ABSTRACT
Urgent treatment of gastrointestinal bleeding is multidisciplinary and often variable by institution. In general, medical management is the first-line therapy for both upper and lower gastrointestinal hemorrhage. In severe upper gastrointestinal hemorrhage, endoscopy is performed prior to other interventions as it is often both diagnostic and therapeutic. Embolization is performed for refractory arterial bleeding. Transjugular portosystemic shunt insertion may be performed to treat refractory variceal bleeding although its use at night is controversial. The treatment algorithm for lower gastrointestinal bleeding is less clear but in general, severe bleeding is handled in the interventional suite by superselective embolization and less severe bleeding is initially treated by endoscopy after an 8- to 12-hour bowel prep. This article will summarize the current approach in my hospital for treating patients with acute gastrointestinal hemorrhage.
Keywords: Embolization, urgent, gastrointestinal bleeding, variceal bleeding, TIPS, microcatheter, colon, endovascular therapy
In an ideal world, all gastrointestinal bleeding would be approached similarly, irrespective of time or day. In reality, this approach is impossible to implement given obvious after-hours limitations on staffing and facilities. Thus, each hospital needs a diagnostic and therapeutic algorithm for the patient with gastrointestinal bleeding during the day and at night. Optimal treatment is multidisciplinary and variable by institution. Endoscopic and endovascular procedures have supplanted surgery as the preferred initial therapies for patients with bleeding refractory to medical management.
In upper gastrointestinal hemorrhage, endoscopic diagnosis and therapy is the initial treatment of choice followed by embolization or transjugular portosystemic shunt (TIPS) insertion depending on whether bleeding is arterial or variceal. The treatment algorithm for lower gastrointestinal bleeding is less clear but in general, severe bleeding is handled in the interventional suite by superselective embolization with less severe bleeding initially treated by endoscopists after a 8- to 12-hour bowel prep. This article will summarize the current approach in my hospital for treating patients with acute gastrointestinal hemorrhage.
UPPER GASTROINTESTINAL HEMORRHAGE
Upper gastrointestinal hemorrhage is initially treated with medical management and endoscopy. Medical therapy includes volume restoration, hemodynamic stabilization, and correction of coagulopathy. Therapeutic endoscopy and omeprazole have decreased the need for emergent surgery for patients with upper gastrointestinal bleeding.1,2,3,4,5 In the vast majority of patients, endoscopy should be performed prior to other invasive maneuvers as it is imperative to determine whether bleeding is variceal or arterial. Moreover, in the majority of patients, endoscopic treatments such as epinephrine injection, variceal banding, or thermocoagulation will control bleeding. In refractory cases, patients are often referred to interventional radiologists for further care.
Arterial Hemorrhage
The most common etiologies of arterial upper gastrointestinal hemorrhage are peptic ulcer disease, variceal bleeding, hemorrhagic gastritis, trauma, and Mallory-Weiss tears. Our treatment algorithm for upper gastrointestinal arterial hemorrhage is the same at day and night. In most cases, the endovascular treatment is similar regardless of bleeding etiology. The basic principles of angiographic diagnosis and management of arterial upper gastrointestinal hemorrhage have not changed significantly in the past 20 years. However, the equipment available to interventional radiologists has evolved considerably, simplifying the technical aspects of the procedure.
Prior to angiography, renal and coagulation status should be assessed. Intravenous hydration should be performed if necessary and any coagulopathy (prothrombin time greater than 17 seconds or platelets less than 50,000/mm3) should be corrected with packed erythrocyte, platelet, or fresh frozen plasma transfusions. In short, medical management must be optimized as much as reasonably possible. If necessary, blood products may be given during angiographic procedures. It is important for patients and referring physicians to understand that embolization is unlikely to succeed if an underlying coagulopathy cannot be corrected. Patients must be able to lie supine for the procedure and it is helpful if they are able to cooperate with simple breathing instructions.
The results of endoscopy should be discussed prior to angiography. If the bleeding site is identified (which should be the case in nearly all patients), angiography is performed with the intention to treat. When necessary, intravenous midazolam hydrochloride (Versed; Roche Pharmaceuticals, Nutley, NJ) and fentanyl citrate (Sublimaze; Abbott Laboratories, North Chicago, IL) are used for sedation and analgesia. Intravenous glucagon (1 mg, Eli Lilly and Company, Indianapolis, IN) may be administered to decrease bowel peristalsis. Oxygen saturation, blood pressure, and heart rate and rhythm are monitored noninvasively in all patients. In our practice, a member of the referring medical service is usually immediately available to help manage patient care.
Diagnostic angiography is performed by catheterizing one of the common femoral arteries using the Seldinger technique and inserting a 5F sheath. A 5F catheter is then advanced through the sheath and used to selectively engage the celiac or superior mesenteric artery. I prefer the RC-1 catheter (Boston Scientific, Natick, MA) for celiac and superior mesenteric artery catheterization but each interventional radiologist has his or her own favorite. Digital angiography is performed and active bleeding is typically characterized by persistent pooling of contrast throughout the venous phase of injection on unsubtracted images. Often, contrast extravasation is directly visualized when digital subtraction angiographic runs are displayed in a cine-loop fashion.
Endovascular Therapy
If either arteriography or endoscopy discloses the site of bleeding, two catheter-based treatment options exist: local vasoconstrictive therapy and embolization. Although local vasoconstrictive therapy is reported to be successful in ~50 to 90% of patients,6,7,8 in my practice, it is only used as a last resort option if subselection of either the gastroduodenal artery or left gastric artery is not possible. Parenthetically given the wide availability of microcatheters that facilitate embolization in even the most difficult catheterizations, I have not used vasopressin for gastrointestinal bleeding in the past 5 years. Typically, vasopressin is infused into a proximal visceral artery at 0.2 U per minute and may be increased to 0.4 U per minute in refractory bleeding. If successful, the infusion is slowly tapered over 12 to 24 hours. There are several disadvantages of vasopressin: an intensive care unit bed is required to manage a femoral artery catheter, a protracted infusion fails to control bleeding in 20 to 40% of patients, and bleeding recurs after successful therapy in up to 20% of patients. Moreover, vasopressin infusion is relatively contraindicated in patients with coronary artery disease. Side effects are encountered in 20% of patients and include abdominal cramps, bowel infarction, congestive heart failure, and ischemia of other organs such as the brain and kidneys.6,7,8
The prevailing endovascular therapy for arterial hemorrhage is embolization, which can be performed with a variety of materials. Gelfoam pledgets, coils, and polyvinyl alcohol particles are used most frequently for upper gastrointestinal hemorrhage. In some patients, combining coils and gelfoam pledgets (“gelfoam-coil sandwiches”) helps to achieve optimal hemostasis. I typically use coils primarily. I add gelfoam to “plug the holes” if bleeding persists through coils. Due to the rich collateral supply of the stomach and duodenum, the risk of bowel ischemia is minimal when embolization is performed with these agents, although caution should be exercised if prior bowel surgery has been performed because collateral vessels may have been sacrificed. Most often, upper gastrointestinal embolization can be performed with conventional 5F catheters. Microcatheters are helpful in select cases (e.g., marked vasospasm of target artery).
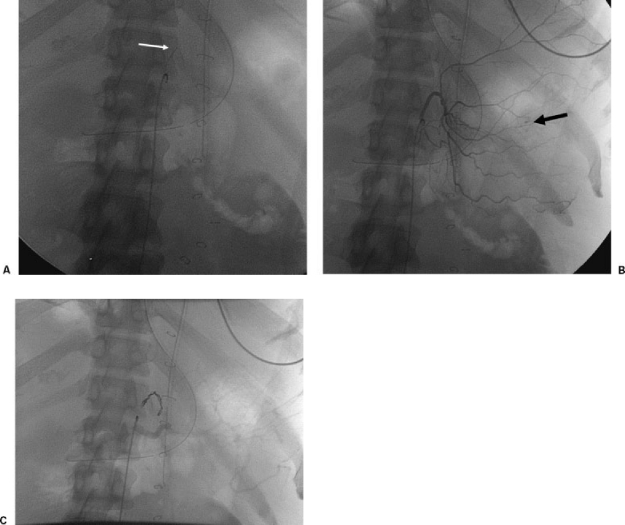
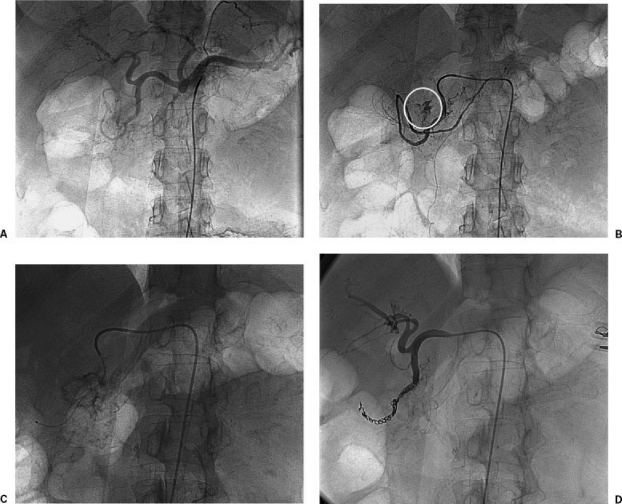
Bleeding in the fundus of the stomach is treated by left gastric artery embolization (Fig. 1), and bleeding in the gastric antrum or proximal duodenum is treated by gastroduodenal artery embolization (Fig. 2). When embolizing the gastroduodenal artery, it is imperative to isolate the source of bleeding. If only the proximal portion of the gastroduodenal artery is occluded, bleeding may persist via the pancreaticoduodenal arcade supplied by the superior mesenteric artery (i.e., collateral bleeding via the “back door”). Occasionally, it may be necessary to embolize these arteries in superselective fashion via the superior mesenteric artery using coaxial microcatheters. Usually, a catheter can be advanced across the bleeding site and coils deposited on both sides. Left gastric artery catheterization can be challenging, especially as this artery arises on the cephalad aspect of the celiac axis. A few options are available including forming a Waltman's loop or using a catheter with a preformed upward pointing tip and a microcatheter. A replaced left gastric artery is larger in caliber and therefore easier to catheterize but if encountered, it is generally advisable to perform embolization distal to the origins of the segment 3 and 4 left hepatic arteries.
Figure 1.
A 64-year-old man with gastric fundal bleeding diagnosed on endoscopy. (A) Fluoroscopic image showing microcatheter in left gastric artery (white arrow). (B) Selective left gastric angiogram showing extravasation of contrast (black arrow). (C) Fluoroscopic image showing coil embolization of left gastric artery.
Figure 2.
A 45-year-old woman with duodenal hemorrhage. (A) Celiac arteriogram showing left gastric, splenic, common hepatic, gastroduodenal, and proper hepatic arteries. The left gastric is enlarged and supplying a replaced left hepatic artery. There is no evidence of active bleeding on this image. (B) Selective gastroduodenal arteriogram showing active extravasation (white circle). (C) Fluoroscopic image showing microcatheter advanced beyond the bleeding site in gastroduodenal artery. (D) Follow-up arteriogram after embolization showing occlusion of gastroduodenal artery on both sides of bleeding vessel.
In 1986, Gomes et al9 compared vasoconstrictive therapy with embolotherapy and reported an 88% success rate with embolization compared with a 52% success rate with vasopressin. Since that time, most interventional radiologists have adopted embolization as their initial endovascular therapy of choice. Empiric embolization has proven to be effective in numerous studies10,11 and is used routinely in my hospital. Lang et al10 reported the value of prophylactic embolization of the left gastric artery in patients with massive upper gastrointestinal hemorrhage with normal findings on arteriography. Iatrogenic bleeding from gastrostomy, endoscopic sphincterotomy, or percutaneous transhepatic interventions is particularly well suited for embolotherapy. Other uncommon causes of bleeding that respond well to embolotherapy include aneurysms associated with visceral artery stenosis and pseudoaneurysms caused by pancreatitis.
Schenker et al11 studied the role of clinical and technical factors on the success and survival of patients undergoing upper gastrointestinal embolotherapy. Using logistic regression, these researchers found that patients who underwent clinically successful embolization were 13.3 times more likely to survive compared with those who had an unsuccessful procedure. Patients with multiorgan system failure were 17.5 times more likely to die, independent of the outcome of embolization. Because ongoing bleeding typically coincides with clinical deterioration of the patient, embolization should be pursued early in the hospitalization of any patient with upper gastrointestinal bleeding that fails therapeutic endoscopy.
Variceal Bleeding
After-hours treatment of variceal hemorrhage is difficult and highly controversial. In my hospital, we generally have stopped performing TIPS at night (i.e., in patients who cannot be stabilized with medical management until morning) but do perform this procedure on weekends during the day. At night, medical and endoscopic management is maximized. The goal is to stabilize the patient, correct any coagulopathies that may be present, and optimize medical management prior to TIPS (which is performed with sedation provided by the Department of Anesthesia). The patient should be resuscitated with blood products, clotting factors, and intravenous fluids. Prophylactic antibiotics are administered. Pharmacological therapy with vasopressin and somatostatin or their analogs may provide excellent short-term control of bleeding. Sclerotherapy and/or band ligation should be performed, and if necessary, balloon tamponade may be pursued with Sengstaken-Blakemore catheters.
There are several prognostic algorithms to decide whether a TIPS may be indicated emergently. In general, a Child-Pugh score of > 12 or APACHE II score > 18 portend early mortality and in most of these patients, TIPS is not recommended. In my own anecdotal experience, I have found that patients who are so critical as to require a TIPS urgently at night are unlikely to survive long even with a successful procedure. For a detailed discussion of the role of emergency TIPS, please see the excellent article by Jorge E. Lopera in the December 2005 issue of Seminars in Interventional Radiology.12
LOWER GASTROINTESTINAL HEMORRHAGE
Like the therapy for upper gastrointestinal bleeding, the treatment of severe lower gastrointestinal hemorrhage includes endoscopy, surgical resection, local vasoconstrictive therapy, and embolotherapy. In general, in our hospital, the following algorithm is used during the day and at night. All patients with minor bleeding either receive medical management only or undergo bowel preparation and colonoscopy during working hours. With severe, life-threatening intermittent bleeding with hemodynamic stability in between episodes of hemorrhage, patients are first screened with 64-slice computed tomographic angiography (CTA) (arterial and venous phase, no oral contrast—same protocol as to detect aortic endoleaks) to determine whether active bleeding is ongoing. If so, patients undergo immediate angiography targeted to the site of bleeding. If CTA fails to disclose bleeding, Tc-99M red blood cell scans may be obtained to identify intermittent bleeding. Nuclear medicine scanning is advantageous for intermittent bleeding because imaging is performed continuously during a 1- to 2-hour period. Angiography should be performed as quickly as reasonably possible after a positive study. In our hospital, we strive to perform angiography within 1 hour of a positive study. By using this algorithm, the yield of angiography increases substantially.13
Patients who have severe, ongoing bleeding will occasionally forego noninvasive imaging and instead receive urgent angiography. Most often, these patients are hypotensive and tachycardic. It is important to recognize the fact that brisk upper gastrointestinal bleeding can sometimes present with bright red blood per rectum. Therefore, a patient seen by an interventional radiologist with purported lower gastrointestinal hemorrhage should always receive complete mesenteric angiography (celiac axis, superior mesenteric artery, inferior mesenteric artery, and internal iliac arteries) to exclude either both an upper or lower gastrointestinal source.
Angiography
Mesenteric angiography is performed as described above. When obtained, nuclear medicine studies or CTA are used to guide catheterization. If bleeding appears to originate in the distal colon, the inferior mesenteric artery is catheterized initially (I use a Rosch Inferior Mesenteric catheter) and if bleeding appears to originate more proximally, the superior mesenteric artery is interrogated first. In distal bleeding or in an otherwise negative study, it is important to remember to interrogate the internal iliac arteries as both the middle and inferior rectal arteries can be a source of colonic hemorrhage. Active extravasation in the lower gastrointestinal tract appears identical to bleeding in the upper gastrointestinal tract. Contrast extravasation persists throughout the venous phase and is distinguished from bowel peristalsis on unsubtracted images.
Endovascular Therapy
Similar to upper gastrointestinal bleeding, local vasoconstrictive therapy with vasopressin infusion was initially established as the first-line catheter-directed treatment for lower gastrointestinal hemorrhage. The procedural details and disadvantages have been described above. As noted previously, in my hospital, vasopressin is used only if embolotherapy cannot be successfully performed.
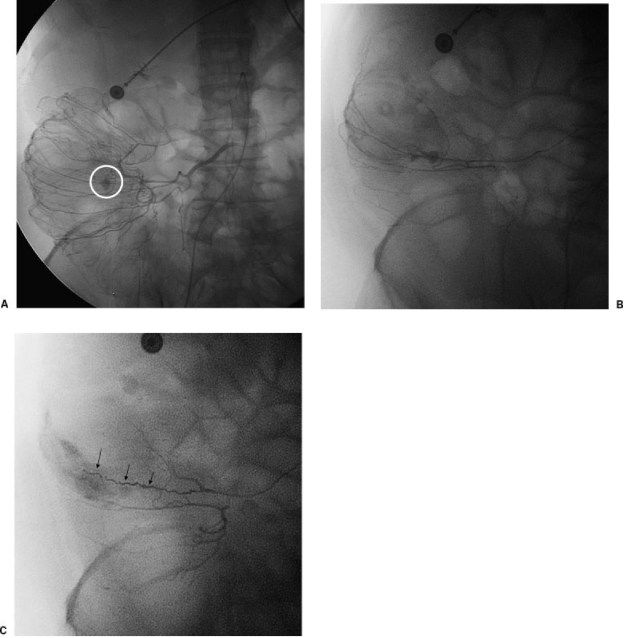
If a bleeding source is seen angiographically, a 3F microcatheter (Target 18, 325 or HiFlow, Boston Scientific) is advanced coaxially to the site through the indwelling 5F catheter. The catheter is positioned as close to the bleeding site as possible. In the inferior mesenteric artery distribution, the catheter is advanced to the marginal artery or terminal artery if possible, and in the superior mesenteric artery, the catheter is advanced to the vasa rectum (Fig. 3). If vasospasm impedes the catheter, intra-arterial papaverine (25 mg, American Regent Laboratories, Inc., Shirley, NY) or simply waiting for several minutes may relieve the obstruction. Except in rare circumstances, embolization should be performed only when the catheter has been advanced to or distal to the mesenteric border of the colon (i.e., to the marginal artery or vasa rectae).
Figure 3.
A 68-year-old woman with colonic bleeding. (A) Subselective superior mesenteric artery injection via microcatheter shows bleeding in right colon (white circle). (B) Superselective angiogram shows bleeding artery. (C) Superselective angiogram after embolization of bleeding artery shows no further extravasation. Note multiple coils (arrows).
Unlike the stomach and duodenum, the bowel distal to the ligament of Treitz (with the exception of the rectum) does not have a dual blood supply. Therefore, caution must be exercised when performing embolization. Therapy is a compromise between selective arterial inflow reduction and maintenance of collateral arterial blood flow. Arterial inflow must be decreased enough to allow hemostasis but not to the degree to cause total devascularization. The preferred level of embolization remains controversial. In general, any embolic agent should be deployed as distally as possible to limit possible ischemia to as small an area as possible. The risk of infarction is related to both the embolic agent as well as the proximity of embolization. Ischemia and infarction may result from embolization of proximal colonic branches supplying a large area of bowel or embolization of multiple distal arteries that are devoid of collateral flow.14,15 Numerous reports indicate that superselective embolization for lower gastrointestinal tract hemorrhage rapidly arrests bleeding while minimizing the risk of ischemia.15,16,17,18,19,20,21,22,23,24,25,26,27,28
The embolic agent is generally selected based on the suspected etiology of bleeding. In the vast majority of patients, I use microcoils as they are easily visualized fluoroscopically, allowing precise deployment and in most cases fulfilling the objective of decreasing the perfusion pressure while allowing enough collateral flow to prevent infarction. Microcoils are ideal for diverticular bleeding. Others materials such as polyvinyl alcohol have also been used successfully; however, polyvinyl alcohol is more difficult to control than microcoils because it cannot be directly visualized by fluoroscopy. An additional disadvantage of polyvinyl alcohol particles is that small particles as well as larger particles (if they fragment) may reach intramural circulation beyond the level of collateralization23 or reflux into nontarget arteries. Finally, embolization with polyvinyl alcohol is not advised if the terminal artery cannot be catheterized. Nonetheless, polyvinyl alcohol particles may have advantages in some instances, such as in patients with bleeding tumor masses or angiodysplasia.
As discussed, the etiology of hemorrhage has important implications on both the short- and long-term success of the procedure. Because embolization treats a symptom of the underlying disorder rather than the disease itself, bleeding may recur in some patients, particularly those with multifocal disease (e.g., extensive diverticulosis, bowel metastases). Nonetheless, like many percutaneous procedures, successful embolization does not preclude repeat attempts and when successful, embolization often averts bowel resection and associated colostomy.
CONCLUSION
The treatment of arterial gastrointestinal hemorrhage continues to evolve. Currently, most interventional radiologists approach bleeding both in the upper and lower gastrointestinal tract with intention to treat. Embolization has replaced local vasoconstrictive therapy as the catheter-based treatment of choice. Coaxial microcatheters have simplified embolotherapy and enabled safe and effective treatment of lower gastrointestinal bleeding. TIPS remains the second-line treatment of choice for refractory variceal bleeding, although its use at night remains controversial.
REFERENCES
- Cook D J, Guyatt G H, Salena B J, et al. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: a meta-analysis. Gastroenterology. 1992;102:139–148. doi: 10.1016/0016-5085(92)91793-4. [DOI] [PubMed] [Google Scholar]
- Cooper G S, Chak A, Way L E, et al. Early endoscopy in upper gastrointestinal hemorrhage: associations with recurrent bleeding, surgery, and length of hospital stay. Gastrointest Endosc. 1999;49:145–152. doi: 10.1016/s0016-5107(99)70478-5. [DOI] [PubMed] [Google Scholar]
- Khuroo M S, Yattoo G N, Javid G, et al. A comparison of omeprazole and placebo for bleeding peptic ulcer. N Engl J Med. 1997;336:1054–1058. doi: 10.1056/NEJM199704103361503. [DOI] [PubMed] [Google Scholar]
- Gralnek I M, Jensen D M, Gornbein J, et al. Clinical and economic outcomes of individuals with severe peptic ulcer hemorrhage and nonbleeding visible vessel: an analysis of two prospective clinical trials. Am J Gastroenterol. 1998;93:2047–2056. doi: 10.1111/j.1572-0241.1998.00590.x. [DOI] [PubMed] [Google Scholar]
- Libby E D. Omeprazole to prevent recurrent bleeding after endoscopic treatment of ulcers. N Engl J Med. 2000;343:358–359. doi: 10.1056/NEJM200008033430509. [DOI] [PubMed] [Google Scholar]
- Rosch J, Dotter C T, Antonovic R. Selective vasoconstrictor infusion in the management of arterio-capillary gastrointestinal hemorrhage. Am J Roentgenol Radium Ther Nucl Med. 1972;116:279–288. doi: 10.2214/ajr.116.2.279. [DOI] [PubMed] [Google Scholar]
- Athanasoulis C A, Baum S, Rosch J, et al. Mesenteric arterial infusion of vasopressin for hemorrhage from colonic diverticulosis. Am J Surg. 1975;129:212–216. doi: 10.1016/0002-9610(75)90300-1. [DOI] [PubMed] [Google Scholar]
- Eckstein M R, Kelemouridis V, Athanasoulis C A, et al. Gastric bleeding: therapy with intraarterial vasopressin and transcatheter embolization. Radiology. 1984;152:643–646. doi: 10.1148/radiology.152.3.6611562. [DOI] [PubMed] [Google Scholar]
- Gomes A S, Lois J F, McCoy R D. Angiographic treatment of gastrointestinal hemorrhage: comparison of vasopressin infusion and embolization. AJR Am J Roentgenol. 1986;146:1031–1037. doi: 10.2214/ajr.146.5.1031. [DOI] [PubMed] [Google Scholar]
- Lang E V, Picus D, Marx M V, et al. Massive upper gastrointestinal hemorrhage with normal findings on arteriography: value of prophylactic embolization of the left gastric artery. AJR Am J Roentgenol. 1992;158:547–549. doi: 10.2214/ajr.158.3.1738991. [DOI] [PubMed] [Google Scholar]
- Schenker M P, Duszak R, Soulen M C, et al. Upper gastrointestinal hemorrhage and transcatheter embolotherapy: clinical and technical factors impacting success and survival. J Vasc Interv Radiol. 2001;12:1263–1271. doi: 10.1016/s1051-0443(07)61549-8. [DOI] [PubMed] [Google Scholar]
- Lopera J E. Role of emergency transjugular intrahepatic portosystemic shunts. Semin Intervent Radiol. 2005;22:253–265. doi: 10.1055/s-2005-925551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderman R, Leef J, Ong K, et al. Scintigraphic screening prior to visceral arteriography in acute lower gastrointestinal bleeding. J Nucl Med. 1998;39:1081–1083. [PubMed] [Google Scholar]
- Rosenkrantz H, Bookstein J J, Rosen R J, et al. Postembolic colonic infarction. Radiology. 1982;142:47–51. doi: 10.1148/radiology.142.1.6975953. [DOI] [PubMed] [Google Scholar]
- Funaki B, Kostelic J K, Lorenz J, et al. Superselective microcoil embolization of colonic hemorrhage. AJR Am J Roentgenol. 2001;177:829–836. doi: 10.2214/ajr.177.4.1770829. [DOI] [PubMed] [Google Scholar]
- Evangelista P T, Hallisey M J. Transcatheter embolization for acute lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2000;11:601–606. doi: 10.1016/s1051-0443(07)61612-1. [DOI] [PubMed] [Google Scholar]
- Gordon R L, Ahl K L, Kerlan R K, et al. Selective arterial embolization for the control of lower gastrointestinal bleeding. Am J Surg. 1997;174:24–28. doi: 10.1016/S0002-9610(97)00044-5. [DOI] [PubMed] [Google Scholar]
- Guy G E, Shetty P C, Sharma R P, et al. Acute lower gastrointestinal hemorrhage: treatment by superselective embolization with polyvinyl alcohol particles. AJR Am J Roentgenol. 1992;159:521–526. doi: 10.2214/ajr.159.3.1503016. [DOI] [PubMed] [Google Scholar]
- Ledermann H P, Schoch E, Jost R, et al. Embolization of the vasa recta in acute lower gastrointestinal hemorrhage: a report of five cases. Cardiovasc Intervent Radiol. 1999;22:315–320. doi: 10.1007/s002709900395. [DOI] [PubMed] [Google Scholar]
- Ledermann H P, Schoch E, Jost R, et al. Superselective coil embolization in acute gastrointestinal hemorrhage: personal experience in 10 patients and review of the literature. J Vasc Interv Radiol. 1998;9:753–760. doi: 10.1016/s1051-0443(98)70387-2. [DOI] [PubMed] [Google Scholar]
- Luchtefeld M A, Senagore A J, Szomstein M, et al. Evaluation of transarterial embolization for lower gastrointestinal bleeding. Dis Colon Rectum. 2000;43:532–534. doi: 10.1007/BF02237200. [DOI] [PubMed] [Google Scholar]
- Kramer S C, Gorich J, Rilinger N, et al. Embolization for gastrointestinal hemorrhages. Eur Radiol. 2000;10:802–805. doi: 10.1007/s003300051007. [DOI] [PubMed] [Google Scholar]
- Kusano S, Murata K, Ohuchi H, et al. Low-dose particulate polyvinylalcohol embolization in massive small artery intestinal hemorrhage: experimental and clinical results. Invest Radiol. 1987;22:388–392. doi: 10.1097/00004424-198705000-00006. [DOI] [PubMed] [Google Scholar]
- Nicholson A A, Ettles D F, Hartley J E, et al. Transcatheter coil embolotherapy: a safe and effective option for major colonic haemorrhage. Gut. 1998;43:79–84. doi: 10.1136/gut.43.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck D J, McLoughlin R F, Hughson M N, et al. Percutaneous embolotherapy of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 1998;9:747–751. doi: 10.1016/s1051-0443(98)70386-0. [DOI] [PubMed] [Google Scholar]
- d'Othee B J, Surapaneni P, Rabkin D, Nasser I, Clouse M. Microcoil embolization for acute lower gastrointestinal bleeding. Cardiovasc Intervent Radiol. 2006;29:49–58. doi: 10.1007/s00270-004-0301-4. [DOI] [PubMed] [Google Scholar]
- Kuo W T, Lee D E, Saad W E, Patel N, Sahler L G, Waldman D L. Superselective microcoil embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2003;14:1503–1509. doi: 10.1097/01.rvi.0000099780.23569.e6. [DOI] [PubMed] [Google Scholar]
- Bandi R, Shetty P C, Sharma R P, Burke T H, Burke M W, Kastan D. Superselective arterial embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2001;12:1399–1405. doi: 10.1016/s1051-0443(07)61697-2. [DOI] [PubMed] [Google Scholar]