholar]
- Barbieri R. L., Ryan K. J. Hyperandrogenism, insulin resistance, and acanthosis nigricans syndrome: a common endocrinopathy with distinct pathophysiologic features. Am J Obstet Gynecol. 1983 Sep 1;147(1):90–101. doi: 10.1016/0002-9378(83)90091-1. [DOI] [PubMed] [Google Scholar]
- Burghen G. A., Givens J. R., Kitabchi A. E. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980 Jan;50(1):113–116. doi: 10.1210/jcem-50-1-113. [DOI] [PubMed] [Google Scholar]
- Chang R. J., Laufer L. R., Meldrum D. R., DeFazio J., Lu J. K., Vale W. W., Rivier J. E., Judd H. L. Steroid secretion in polycystic ovarian disease after ovarian suppression by a long-acting gonadotropin-releasing hormone agonist. J Clin Endocrinol Metab. 1983 May;56(5):897–903. doi: 10.1210/jcem-56-5-897. [DOI] [PubMed] [Google Scholar]
- Chang R. J., Nakamura R. M., Judd H. L., Kaplan S. A. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1983 Aug;57(2):356–359. doi: 10.1210/jcem-57-2-356. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Deslypere J. P., Verdonck L., Vermeulen A. Fat tissue: a steroid reservoir and site of steroid metabolism. J Clin Endocrinol Metab. 1985 Sep;61(3):564–570. doi: 10.1210/jcem-61-3-564. [DOI] [PubMed] [Google Scholar]
- Dunaif A. Do androgens directly regulate gonadotropin secretion in the polycystic ovary syndrome? J Clin Endocrinol Metab. 1986 Jul;63(1):215–221. doi: 10.1210/jcem-63-1-215. [DOI] [PubMed] [Google Scholar]
- Dunaif A., Graf M., Mandeli J., Laumas V., Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab. 1987 Sep;65(3):499–507. doi: 10.1210/jcem-65-3-499. [DOI] [PubMed] [Google Scholar]
- Dunaif A., Hoffman A. R., Scully R. E., Flier J. S., Longcope C., Levy L. J., Crowley W. F., Jr Clinical, biochemical, and ovarian morphologic features in women with acanthosis nigricans and masculinization. Obstet Gynecol. 1985 Oct;66(4):545–552. [PubMed] [Google Scholar]
- Dunaif A., Mandeli J., Fluhr H., Dobrjansky A. The impact of obesity and chronic hyperinsulinemia on gonadotropin release and gonadal steroid secretion in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1988 Jan;66(1):131–139. doi: 10.1210/jcem-66-1-131. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Eastman R. C., Minaker K. L., Matteson D., Rowe J. W. Acanthosis nigricans in obese women with hyperandrogenism. Characterization of an insulin-resistant state distinct from the type A and B syndromes. Diabetes. 1985 Feb;34(2):101–107. doi: 10.2337/diab.34.2.101. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Minaker K. L., Landsberg L., Young J. B., Pallotta J., Rowe J. W. Impaired in vivo insulin clearance in patients with severe target-cell resistance to insulin. Diabetes. 1982 Feb;31(2):132–135. doi: 10.2337/diab.31.2.132. [DOI] [PubMed] [Google Scholar]
- Garzo V. G., Dorrington J. H. Aromatase activity in human granulosa cells during follicular development and the modulation by follicle-stimulating hormone and insulin. Am J Obstet Gynecol. 1984 Mar 1;148(5):657–662. doi: 10.1016/0002-9378(84)90769-5. [DOI] [PubMed] [Google Scholar]
- Goodyer C. G., De Stéphano L., Lai W. H., Guyda H. J., Posner B. I. Characterization of insulin-like growth factor receptors in rat anterior pituitary, hypothalamus, and brain. Endocrinology. 1984 Apr;114(4):1187–1195. doi: 10.1210/endo-114-4-1187. [DOI] [PubMed] [Google Scholar]
- Havrankova J., Roth J., Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978 Apr 27;272(5656):827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- Huseman C. A., Johanson A. J., Varma M. M., Blizzard R. M. Congenital lipodystrophy. II. Association with polycystic ovarian disease. J Pediatr. 1979 Jul;95(1):72–74. doi: 10.1016/s0022-3476(79)80087-6. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J., Peterson R. E., Sturla E., Dawood Y., Bar R. S. Primary amenorrhea associated with hirsutism, acanthosis nigricans, dermoid cysts of the ovaries and a new type of insulin resistance. Am J Med. 1978 Aug;65(2):389–395. doi: 10.1016/0002-9343(78)90838-0. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Flier J. S., Bar R. S., Archer J. A., Gorden P., Martin M. M., Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976 Apr 1;294(14):739–745. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- Kim M. H., Rosenfield R. L., Hosseinian A. H., Schneir H. G. Ovarian hyperandrogenism with normal and abnormal histologic findings of the ovaries. Am J Obstet Gynecol. 1979 Jun 15;134(4):445–452. doi: 10.1016/s0002-9378(16)33087-3. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY B. P., ENGELBERG W., PATTEE C. J. Simple method for the determination of plasma corticoids. J Clin Endocrinol Metab. 1963 Mar;23:293–300. doi: 10.1210/jcem-23-3-293. [DOI] [PubMed] [Google Scholar]
- Moltz L., Sörensen R., Römmler A., Schwartz U., Hammerstein J. Polyzystische Ovarien: eigenständiges Krankheitsbild oder unspezifisches Symptom? Geburtshilfe Frauenheilkd. 1985 Feb;45(2):107–114. doi: 10.1055/s-2008-1036216. [DOI] [PubMed] [Google Scholar]
- Nestler J. E., Clore J. N., Strauss J. F., 3rd, Blackard W. G. The effects of hyperinsulinemia on serum testosterone, progesterone, dehydroepiandrosterone sulfate, and cortisol levels in normal women and in a woman with hyperandrogenism, insulin resistance, and acanthosis nigricans. J Clin Endocrinol Metab. 1987 Jan;64(1):180–184. doi: 10.1210/jcem-64-1-180. [DOI] [PubMed] [Google Scholar]
- New M. I., Lorenzen F., Lerner A. J., Kohn B., Oberfield S. E., Pollack M. S., Dupont B., Stoner E., Levy D. J., Pang S. Genotyping steroid 21-hydroxylase deficiency: hormonal reference data. J Clin Endocrinol Metab. 1983 Aug;57(2):320–326. doi: 10.1210/jcem-57-2-320. [DOI] [PubMed] [Google Scholar]
- Pang S. Y., Softness B., Sweeney W. J., 3rd, New M. I. Hirsutism, polycystic ovarian disease, and ovarian 17-ketosteroid reductase deficiency. N Engl J Med. 1987 May 21;316(21):1295–1301. doi: 10.1056/NEJM198705213162102. [DOI] [PubMed] [Google Scholar]
- Pasquali R., Venturoli S., Paradisi R., Capelli M., Parenti M., Melchionda N. Insulin and C-peptide levels in obese patients with polycystic ovaries. Horm Metab Res. 1982 Jun;14(6):284–287. doi: 10.1055/s-2007-1018996. [DOI] [PubMed] [Google Scholar]
- Poretsky L., Glover B., Laumas V., Kalin M., Dunaif A. The effects of experimental hyperinsulinemia on steroid secretion, ovarian [125I]insulin binding, and ovarian [125I]insulin-like growth-factor I binding in the rat. Endocrinology. 1988 Feb;122(2):581–585. doi: 10.1210/endo-122-2-581. [DOI] [PubMed] [Google Scholar]
- Poretsky L., Kalin M. F. The gonadotropic function of insulin. Endocr Rev. 1987 May;8(2):132–141. doi: 10.1210/edrv-8-2-132. [DOI] [PubMed] [Google Scholar]
- Poretsky L., Smith D., Seibel M., Pazianos A., Moses A. C., Flier J. S. Specific insulin binding sites in human ovary. J Clin Endocrinol Metab. 1984 Oct;59(4):809–811. doi: 10.1210/jcem-59-4-809. [DOI] [PubMed] [Google Scholar]
- Shoupe D., Kumar D. D., Lobo R. A. Insulin resistance in polycystic ovary syndrome. Am J Obstet Gynecol. 1983 Nov 1;147(5):588–592. doi: 10.1016/0002-9378(83)90023-6. [DOI] [PubMed] [Google Scholar]
- Stuart C. A., Prince M. J., Peters E. J., Meyer W. J., 3rd Hyperinsulinemia and hyperandrogenemia: in vivo androgen response to insulin infusion. Obstet Gynecol. 1987 Jun;69(6):921–925. [PubMed] [Google Scholar]
- Taylor S. I., Dons R. F., Hernandez E., Roth J., Gorden P. Insulin resistance associated with androgen excess in women with autoantibodies to the insulin receptor. Ann Intern Med. 1982 Dec;97(6):851–855. doi: 10.7326/0003-4819-97-6-851. [DOI] [PubMed] [Google Scholar]
- Wallum B. J., Taborsky G. J., Jr, Porte D., Jr, Figlewicz D. P., Jacobson L., Beard J. C., Ward W. K., Dorsa D. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab. 1987 Jan;64(1):190–194. doi: 10.1210/jcem-64-1-190. [DOI] [PubMed] [Google Scholar]



