ABSTRACT
The liver represents a frequent site for primary and secondary neoplasia. Cytoreductive techniques positively influence the outcome of disease progression in these patients. Transhepatic arterial radioembolotherapy utilizing yttrium-90 microspheres represents a recently available in situ therapy that has shown encouraging results in the treatment of these patients. Harnessing the skills of many different specialties, such as interventional radiology, surgical oncology, medical oncology, nuclear medicine, radiation oncology, medical physics, and radiation safety, brings invaluable expertise to the treatment process for a safe and effective radioembolization treatment program.
Keywords: Liver cancer, yttrium-90, radioembolization
Hepatic neoplasia represents a major cause of cancer-related morbidity and mortality. The liver is a predominant focus of metastatic disease from a wide variety of neoplasms. Autopsy studies have demonstrated that the liver is involved in 50 to 70% of metastases from melanoma, lymphoma, and common malignancies that originate in the breast, lung, and gastrointestinal tract, including colorectal cancer.1
A total of 52,180 U.S. deaths are estimated for colorectal cancer in 2007, making it the third most important cause of U.S. cancer mortality.2 Surgical resection with curative intent with or without adjuvant chemotherapy for colorectal cancer is considered to offer the highest survival rates, which range from 30 to 58% at 5 years.3,4 Recurrent, most often unresectable disease, in up to 50% of patients in the hepatic remnant contributes significantly to this inability to achieve long-term cure rates for colorectal cancer patients. Hepatocellular cancer is one of the most common visceral malignancies worldwide and the fourth most common cancer-related death whose incidence is rapidly increasing in the United States.5 Five-year survival for hepatocellular cancer ranges between 30% and 50% following hepatic resection and < 20% following transplantation.
PRINCIPLE OF YTTRIUM-90 RADIOEMBOLIZATION
The regional application of in situ hepatic cytoreductive therapies may favorably alter the natural history of the disease because liver involvement represents a major adverse prognostic variable. In this regard the transarterial route has been exploited on the premise that neoplasia receive blood supply from the arterial rather than the portal circulation, unlike normal hepatocytes.6 Hepatic artery injection therefore allows preferential delivery of therapeutic material to the tumor. A suspension of appropriately calibrated microspheres injected via the hepatic artery preferentially lodge in the peritumoral vessels, a process termed embolization, by which tumors are deprived of their nutrient arterial supply.
Radiation is tumoricidal if sufficient doses can be delivered selectively without compromising innocent bystander tissue. In that context, external beam whole liver radiotherapy is limited in efficacy in the presence of multifocal or large tumors in the liver because the unavoidable exposure of normal hepatocytes results in hepatocellular dysfunction before tumoricidal doses can be achieved.7 Brachytherapy allows the therapeutic radiation source to be implanted within the tumor, circumventing the limitation of nonselectivity of extracorporeal radiotherapy. The clinical application of this effective technology is hampered by the traditional requirement of intraoperative exposure of the liver and is technically restrictive in the presence of multifocal disease.
The hypervascular arterial supply to hepatic tumors could potentially be exploited to deliver lethal doses of radiation. A high-energy radiation source combined with an appropriate-size embolic microscopic particle administered transarterially would allow radiation to be delivered preferentially to the tumor.8 Incorporation of a high energy β-emitter, such as yttrium-90 (Y90), would create a zone of radiation exposure confined to the vicinity of the tumor while maintaining nontumorous hepatic parenchymal exposure to tolerable levels. This forms the premise for radioembolization, also known as selective internal radiation therapy or microsphere brachytherapy. In clinical practice, millions of microspheres, measuring ~30 μ in diameter incorporating yttrium-90, are injected via an arterial catheter to the hepatic arterial supply of the tumor. This technique allows large doses of radiation (200 to 300 Gy) to be given to liver tumors with minimal serious effect on the nontumorous liver.9
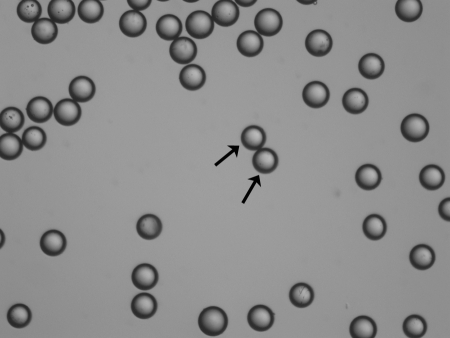
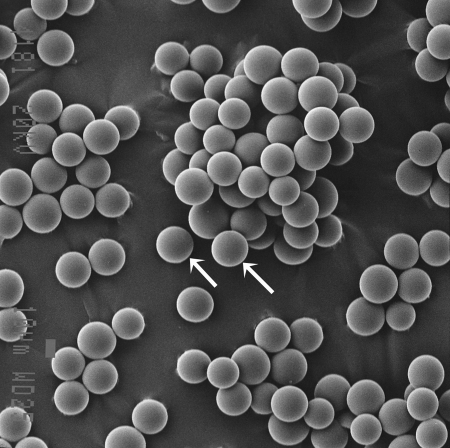
Y90, a pure β emitter, is produced by neutron bombardment of yttrium-89 in a reactor. Y90 has a physical half-life of 64.2 hours (2.67 days) and decays to stable zirconium 90. The average energy of the emissions from the Y90 is 0.9367 MeV, with an average/maximal penetration range of 2.5 mm and 11 mm, respectively, in tissue. One gigabecquerel (27 mCi) delivers a total absorbed radiation dose of 50 Gy/kg. In therapeutic use, in which the isotope decays to infinity, 94% of the radiation is delivered in 11 days. Y90 is the active moiety in a number of targeted radioimmunotherapies used in the treatment of a variety of solid organ and hematological malignancies. The two commercially available Y90 microsphere products are TheraSphere (MDS Nordion, Ottawa, Canada) (Fig. 1) and SIR-Spheres (Sirtex Medical, Sydney, Australia) (Fig. 2), and they vary in their regulatory handling, physical properties, and radioactivity levels (Table 1). They are approved for human use in North America, Europe, Asia, and Australia for the treatment of hepatic neoplasia with the exception of the United States. The U.S. Food and Drug Administration (FDA) has approved TheraSphere under the auspices of a humanitarian device exemption for use as a neoadjuvant to surgery in the treatment of unresectable hepatocellular carcinoma in the presence or absence of portal vein thrombosis. SIR-Spheres are FDA approved for use in the treatment of hepatic metastases from colorectal primary with adjuvant intra-arterial floxuridine.
Figure 1.
TheraSphere. Electron micrograph. Device is represented by individual spherical microspheres (arrows).
Figure 2.
SIR-Spheres. Electron micrograph. Device is represented by individual spherical microspheres (arrows). (Courtesy of Ms. Mara Cvejic, SEM Unit, Institute of Dental Research, Westmead Hospital, Sydney, Australia.)
Table 1.
Characteristics of Microspheres
| Parameter | Resin | Glass |
|---|---|---|
| Trade name | SIR-Spheres | TheraSphere |
| Diameter | 22 ± 10 μm | 32 ± 10 μm |
| Specific gravity | 1.6 g/dL | 3.6 g/dL |
| Activity per particle | 50 Bq | 2500 Bq |
| Average number of microspheres per administered activity | 40–80 million | 1.2–8 million |
| Material | Resin with bound yttrium | Glass with yttrium in matrix |
INDICATIONS AND CONTRAINDICATIONS
The indications for radioembolization are evolving. At the time of publication it is reasonable to consider patients with unresectable hepatic primary or metastatic cancer who harbor liver-dominant tumor burden with a life expectancy > 3 months.
There are four absolute contraindications for liver-directed therapy with Y90 microspheres. These include excessive hepatopulmonary and demonstrable gastrointestinal deposition that can lead to fatal and morbid complications of radiation pneumonitis and gastrointestinal ulceration, respectively; their unique association with radioembolization and their preventability warrants further discussion (see later). Radioembolization should not be performed in pregnancy or in women of childbearing potential without appropriate contraception or breastfeeding because the risks to the developing fetus or infant are unknown. As per the package insert for the resin microsphere device, use of capecitabine is contraindicated for 4 weeks before radioembolization and cannot be resumed after implantation. Relative contraindications include portal venous compromise, liver failure, renal insufficiency, prior hepatic radiotherapy, biliary obstruction, and right-left cardiopulmonary shunting (e.g., patent foramen ovale)10 (Table 2).
Table 2.
Contraindications
| Contraindication | Criterion |
|---|---|
| Absolute | Exaggerated hepatopulmonary shunting |
| Absolute | Propensity for uncorrectable gastrointestinal reflux |
| Absolute | Pregnancy |
| Absolute | Capecitabine therapy/breastfeeding (SIR-Spheres) |
| Relative | Prior hepatic radiotherapy |
| Relative | Portal vein compromise |
| Relative | Liver failure |
| Relative | Renal failure |
| Relative | Right to left cardiopulmonary shunting |
| Relative | Biliary obstruction |
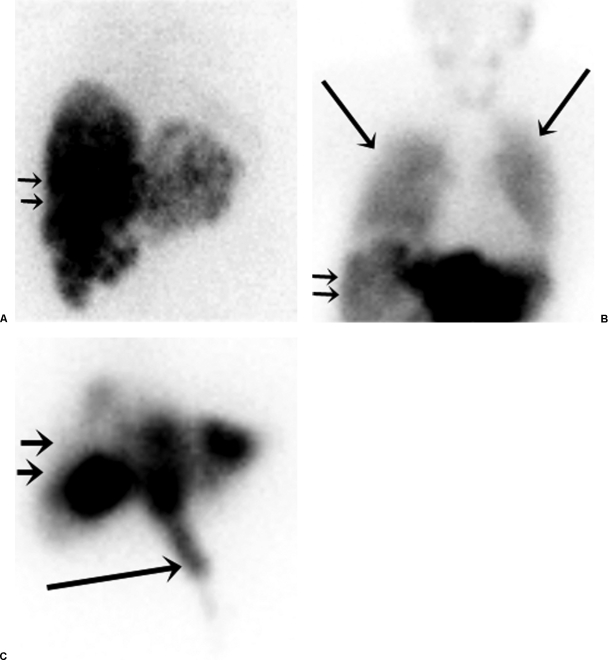
Microspheres injected into the hepatic artery pass through tumor-related arteriovenous shunts and eventually embolize within pulmonary arteriolar branches. Radioactive microspheres can reach the lungs causing clinically significant radiation pneumonitis if the magnitude of shunting is large. Maintenance of lung exposure below a mean dose of 30 Gy has been demonstrated in clinical practice to avoid this complication.11 The likelihood of developing this complication fortunately can be detected before definitive treatment by utilizing technetium 99m (Tc99m) macroaggregate albumin (MAA) as a surrogate that mimics the distribution of the Y90 microspheres (Fig. 3). The magnitude of this shunting phenomenon is calculated by a quantitative assessment of the ratio of the gamma emission count in the lung to that in the liver corrected for background. This value assists in activity modification when the resin microspheres are used.
Figure 3.
Nuclear scintigraphy following hepatic arterial injection of Tc99m MAA (liver depicted with small arrows). (A) A 48-year-old woman with intermediate-grade neuroendocrine hepatic metastases demonstrating normal radionuclide distribution with no significant pulmonary or gastrointestinal activity. (B) A 66-year-old woman with hepatic metastatic high-grade neuroendocrine tumor depicting excessive hepatopulmonary shunting (large arrows) that precluded safe treatment. (C) A 55-year-old man with hepatic metastatic colorectal cancer demonstrating gastrointestinal deposition (large arrow) from small unnamed extrahepatic arteries arising from the proper hepatic artery.
Microspheres may inadvertently reflux into extrahepatic arteries arising from the hepatic arteries that supply the adjacent gastrointestinal tract during the delivery process. This results in their embolization into the gastrointestinal submucosal visceral arterioles, resulting in focal ischemia and radiation. Frequently a treatment-refractory ulcerative diathesis ensues. These extrahepatic arteries (typically the right gastric and the gastroduodenal) are identified angiographically. Extrahepatic scintigraphic activity detected on the Tc99m MAA shunt study allows for the identification of occult extrahepatic arteries or those that are misidentified on arteriography. The incidence of this complication is mitigated by blocking the arterial channels that provide access to this region by coil embolization of their hepatic origins.12
THERAPY PLANNING
Serum chemical analyses are also performed to evaluate hepatic and renal function, traditionally measured by serum bilirubin and creatinine, respectively. The presence and magnitude of the elevation of tumor markers specific to the tumor type being treated are ascertained.
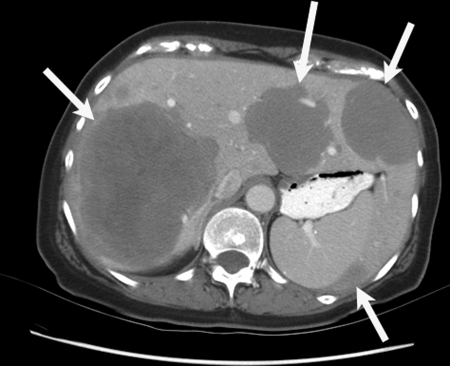
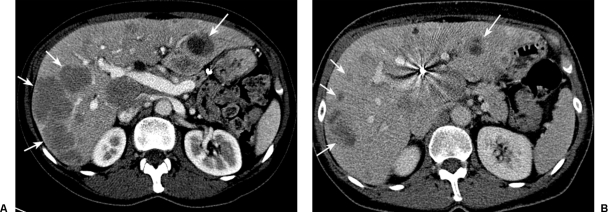
Treatment with Y90 microspheres must be based on cross-sectional images and arteriograms in the individual patient. The workup should include computed tomography (CT) or magnetic resonance (MR) imaging of the liver for assessment of tumoral and nontumoral volume, portal vein patency, and extent of extrahepatic disease. A triple-phase CT to delineate the geographical distribution, the volume, and the partition between hepatic parenchyma and tumor is essential (Fig. 4). Distribution of the disease is typically characterized as unilobar or bilobar; however the correlation of tumor-related hepatic arterial supply is variable and can only be confirmed with selective arteriography. Ascites indicates poor hepatic reserve or peritoneal metastasis, both of which have a poor prognosis.
Figure 4.
Triple-phase computed tomography scan. A 68-year-old woman with low-grade hepatic metastatic neuroendocrine cancer assessed for radioembolotherapy. Multiple low attenuation lesions (white arrows) within the liver are noted. Normal hepatic parenchyma and tumor volume is required for dosimetry.
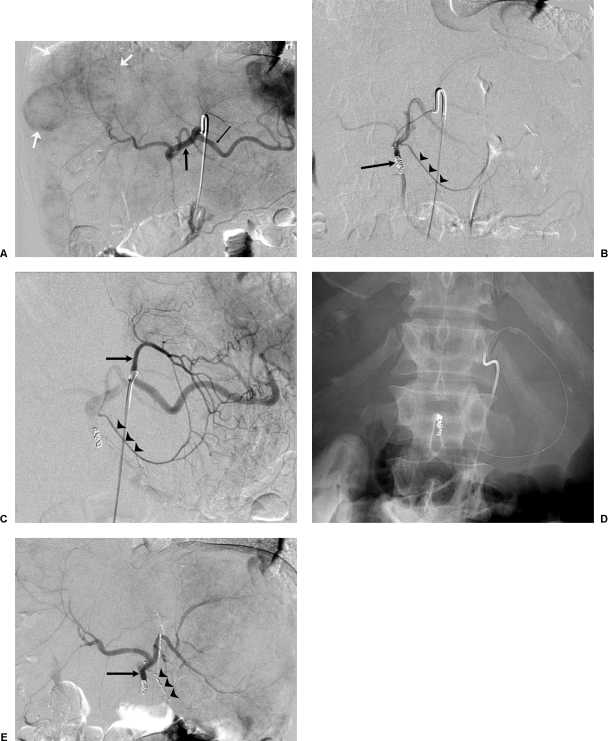
Arteriography is essential to map the hepatic arterial supply from the celiac and the superior mesenteric artery, and it is the single most important test to mitigate gastrointestinal complications (Fig. 5). Using a percutaneously inserted catheter, the hepatic arteries are accessed and the supply to the liver and the adjacent gastrointestinal tract is identified. Once identified, these gastrointestinal tract arteries are coil embolized to ensure prevention of reflux of microspheres into the gut. When such arteries are not apparent arteriographically, the hepatic arterial infusion of 5 mCi of Tc99m MAA assists to detect occult extrahepatic perfusion. This is manifested by extrahepatic scintigraphic activity on nuclear medicine imaging. The culprit artery can usually be identified retrospectively on the angiogram and then coil embolized before radioembolic delivery.
Figure 5.
Pre-radioembolotherapy arteriography in a 55-year-old man with hepatic metastatic colorectal cancer. (A) Celiac arteriogram. Splenic artery (thin black arrow) and common hepatic artery (thick black arrow) arise normally. Note numerous hepatic metastases (white arrows). (B) Proper hepatic arteriogram. The gastroduodenal artery has been coil embolized (single arrow). Note opacification of the right gastric artery arising from the proper hepatic artery (arrowheads). (C) Left gastric arteriogram. The left gastric artery (arrow) anastomoses with the right gastric artery (arrowheads) along the lesser curvature of the stomach. (D) Retrograde access of the right gastric artery. Digital Photospot image demonstrating a microcatheter and wire at the origin of the right gastric artery in preparation for coil embolization. (E) Postembolization proper hepatic arteriogram. The gastroduodenal (single arrow) and right gastric artery (arrowheads) have been coil embolized and demonstrate absence of flow to the gastroduodenal region.
Solitary or multiple lesions distributed in a lobe or both lobes can be treated with single and multiple microsphere treatments successfully. Nomenclature for the current convention for whole liver treatment by first treating one lobe and then the other in 4 to 6 weeks is termed “sequential” or “lobar” delivery, as opposed to both lobes treated at one setting, termed “whole liver delivery” in the absence of a lobectomy.10 The current practice is to allow a 4- to 6-week interval between infusions if treatment was intended to be delivered sequentially to allow for resolution of any treatment-related toxicities.
ACTIVITY DETERMINATION
Y90 microspheres are unlike any traditional radiopharmaceutical or brachytherapy device because they share characteristics of both. At present the activity calculation methodology described in the package insert is recommended; however improvements in dosimetry represent an area of intense investigation.13 CT treatment planning with reconstruction of the liver volumes assists to calculate the required activity for treatment.
Glass Y90 Microsphere Activity Calculation
The dose determination for glass microspheres is based on a nominal average target dose (150 Gy/kg), and the patient's liver mass is determined from the CT data and assumes the uniform distribution of the microsphere throughout liver volume as:
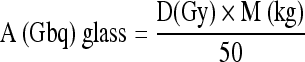 |
In this equation, A is the activity, D is the nominal target dose, and M is the mass of the targeted liver tissue.
Resin Y90 Microsphere Activity Calculation
Resin microspheres are received in a vial as a 3 GBq dose, and the individual medical centers remove the prescribed activity. This process differs from that for glass microspheres where a predetermined activity is delivered to the facility. The manufacturer recommends one of two methods for activity determination for the resin microsphere: the body surface area (BSA) method and the empirical method (EM). However, most experienced practicing physicians recommend the use of the BSA for resin microsphere dose calculation because the delivered dose more closely resembles the activity calculated by the BSA methodology.10
BODY SURFACE AREA METHOD
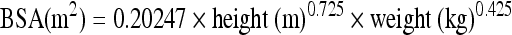 |
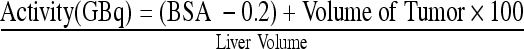 |
EMPIRICAL METHOD
Activity calculated for whole liver treatment is based on tumor replacement as demonstrated on CT (Table 3).
Table 3.
Empirical Method
| A. Calculation of Dose | |
|---|---|
| Liver Involvement by Tumor (%) | Recommended Dose (Gbq) |
| < 25 | 2.0 |
| 25–50 | 2.5 |
| > 50 | 3.0 |
| B. Calculation of Dose Reduction | |
|
Hepatopulmonary Shunting (%) |
Recommended Dose Reduction (%) |
| < 10 | 0 |
| 10–15 | 20 |
| 15–20 | 40 |
| > 20 | 100 |
RADIOEMBOLIZATION
Conventional catheter systems utilized for hepatic arteriography (including large inner diameter microcatheters) are utilized for Y90 radioembolization. The delivery of the radioactive microspheres is based on the principle of fluid displacement and suspension of the particles in the infusate that created the displacement per se. The device-specific proprietary infusion kits protect the operator by separating him or her from the radiotherapy source and are directly connected to the hepatic arterial catheter. The difference in specific gravity influences the relative pressure required to suspend and eject the microspheres from the vial (i.e., more for glass microspheres). The higher specific activity with glass microspheres implies a low volume of microspheres per activity dispensed, and therefore embolic occlusion of the parent artery has not been observed arteriographically.14 On the contrary, the prescribed activity of resin spheres cannot always be delivered completely due to embolic arterial occlusion related to the lower specific activity and therefore a higher volume of microspheres per activity level.15 The delivery kit of the resin microsphere allows for the injection of contrast alternating with the microspheres to determine the appropriate endpoint for microsphere delivery. In these instances, the residual activity in the delivery vial is measured and the delivered activity is the difference between the prescribed and the residual activity.
BREMSSTRAHLUNG SCAN
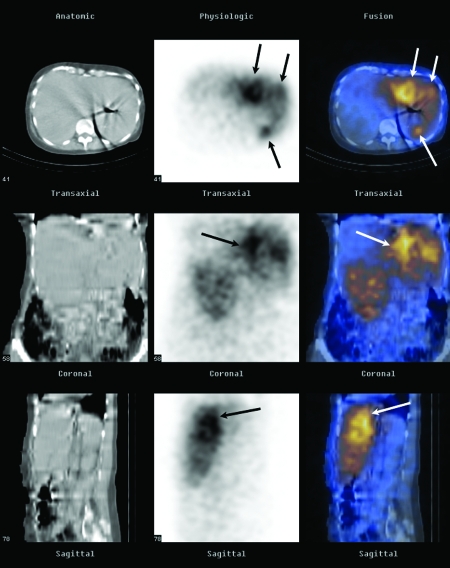
Secondary gamma, Bremsstrahlung, emission scans are possible due to the interaction of the high-energy β-emission interacting with matter. Unfortunately, such Bremsstrahlung emissions represent a broad spectrum of energy emissions, rendering relatively poor spatial discrimination. Planar and/or single-photon emission computed tomography images obtained from such an acquisition are qualitative and allow the operator indirectly to discern the relative distribution of the Y90 microspheres within the liver (Fig. 6). Extrahepatic activity may warn clinicians of impending gastrointestinal complications and serve as a quality assurance tool that assists in retrospective identification of the etiology of extrahepatic perfusion.
Figure 6.
Bremsstrahlung imaging. Successful targeting of hepatic metastases in a 68-year-old woman (same as Fig. 4). Representative axial, coronal, and sagittal computed tomography (CT) images (first column), single-photon emission computed tomography (SPECT) images (second column), and SPECT/CT fusion images (third column) obtained with a dual-modality imaging system (Hawkeye; GE Medical Systems, Milwaukee, WI) show selective activity in the left hepatic lobe metastases (arrows) ~24 hours after intra-arterial infusion of 35 mCi (945 MBq) of Y90 resin microspheres.
POST-TREATMENT COURSE
Patients are usually seen in the clinic weekly or every 2 weeks for a month and then once every month. At the time of clinic visits complete blood count, serum tumor markers, and liver function tests are assayed. Cross-sectional imaging with CT/MRI is performed between 60 and 90 days following treatment to avoid attenuation changes of the hepatic parenchyma to be interpreted erroneously as progression. These decreased attenuation changes in the hepatic parenchyma may be noted on CT and are largely reversible.16 An 18-Fluoro-deoxyglucose positron emission tomography scan may be useful in cases of discordance where tumor markers are not elevated, and CT scans suggest progression or to distinguish the site of progression in the presence of multiorgan disease when not evident by conventional techniques.17
CLINICAL APPLICATIONS
Early studies demonstrated the feasibility of Y90 microsphere therapy for a variety of disease types.18,19 The systematic application toward the treatment of a specific tumor type was seen for colorectal cancer.20 The application of Y90 resin microspheres to a patient population with hepatic colorectal metastases demonstrated favorable responses that were augmented with the addition of hepatic arterial 5FU.21,22 Encouraged by these results, a phase III trial in patients with laparotomy-proven hepatic-only metastatic disease was performed. In this pivotal trial, 72 patients were randomized to receive intra-arterial floxuridine with or without a single dose of the resin microspheres.23 The results demonstrated a benefit in all clinical indexes favoring the combination therapy, specifically a time-to-tumor progression of 15.9 versus 9.7 months (p < 0.01) and formed the basis for FDA approval in the United States. During this trial the majority of patients developed extrahepatic disease that adversely affected survival; this observation was supported by a separate large clinical experience.24 Intra-arterial FUDR was no longer the standard of care at the time of trial completion because irinotecan and oxaliplatin were introduced. To address these two major shortcomings, a small phase II randomized trial and two phase I trials that combined systemic 5FU/LV and the 5FU-based regimens of oxaliplatin and irinotecan, respectively, were performed with enrollment in Australia and Europe. In the randomized phase II trial, responses were significantly augmented with the addition of the Y90 microspheres (8 partial response [PR] versus 0 PR).25 The phase I trials demonstrated that the bimodality approach was safe within the chemotherapy dose ranges used in clinical practice. Furthermore, such combinations generated robust responses (PR + complete response [CR] of 90% with FOLFOX4 [oxaliplatin, leucovorin, and fluorouracil]).26,27 To date, in the United States the application of this therapy has been relegated to patients who have failed multiple chemotherapy regimens, most often as a single agent without combinatorial systemic chemotherapy.15,28 Responses in this highly pretreated cohort are significantly lower than the chemo-naïve population (Fig. 7).
Figure 7.
Colorectal cancer. 50-year-old woman with hepatic metastatic colorectal cancer refractory to oxaliplatin and irinotecan chemotherapy. (A) Pretreatment contrast-enhanced axial CT scan demonstrates a multiple bilobar hepatic metastases (arrows). Radioembolization was performed with resin microspheres on a lobar basis. First treatment was to the left lobe, which received 1.16 GBq. Forty days later, the right lobe was treated with 1.62 GBq. (B) Partial response by RECIST (arrows).
HEPATOCELLULAR CARCINOMA
The other major therapeutic area used with Y90 microsphere therapy has been in the treatment of unresectable hepatocellular carcinoma (HCC). Extensive experience has been gained in the treatment of HCC with resin microspheres in Asia and with glass spheres in the United States.
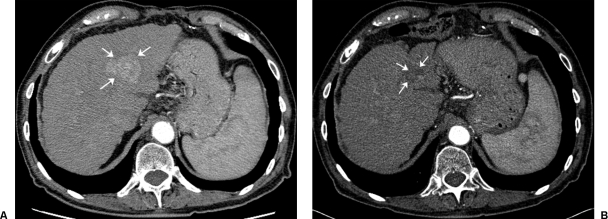
In Asia, Y90 resin microspheres as an effective treatment option was first delineated by an 18-patient phase I/II trial and supported by an observational study in 71 patients conducted by the same group. These studies found that tumor response and clinical benefit was proportional to the dose delivered; patients receiving > 120 Gy survived 55.9 weeks compared with 26.2 weeks for those patients that received < 120 Gy. Repeat treatments with Y90 microspheres provided additional survival benefits.29,30 A Canadian study on 22 patients was conducted to determine response parameters, survival, and toxicity after intra-arterial injection of Y90 glass microspheres.31 Twenty were evaluated for efficacy, including 9 patients who were Okuda stage I and II and 11 patients who were Okuda stage III. The median dose delivered was 104 Gy (range, 45 to 145 Gy). Interestingly, the median survival of 54 weeks (range, 7 to 180 weeks) and the trend for enhanced survival with higher doses (> 104 Gy) was similar to the results seen for resin microspheres. Several retrospective patient studies have emerged from the centers treating with glass Y90 microspheres in the United States. In an analysis by Carr in 65 patients, 38% had partial responses; the median survival duration for Okuda stage I and II patients was 649 and 302 days, respectively.32 Geschwind reported on 80 patients from a relatively large database of 121 patients who were treated with glass microspheres.33 Patients were staged using the Child-Pugh, Okuda, or Cancer of the Liver Italian Program (CLIP) scoring systems. Survival was found to be 628 and 324 days for Okuda I (68%) and II (32%) patients, respectively. Data from an in-depth subset analysis in 121 patients elucidated factors that predicted high 3-month mortality. These included infiltrative tumor, liver replacement by tumor ≥ 70%, elevation in liver enzymes (ALT/AST) ≥ 5 × ULN (upper limits of normal), a combination of tumor volume ≥ 50% and albumin < 3 g/dL, and bilirubin elevation ≥ 2 mg/dL34. Y90 microsphere treatment has resulted in the downstaging of nonresectable disease to be treatable by transplantation, resection or radiofrequency ablation,35 or transplant36 (Fig. 8).
Figure 8.
Hepatocellular cancer. 75-year-old male with hepatitis C–induced cirrhosis and left lobe hepatocellular carcinoma. (A) Pretreatment contrast-enhanced axial CT scan demonstrates a single lesion in the right lobe (arrows). (B) Following a single 1.67 GBq glass microsphere radioembolization (134 Gy dose), a near complete response (arrows) is noted after a 2-year follow-up period.
TOXICITY
The incidence of complications is low if patient selection is appropriate and the delivery technique meticulous. The most common side effect following treatment is transient mild to moderate fatigue and abdominal pain that occurs in ~25% of patients. Nausea and vomiting are managed conservatively; if severe they may be a harbinger for gastrointestinal ulceration. Endoscopy may be indicated. Pancytopenia that had been reported in the earliest version of the Y90 microsphere has not been reported with the newer agents that are in current clinical use.19 Radiation pneumonitis following lung exposure occurs when the dose > 30 Gy.11 No cases of this complication have been reported in > 3000 treatments delivered in the United States, serving as a testament to the validity of utilizing the Tc99m MAA scan in calculating potential lung exposure. Radiation gastritis and gastrointestinal ulceration occur in < 8% of cases; the vast majority of such cases have been managed conservatively without sequelae.12 Gallbladder wall edema is a common finding following treatment, but cholecystitis requiring a cholecystectomy is rare.37 Radiation-induced liver disease (RILD) is a forme fruste of hepatic veno-occlusive disease and manifests clinically as a triad of hepatomegaly and anicteric ascites. Steroids have been the mainstay of therapy and have a poor and variable success at preventing hepatic insufficiency. Although the true incidence is unknown at this point, the perceived incidence of RILD is low.
CONCLUSION
Radioembolization represents an emerging therapy for the treatment of liver cancer. The utility of Y90 microsphere therapy within the context of the other currently available therapies is being evaluated via a series of ongoing clinical trials. Registry data will provide guidance on clinical and therapeutic effectiveness for disease types for which clinical trials are not feasible due to their low incidence or for the vast majority of those afflicted who do not meet eligibility criteria.
REFERENCES
- Gilbert H A, Kagan A R. In: Weiss L, editor. Fundamental Aspects of Metastasis. Amsterdam: North Holland; 1976. Metastasis: incidence, detection and evaluation without histological confirmation. pp. 385–405.
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun M J. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Fong Y, Cohen A, Fortner J, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15(3):938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- Abdalla E K, Vauthey J N, Ellis L M, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239(6):818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag H B, Davila J A, Petersen N J, McGlynn K A. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139(10):817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- Breedis C. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–77. [PMC free article] [PubMed] [Google Scholar]
- Dawson L A, Normolle D, Balter J M, McGinn C J, Lawrence T S, Ten Haken R K. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53(4):810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- Fox R A, Klemp P F, Egan G, Mina L L, Burton M A, Gray B N. Dose distribution following selective internal radiation therapy. Int J Radiat Oncol Biol Phys. 1991;21(2):463–467. doi: 10.1016/0360-3016(91)90797-8. [DOI] [PubMed] [Google Scholar]
- Kennedy A S, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60(5):1552–1563. doi: 10.1016/j.ijrobp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Kennedy A, Salem R, Murthy R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 (Y90) microsphere brachytherapy: a Consensus Panel Report from the Radioembolization Brachytherapy Oncology Consortium. Int J Radiat Oncol Biol Phys. 2007;68(1):13–23. doi: 10.1016/j.ijrobp.2006.11.060. [DOI] [PubMed] [Google Scholar]
- Leung T W, Lau W Y, Ho S K, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys. 1995;33(4):919–924. doi: 10.1016/0360-3016(95)00039-3. [DOI] [PubMed] [Google Scholar]
- Nutting C KA, Coldwell D, Jones B, Quarnberg D. Coil embolization prevents GI ulcers during yttrium-90 hepatic radioembolization. J Vasc Interv Radiol. 2004;15(suppl):2. [Google Scholar]
- Sarfaraz M, Kennedy A, Lodge M, Li X, Wu X, Yu C. Radiation absorbed dose distribution in a patient treated with yttrium-90 microspheres for hepatocellular carcinoma. Med Phys. 2004;31(9):2449–2453. doi: 10.1118/1.1781332. [DOI] [PubMed] [Google Scholar]
- Sato K, Lewandowski R J, Bui J T, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol. 2006;29(4):522–529. doi: 10.1007/s00270-005-0171-4. [DOI] [PubMed] [Google Scholar]
- Murthy R, Xiong H, Nunez R, et al. Yttrium 90 resin microspheres for the treatment of unresectable colorectal hepatic metastases after failure of multiple chemotherapy regimens: preliminary results. J Vasc Interv Radiol. 2005;16(7):937–945. doi: 10.1097/01.RVI.0000161142.12822.66. [DOI] [PubMed] [Google Scholar]
- Marn C S, Andrews J C, Francis I R, Hollett M D, Walker S C, Ensminger W D. Hepatic parenchymal changes after intraarterial Y-90 therapy: CT findings. Radiology. 1993;187(1):125–128. doi: 10.1148/radiology.187.1.8451398. [DOI] [PubMed] [Google Scholar]
- Wong C Y, Salem R, Raman S, Gates V L, Dworkin H J. Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG PET: a comparison with CT or MRI. Eur J Nucl Med Mol Imaging. 2002;29(6):815–820. doi: 10.1007/s00259-002-0787-4. [DOI] [PubMed] [Google Scholar]
- Grady E D. Internal radiation therapy of hepatic cancer. Dis Colon Rectum. 1979;22(6):371–375. doi: 10.1007/BF02586901. [DOI] [PubMed] [Google Scholar]
- Mantravadi R V, Spigos D G, Tan W S, Felix E L. Intraarterial yttrium 90 in the treatment of hepatic malignancy. Radiology. 1982;142(3):783–786. doi: 10.1148/radiology.142.3.7063703. [DOI] [PubMed] [Google Scholar]
- Gray B N, Burton M A, Kelleher D K, Anderson J, Klemp P. Selective internal radiation (SIR) therapy for treatment of liver metastases: measurement of response rate. J Surg Oncol. 1989;42(3):192–196. doi: 10.1002/jso.2930420313. [DOI] [PubMed] [Google Scholar]
- Gray B N, Anderson J E, Burton M A, et al. Regression of liver metastases following treatment with yttrium-90 microspheres. Aust N Z J Surg. 1992;62(2):105–110. doi: 10.1111/j.1445-2197.1992.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Gray B, Hazel G Van, Buck M, Paton G, Burton M, Anderson J. Treatment of colorectal liver metastases with SIR-Spheres plus chemotherapy. GI Cancer. 2000;3(4):249–257. [Google Scholar]
- Gray B, Hazel G Van, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12(12):1711–1720. doi: 10.1023/a:1013569329846. [DOI] [PubMed] [Google Scholar]
- Stubbs R S, Cannan R J, Mitchell A W. Selective internal radiation therapy with 90yttrium microspheres for extensive colorectal liver metastases. J Gastrointest Surg. 2001;5(3):294–302. doi: 10.1016/s1091-255x(01)80051-2. [DOI] [PubMed] [Google Scholar]
- Hazel G Van, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88(2):78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- Sharma R A, Hazel G A Van, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25(9):1099–1106. doi: 10.1200/JCO.2006.08.7916. [DOI] [PubMed] [Google Scholar]
- Hazel G Van. Selective internal radiation therapy (SIRT) plus systemic chemotherapy with Irinotecan: a phase I dose escalation study. ASCO GI Symposium; 2005.
- Kennedy A S, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65(2):412–425. doi: 10.1016/j.ijrobp.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Lau W Y, Leung W T, Ho S, et al. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer. 1994;70(5):994–999. doi: 10.1038/bjc.1994.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau W Y, Ho S, Leung T, et al. Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90yttrium microspheres. Int J Radiat Oncol Biol Phys. 1998;40(3):583–592. doi: 10.1016/s0360-3016(97)00818-3. [DOI] [PubMed] [Google Scholar]
- Dancey J E, Shepherd F A, Paul K, et al. Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. J Nucl Med. 2000;41(10):1673–1681. [PubMed] [Google Scholar]
- Carr B I. Hepatic arterial 90Yttrium glass microspheres (TheraSphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl. 2004;10(suppl 1):S107–S110. doi: 10.1002/lt.20036. [DOI] [PubMed] [Google Scholar]
- Geschwind J F, Salem R, Carr B I, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127(suppl 1):S194–S205. doi: 10.1053/j.gastro.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Goin J E, Salem R, Carr B I, et al. Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: factors associated with liver toxicities. J Vasc Interv Radiol. 2005;16:205–213. doi: 10.1097/01.rvi.00001142592.89564.f9. [DOI] [PubMed] [Google Scholar]
- Lau W Y, Ho S K, Yu S C, Lai E C, Liew C T, Leung T W. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240:299–305. doi: 10.1097/01.sla.0000133123.11932.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D Y, Kwon D S, Salem R, Ma C K, Abouljoud M S. Successful embolization of hepatocellular carcinoma with yttrium-90 glass microspheres prior to liver transplantation. J Gastrointest Surg. 2006;10:413–416. doi: 10.1016/j.gassur.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Thamboo T, Tan K B, Wang S C, Salto-Tellez M. Extra-hepatic embolisation of Y-90 microspheres from selective internal radiation therapy (SIRT) of the liver. Pathology. 2003;35(4):351–353. doi: 10.1080/0031302031000152892. [DOI] [PubMed] [Google Scholar]