ABSTRACT
For patients dependent on permanent venous catheters for survival, the progressive loss of venous access sites should prompt a systematic approach to alternative sites and techniques to maximize patient survival and minimize complications. Interventional radiologists should be familiar with the appropriate use of both conventional and unconventional types of venous access and their associated risks. This article discusses the use of venous access sites available as alternatives to occluded internal jugular veins, including the subclavian veins, the femoral veins, the inferior vena cava, and the hepatic veins. In addition, unconventional techniques for venous access are reviewed, including recannulization of occluded neck and chest veins, catheterization of small thyrocervical veins, and sharp recannulization of occluded central veins.
Keywords: Venous access, unconventional, translumbar, hemodialysis
Gradual exhaustion of central venous access options is an inevitable, potentially life-threatening outcome in patients dependent on long-term central venous catheters for total parental nutrition or hemodialysis. The internal jugular veins remain the first-line permanent access site due to a lower risk of procedural and delayed complications compared with alternative sites. However, physicians who care for catheter-dependent patients should anticipate eventual occlusion of the internal jugular veins and plan alternative routes of access in a systemic, objective manner with the intention of preserving future venous access options, minimizing associated complications, and thereby maximizing patient survival. This article reviews the use of alternative central venous access sites for placement of permanent central lines in line-dependent patients with progressive venous occlusion.
PERMANENT CATHETER EXCHANGE
When catheter dysfunction occurs, permanent catheter exchange should be considered before an alternative site is sought. As a means for preserving central venous access as long as possible, permanent catheters can be exchanged over stiff, hydrophilic glide wires with an extremely low rate of associated complications.1,2,3 Indications for this procedure include mild catheter infections that respond well to antibiotics and catheter dysfunction caused by a variety of factors such as fibrin-sheath formation, catheter kinking or malposition, and thrombosis refractory to thrombolytic infusion. Catheters placed through occluded veins are optimal candidates for catheter exchange as loss of access means alternative sites must be sought. Catheter exchange has resulted in improved patency rates compared with the older technique of fibrin-sheath stripping from the femoral vein approach using a loop snare.4
SUBCLAVIAN VEINS
Avoidance of the subclavian veins as a first-line choice for venous catheters is based on of a higher rate of venous stenosis and thrombosis compared with internal jugular access—well documented in the published literature.5,6,7 In addition, some studies have demonstrated a higher rate of pneumothorax associated with subclavian vein access.8 Catheter-related subclavian vein occlusion results in obstruction of the venous outflow of the arm with several potential adverse consequences, including clinical symptoms of arm swelling and pain as well as loss of future access options for upper extremity hemodialysis arteriovenous shunt creation. The Dialysis Outcomes Quality Initiative (DOQI)9 recommends avoiding the subclavian veins when upper extremity options for peripheral hemodialysis shunts remain viable in patients with renal failure (DOQI guidelines 5 and 6); in our practice, the subclavian veins are avoided as first-line access sites for all hemodialysis patients. When upper extremity peripheral veins have been exhausted for shunt creation and bilateral jugular veins are occluded, subclavian vein catheterization is considered the next option.
OCCLUDED OR SMALL CHEST AND THYROCERVICAL VEINS
Inevitably, multiple permanent venous catheters inserted in the central veins of the chest will result in bilateral subclavian, jugular venous occlusion. In addition, occlusion of the brachiocephalic veins and superior vena cava (SVC) may occur. In patients requiring chronic central venous access, occlusion of the central veins is gradual, and the development of symptoms related to SVC syndrome is typically avoided due to well-compensated collateral drainage via mediastinal, chest wall, paraspinal, and azygous venous pathways. Therefore, before proceeding to alternative sites such as the femoral veins, inferior vena cava, and hepatic veins, physicians should recognize the potential for placement of catheters by recannulization of the occluded central veins or selection of small thyrocervical collateral veins. In well-compensated patients, such options carry a low risk of catheter-related symptoms of central venous obstruction and are associated with low rates of peri- and postprocedural complications—comparable to rates associated with traditional sites of venous access in the chest.10
In performing central venous recannulization, access to the femoral vein is achieved, a long vascular sheath is advanced to the right atrium, and using the combination of a hydrophilic glide wire and glide catheter, occluded chest veins are eventually crossed (Fig. 1). Successful selection of a variety of options above the level of the central venous obstruction, including still-patent portions of the internal or external jugular veins or small thyrocervical veins, can facilitate catheter placement. The selected target vein should be superficial, without intervening structures such as muscle or artery as verified by fluoroscopic and sonographic evaluation. By the fluoroscopic method, a clamp placed on the surface of the skin overlying the tip of the glide catheter displaces the tip with slight pressure or follows the tip closely as the image intensifier is obliqued. Following successful selection, a snare is placed in the target vein and punctured under fluoroscopic guidance. A wire is advanced, captured by the snare, pulled into the right atrium, and used for otherwise routine placement of a permanent central venous catheter, tunneled subcutaneously from the anterior chest. Serial dilatation or venoplasty of the occluded central veins may be necessary to facilitate advancement of the peel-away sheath during catheter placement.
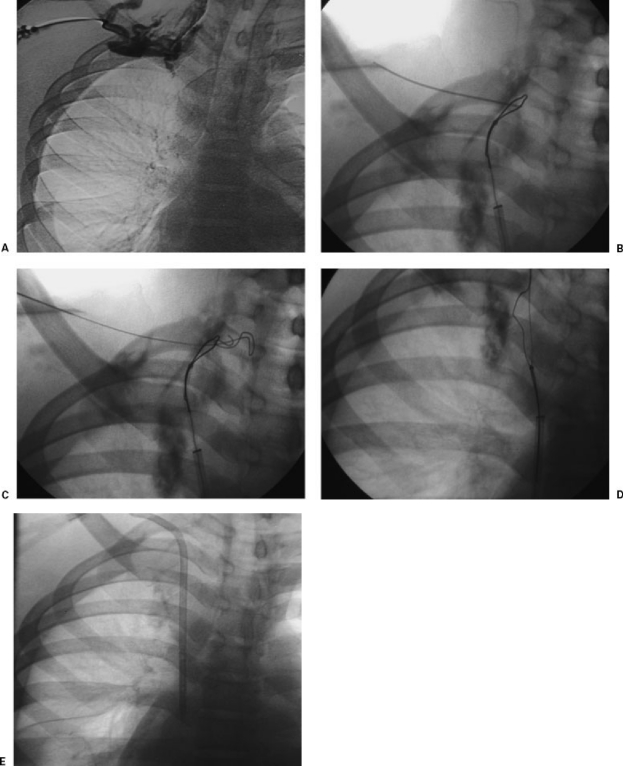
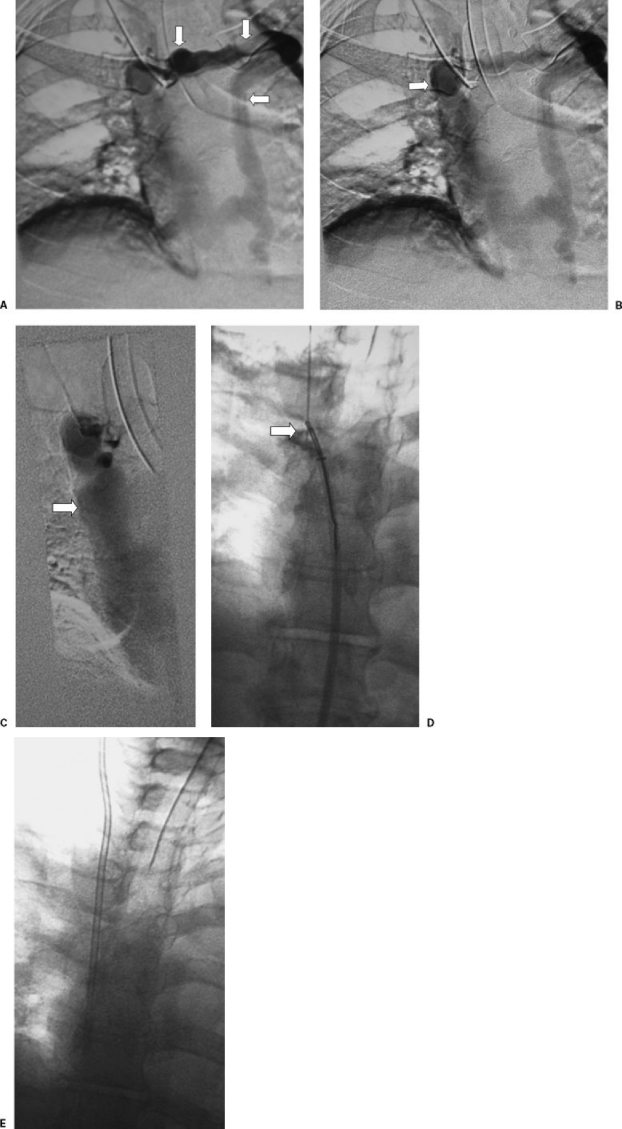
Figure 1.
(A) Venous recannulization in a patient requiring permanent hemodialysis catheter placement. Sonography demonstrated bilateral occlusion of the jugular and subclavian veins. Venography from a right-sided collateral vein verified complete right-sided venous occlusion. (B) A long sheath was placed in the superior vena cava from the femoral venous approach, a glide catheter and wire were used to cross the occluded brachiocephalic vein into a small cervical collateral vein, and a loop snare was opened. This image shows fluoroscopically guided venipuncture using the loop snare as a target. (C) A wire is advanced through the loop snare. (D) The wire is pulled across the occluded veins into the right atrium. (E) A catheter is placed over the wire in standard fashion.
We usually choose a common femoral vein approach rather than a jugular or thyrocervical vein approach for central venous recannulization. In patients with occlusion of the chest veins on both sides, limitations on drainage of the head and upper extremities elevates venous pressure and, in our experience, increases the risks of bleeding and cardiac tamponade associated with inadvertent extravasation or pericardial access during attempts at venous recannulization from the neck. From the femoral approach, we occasionally enter the mediastinum or even the pericardium with a glide wire without adverse sequelae (Fig. 2), presumably due to lower intravenous pressure.
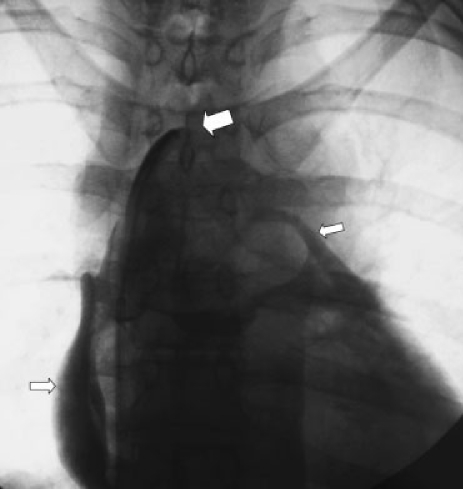
Figure 2.
Attempted central venous recannulization with inadvertent pericardial access. A glide catheter and wire were advanced from the femoral vein with the intention of crossing the occluded bilateral brachiocephalic veins for chest catheter placement. Contrast injection (large arrow at catheter tip) shows spillage into the pericardium (small arrows). The catheter was removed without adverse sequelae.
FEMORAL VEINS
When all options for insertion of permanent chest catheters are exhausted, the remaining alternatives are all associated with an increased risk of complications of long-term catheterization, such as occlusion and infection, especially the hepatic venous route. As a result, the next choice is typically either femoral venous access or direct access of the inferior vena cava via the translumbar approach. The choice may depend on operator and patient preference. Because translumbar inferior vena cava catheters are more technically challenging and time-consuming to place, most physicians choose femoral venous catheters as the next alternative to chest catheters. Before choosing either route for hemodialysis patients, consideration should be given to placement and maintenance of femoral arteriovenous grafts until such options have been completely exhausted. Premature placement of permanent femoral catheters can result in femoral vein, iliac vein, or inferior vena cava obstruction and destroy options for future lower extremity arteriovenous shunts (Fig. 3).
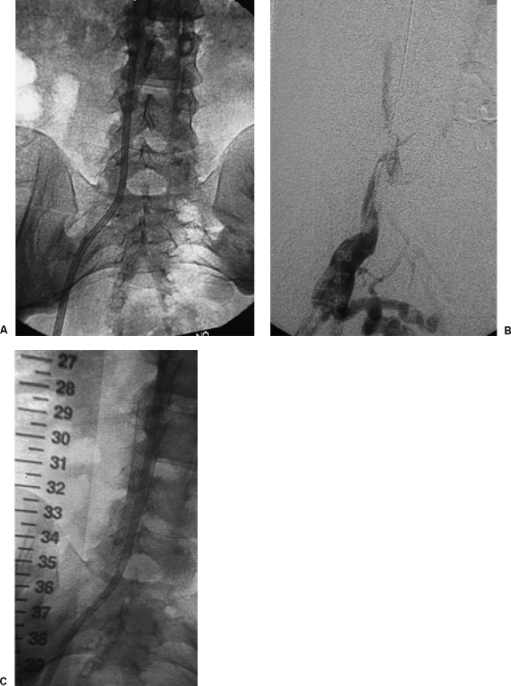
Figure 3.
(A) Patient with occluded right femoral hemodialysis catheter. Catheter tip is in the inferior vena cava. (B) After catheter removal over stiff angled glide wires, venography shows occlusion of the inferior vena cava. (C) A stent was placed to maintain caval patency and assure placement of a functioning catheter. Compared with permanent chest catheters, permanent femoral catheters are associated with an increased rate of malfunction and infection.
Compared with permanent catheters in the chest, femoral catheters are associated with a higher rate of infection and occlusion,11 resulting in more frequent interventions for catheter maintenance. Zaleski et al found a rate of catheter infection of 0.24 per 100 catheter days.11 To improve patency, the tip of the catheter should be placed above the level of the confluence of the common iliac veins. To minimize the risk of infection, the catheter should be tunneled from the common femoral venotomy site inferolaterally on the upper thigh to avoid contamination of the skin exit site by groin bacterial flora.
INFERIOR VENA CAVA
As an alternative to the femoral veins, or in patients with occluded femoral veins, direct puncture of the inferior vena cava is the next option for placement of permanent central venous access. This procedure is more technically challenging (Fig. 4) and time-consuming compared with other techniques but is associated with a low risk of periprocedural complications. The postprocedural complication of catheter malfunction occurs with greater frequency compared with permanent catheters placed in chest veins,12 but rates of infection are comparable. The patient is placed in the prone position on the angiography table. Using a long 20- or 21-gauge needle, a skin access site just above the midpoint of the right iliac crest is chosen, and under direct fluoroscopic monitoring, the needle is advanced to the right lateral aspect of the third lumbar vertebral body. If blood return is achieved, a venogram is performed to verify that access has been achieved into the inferior vena cava and not the paraspinal or azygous veins. A tract is dilated and a peel-away sheath is advanced. A long permanent central line is tunneled from the right lateral skin to the puncture site and advanced through the peel-away sheath until the tip is located at the junction of the right atrium and the inferior vena cava.
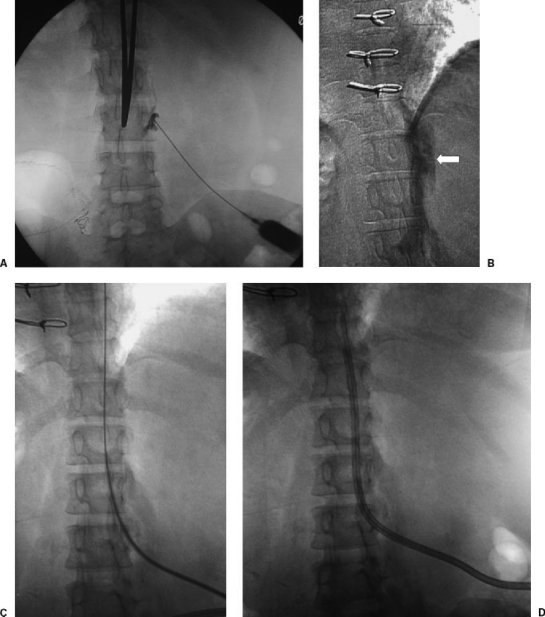
Figure 4.
(A) Translumbar inferior vena cava (IVC) catheter placement in a dialysis patient with occluded jugular, subclavian, and femoral veins. In the prone position, a needle is advanced from the skin overlying the midpoint of the right iliac crest to the right lateral aspect of the L3 vertebral body. (B) Contrast is injected to verify access into the IVC. (C) A wire is advanced to the right atrium. (D) A permanent hemodialysis catheter is tunneled subcutaneously from the right side to the skin access site and advanced through a peel-away sheath until its tip is at the junction of the IVC and right atrium. Direct IVC catheters have a higher rate of catheter malfunction compared with chest catheters.
HEPATIC VEINS
The use of hepatic veins for placement of percutaneous transhepatic permanent central venous catheters is associated with a high rate of postprocedural complications, such as catheter malfunction, and is therefore associated with an increased frequency of required catheter exchanges.13 The inherent problem with transhepatic catheters is the constant movement of the catheter tip from its optimal location in the right atrium resulting from excursion of the liver during inhalation and exhalation. The catheter tip is free to move back and forth, potentially contacting the tricuspid valve or the right atrial wall with associated complications of catheter dislodgement, kinking, withdrawal from the hepatic vein, and associated malfunction. Stavropoulos et al in a series of 36 catheters found a high rate of catheter occlusion, measuring 2.4 per 100 catheter-days.14 The rate of line sepsis was 0.22 per 100 catheter-days. Death from peritoneal hemorrhage resulting from transhepatic catheter placement has been described.13
SHARP RECANNULIZATION
When patients have exhausted all options for central venous access required for the preservation of life, interventional radiologists and patients are faced with the dilemma of abandoning further attempts or performing procedures associated with extremely limited literature precedence and a theoretically high risk of potential complications. When techniques for venous recannulization described above have failed and alternatives have been exhausted, sharp recannulization of occluded subclavian veins and even the azygous arch may be successful in some patients (Fig. 5), although the incidence of morbidity and mortality associated with inadvertent, blind puncture or traversal of mediastinal structures such as the subclavian and brachiocephalic arteries, the aortic arch, and the pericardium has not been established in the published literature. Despite the lack of literature precedent in such circumstances, patients with no other alternatives may accept the risks after detailed, informed consent.
Figure 5.
(A) Sharp venous recannulization in a patient with occluded jugular and subclavian veins. Contrast injection of a cervical vein shows occlusion of the right brachiocephalic vein and filling of a large mediastinal collateral vein (arrows). (B) The collateral vein eventually supplies a dilated azygous arch (arrow). (C) The azygous arch drains into a patent superior vena cava (arrow). (D) Using a 21-gauge needle through a sheath, direct puncture from the cervical collateral vein to a loop snare in the azygous arch was performed. The snare was used to pull a guide wire into the inferior vena cava (arrow). (E) Over the guide wire, a chest catheter was placed in standard fashion.
When performing a sharp recannulization, we start with a detailed venogram performed through an upper extremity or neck vein and performed long enough to image all remaining collateral veins in the ipsilateral chest. The goal is to find an accessible neck or chest vein in very close proximity to a remnant central vein with direct communication to the right atrium. Based on location and distance, the veins are then assessed for the risk of inadvertently crossing or puncturing vital mediastinal structures during blind advancement of a needle. This assessment may be enhanced by a review of all available cross-sectional imaging studies. We typically access the vein, advance a small sheath or dilator to the point of occlusion, and through this sheath a 21-gauge needle is advanced across the gap to a central vein that is patent to the right atrium. Contrast is injected prior to dilating a tract to verify central venous access. As an added measure of safety, access to the right atrium can be achieved with a 0.018-inch wire first, and a 0.035-inch, 4F glide catheter can be advanced into the right atrium. Maintaining wire access, the catheter can be withdrawn gradually into the source vein during active contrast injection under fluoroscopic monitoring to rule out inadvertent crossing of interposed structures prior to tract dilatation. Once access to the right atrium is achieved, a tract is dilated, a peel-away sheath is inserted, and a permanent chest catheter is placed.
SURGICAL ALTERNATIVES
The interventional radiology techniques described above represent the first-line treatments for patients depending on alternative routes of central venous access for survival. Literature precedent for surgical alternatives is extremely limited. Case reports have described direct right atrial catheterization15 and extra-anatomic surgical venous bypass.16
AFTER-HOURS APPROACHES
In our hospital, we perform after-hours venous access only in those patients who have generally exhausted conventional routes or failed bedside or surgical placement and need urgent access. In these cases, the most straightforward approach is taken initially. If the patient is unknown to our service, an ultrasound exam is performed in an attempt to evaluate patency of jugular, subclavian, and femoral veins. Lack of respiratory phasicity may indicate central venous occlusion, but often this will remain unknown until venopuncture and venography are performed.
In some patients with central occlusion and severe peripheral venous disease (i.e., peripheral intravenous access cannot be secured), a small-bore central venous catheter above the occlusion may suffice. In other patients such as those who require dialysis, a large-bore catheter must be placed into a central vein. In general, for those patients requiring urgent dialysis, it is best to place a temporary catheter and initiate dialysis prior to embarking on more protracted procedure such as venous recanalization. After access has been secured, this latter procedure can be performed electively via the temporary access.
REFERENCES
- Garofalo R S, Zaleski G X, Lorenz J M, Funaki B, Rosenblum J D, Leef J D. Exchange of poorly functioning tunneled permanent hemodialysis catheters. AJR Am J Roentgenol. 1999;173:155–158. doi: 10.2214/ajr.173.1.10397118. [DOI] [PubMed] [Google Scholar]
- Mokrzycki M H, Singhal A. Cost-effectiveness of three strategies of managing tunneled, cuffed hemodialysis catheters in clinically mild or asymptomatic bacteremias. Nephrol Dial Transplant. 2002;17:2196–2203. doi: 10.1093/ndt/17.12.2196. [DOI] [PubMed] [Google Scholar]
- Robinson D, Suhocki P, Schwab S J. Treatment of infected tunneled venous access hemodialysis catheters with guidewire exchange. Kidney Int. 1998;53:1792–1794. doi: 10.1046/j.1523-1755.1998.00954.x. [DOI] [PubMed] [Google Scholar]
- Merport M, Murphy T P, Egglin T K, Dubel G J. Fibrin sheath stripping versus catheter exchange for the treatment of failed tunneled hemodialysis catheters: randomized clinical trial. J Vasc Interv Radiol. 2000;11:1115–1120. doi: 10.1016/s1051-0443(07)61351-7. [DOI] [PubMed] [Google Scholar]
- Trerotola S O, Kuhn-Fulton J, Johnson M S, Shah H, Ambrosius W T, Kneebone P H. Tunneled infusion catheters: increased incidence of symptomatic venous thrombosis after subclavian versus internal jugular venous access. Radiology. 2000;217:89–93. doi: 10.1148/radiology.217.1.r00oc2789. [DOI] [PubMed] [Google Scholar]
- Schillinger F, Schillinger D, Montagnac R, Milcent T. Post catheterisation vein stenosis in haemodialysis: comparative angiographic study of 50 subclavian and 50 internal jugular accesses. Nephrol Dial Transplant. 1991;6:722–724. doi: 10.1093/ndt/6.10.722. [DOI] [PubMed] [Google Scholar]
- Cimochowski G E, Worley E, Rutherford W E, Sartain J, Blondin J, Harter H. Superiority of the internal jugular over the subclavian access for temporary dialysis. Nephron. 1990;54:154–161. doi: 10.1159/000185837. [DOI] [PubMed] [Google Scholar]
- Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients. JAMA. 2001;286:700–707. doi: 10.1001/jama.286.6.700. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation Dialysis Outcomes Quality Initiative Available at: http://www.kidney.org/professionals/kdoqi/guidelines. Accessed June 2006. Available at: http://www.kidney.org/professionals/kdoqi/guidelines
- Funaki B, Zaleski G X, Leef J A, Lorenz J, Ha T Van, Rosenblum J D. Radiologic placement of tunneled hemodialysis catheters in occluded neck, chest, or small thyrocervical collateral veins in central venous occlusion. Radiology. 2001;218:471–476. doi: 10.1148/radiology.218.2.r01fe29471. [DOI] [PubMed] [Google Scholar]
- Zaleski G X, Funaki B, Lorenz J M, et al. Experience with tunneled femoral hemodialysis catheters. AJR Am J Roentgenol. 1999;172:493–496. doi: 10.2214/ajr.172.2.9930810. [DOI] [PubMed] [Google Scholar]
- Lund G B, Trerotola S O, Scheel P J., Jr Percutaneous translumbar inferior vena cava cannulation for hemodialysis. Am J Kidney Dis. 1995;25:732–737. doi: 10.1016/0272-6386(95)90549-9. [DOI] [PubMed] [Google Scholar]
- Smith T P, Ryan J M, Reddan D N. Transhepatic catheter access for hemodialysis. Radiology. 2004;232:246–251. doi: 10.1148/radiol.2321030677. [DOI] [PubMed] [Google Scholar]
- Stavropoulos S W, Pan J J, Clark T W, et al. Percutaneous transhepatic venous access for hemodialysis. J Vasc Interv Radiol. 2003;14:1187–1190. doi: 10.1097/01.rvi.0000085770.63355.f2. [DOI] [PubMed] [Google Scholar]
- Chavanon O, Maurizi-Balzan J, Chavanis N, Morel B, Blin D. Successful prolonged use of an intracardiac catheter for dialysis. Nephrol Dial Transplant. 1999;14:2015–2016. doi: 10.1093/ndt/14.8.2015. [DOI] [PubMed] [Google Scholar]
- Chandler N M, Mistry B M, Garvin P J. Surgical bypass for subclavian vein occlusion in hemodialysis patients. J Am Coll Surg. 2002;194:416–421. doi: 10.1016/s1072-7515(02)01127-4. [DOI] [PubMed] [Google Scholar]