ABSTRACT
Atherectomy, the removal of plaque from diseased vessels, is theoretically appealing with respect to various procedures used for revascularization of lower extremity vessels. Instead of damaging the native vessel by pushing aside plaque with a balloon or stent, the plaque is removed. Many atherectomy devices have been designed in attempts to achieve this goal. The SilverHawk device is the latest percutaneous device employing this treatment strategy. We discuss patient selection, technical considerations, and strategies for both above and below the knee revascularization, as well as pitfalls in each location based on our experience with over 200 patients.
Keywords: Atherectomy, SilverHawk, femoropopliteal, tibial
Treatment of peripheral arthrosclerotic disease in the lower extremities offers unique challenges as evidenced by the numerous strategies and devices developed for this purpose. Angioplasty is an accepted treatment for femoropopliteal disease associated with claudication.1 These are characteristically short lesions falling into the TransAtlantic Inter-Society Consensus (TASC) A and B categories. The utility of angioplasty has been extended with subintimal angioplasty.2 Angioplasty is also beneficial for limb salvage in patients with critical limb ischemia in both the femoropopliteal and infrageniculate locations.3,4 Angioplasty has further evolved with the addition of cryoplasty, in an attempt to limit intimal hyperplastic restenosis from barotrauma.5,6
Several novel stent designs have been developed to improve the outcomes of angioplasty in the lower extremities,7,8,9,10 especially in longer length lesions (TASC C and D categories). However, the inhospitable environment of the femoropopliteal region with torsion, flexion, extrinsic compression, and changes in arterial length with daily activities11,12 has limited the success of these devices in the femoropopliteal region.13,14 Below the knee, a different set of problems arises when stents are considered due to the small size of the target vessels, the long segments of disease, and the skip areas without visible disease.
In an effort to avoid the restenosis associated with angioplasty and stenting, other devices have been developed that include laser plaque excision,15,16 mechanical plaque excision, and atherectomy.17,18,19,20,21 These devices attempted to remove plaque from the vessel lumen without damaging the vessel wall, thus reducing post-traumatic restenosis. Despite the initial technical success, these early devices did not yield the patencies that justified the additional procedures and expense.22,23,24,25
One of the most difficult areas to treat in an ischemic limb are the runoff vessels. Angioplasty alone has limited application due to elastic recoil and dissection. Experience with stenting is anecdotal and so far disappointing. However, plaque excision alone or in combination with long balloons may offer a new option for treatment of patients with runoff vessel disease.
ATHERECTOMY DEVICE
The SilverHawk Atherectomy Catheter (FoxHollow Technologies, Redwood City, CA) is the most recent atherectomy device designed for the treatment of de novo and restenotic atherosclerotic lesions. There are several models depending on the size of the vessel and length of lesion. (Table 1) The device tracks over a 0.014-inch guidewire and can be introduced via antegrade or retrograde access depending on the location of the lesion(s). A 7F or 8F sheath is recommended depending on the size of the device, but we have found that we can safely go one size smaller than that recommended for each device.
Table 1.
Currently Available SilverHawk Atherectomy Catheters
| Catalog no. | Vessel Diameter Range | Sheath Compatibility | Crossing Profile | Working Length | Tip Length | |
|---|---|---|---|---|---|---|
| Data courtesy of FoxHollow Corporation (Redwood City, CA). | ||||||
| LS | P4012 | 4.5 mm | 7F/8F | 0.105” (2.7 mm) | 110 cm | 6.0 cm |
| LS-F | P4014 | 4.5 mm | 8F | 0.105” (2.7 mm) | 107 cm | 6.0 cm |
| LX | P4015 | 4.5 mm | 7F/8F | 0.105” (2.7 mm) | 113 cm | 9.0 cm |
| MS | P4016 | 3.5 mm | 7F/8F | 0.105” (2.7 mm) | 110 cm | 6.0 cm |
| MS-F | P4018 | 3.5 mm | 8F | 0.105” (2.7 mm) | 107 cm | 6.0 cm |
| SX | P4023 | 3.0 mm | 7F | 0.095” (2.4 mm) | 136 cm | 4.3 cm |
| SXL | P4033 | 3.0 mm | 7F | 0.095” (2.4 mm) | 136 cm | 7.2 cm |
| SS + | P4030 | 3.0 mm | 7F | 0.090” (2.3 mm) | 135 cm | 2.6 cm |
| ES + | P4034 | 2.0 mm | 6F | 0.075” (1.9 mm) | 135 cm | 2.2 cm |
| DS | P4028 | 1.5 mm | 6F | 0.073” (1.9 mm) | 135 cm | 2.6 cm |
| without wire: 0.062” (1.57 mm) | ||||||
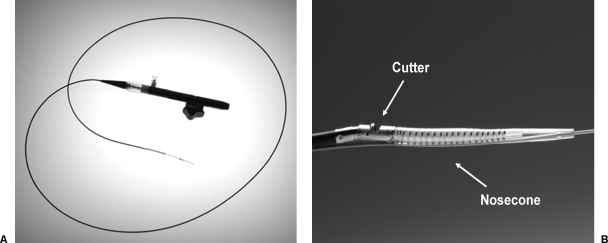
The device consists of two disposable parts, the catheter (Figs. 1A,B) and the battery-driven motor that powers the cutting blade. The distal end of the catheter has a tubular housing that contains the cutting assembly. This consists of a rotating cutting blade and a nosecone, distal to the cutting blade, with a reservoir for collecting the plaque as it is removed. The cutting blade housing is designed such that only a portion of the rotating blade is exposed through a side-looking window. When the motor is off, the reservoir is closed and the cutting blade is contained within the housing, thus protecting the sheath and vessel as the catheter is advanced. When the motor is turned on, the reservoir is opened, the cutting blade exposed, and the window in the tip of the catheter containing the cutting blade is deflected toward the vessel wall to engage the cutting blade against the plaque. The proximal end of the catheter contains the lever that fits into the battery-driven motor unit and simultaneously turns the cutting blade motor on and off and opens and closes the reservoir.
Figure 1.
(A) SilverHawk LS Device without drive motor. (B) Close-up of SilverHawk catheter tip. (Images courtesy of FoxHollow Corporation, Redwood City, CA.)
With the blade spinning at 8000 rpm, the catheter is slowly and smoothly advanced across the lesion, shaving the plaque from the vessel wall. When performing atherectomy, it is recommended that the device only be advanced the length of the nosecone during each pass. This is to reduce the risk of distal embolization. When the cutting process is complete, the lever is advanced, closing the reservoir and covering the cutting blade. The device is then repositioned for the next pass. This is achieved by withdrawing the device and then rotating the cutting blade housing ~30 degrees, to engage another portion of the plaque. This sequence can be repeated as many times as necessary. After two to six passes, depending on the length of the lesion, the device must be removed and cleaned: The atheroma must be removed from the nosecone. Once cleaned, the device may be reinserted and atherectomy continued.
Since the original device there have been several modifications to attempt to overcome its design limitations. The first is the introduction of the LX series of devices. These have a longer nosecone so there is more room to store atheroma. This allows for longer passes as well as fewer removals of the device for cleaning. This should reduce the time for longer lesions. In our experience, the LX series of devices are stiffer than the original devices and with the exception of the superficial femoral artery (SFA) lesions, we do not routinely use them. The LS catheter is our workhorse device for most SFA lesions.
Another design modification is the addition of the MX device. The original design had difficulty in lesions containing visible calcification. The MX device altered the angle of the cutting blade, thus improving device performance in calcified lesions in above-the-knee lesions. The MX device can be used to debulk calcified lesions, and if further atherectomy is required to obtain a satisfactory result, it can be followed with the LS device. The SS device is used for distal popliteal, tibio-perineal trunk, and larger proximal runoff vessels.
The ES and MiniHawk (DS) device were designed to address the difficulties in treatment of stenosis and occlusions below the knee. The ES device is mostly used in mid to distal runoff vessels, and the DS catheter for the calcaneal and dorsalis pedis. The redesigned nosecone has a bulbous tip that improves tracking over the wire. In addition, the wire can be withdrawn reducing the profile of the device and atherectomy performed without the guidewire, due to the improved tracking of the redesigned nosecone.
TECHNICAL CONSIDERATIONS
There is gradually accumulating data in peer-reviewed journals regarding the results of the SilverHawk device.26 The initial results have been quite promising.27,28 Twelve-month follow-up results appear to be encouraging as well.29,30 In our experience, there are two groups of lesions based on location: above the knee (ATK) and below the knee (BTK). Due to their difference in size, as well as the configurations, there are some technical considerations specific to each territory that can facilitate the safe completion of procedures. We review the general considerations as well as specific concerns for interventions in each territory.
Above the Knee: Superficial Femoral and Popliteal Lesions
If possible, our preference is to obtain antegrade access. For ATK lesions, we obtain antegrade percutaneous access with a 7F sheath if the lesion(s) to be treated are ≥ 15 cm, distal to the planned access site. If the lesions(s) are in the common femoral artery or proximal SFA, we perform a retrograde access and then work across the aortic bifurcation. We typically use a 45-cm Pinnacle sheath (Boston Scientific, Natick, MA) or a 45-cm Flexor sheath (Cook, Bloomington, IN). Antegrade access facilitates the procedure by improving torque control of the atherectomy catheter as well as catheters and wires used during the procedure. This is particularly important in the treatment of the runoff vessels. After the sheath has been inserted, we give an intravenous bolus of heparin, 70 U/kg. Activated clotting time is not routinely measured.
Our current practice to use a 0.014-inch filter wire (Boston Scientific, Natick, MA) as our working wire when performing atherectomy of stenosis above the knee. (Fig. 2) Particularly total chronic occlusion and calcified lesions have a high propensity for distal embolization. Distal embolization occurs, albeit infrequently, from lesions in the common femoral, superficial femoral, and popliteal arteries. This has been reported by others as well.31 We have found Rinspiration (FoxHollow Technologies, Redwood City, CA) thrombectomy useful in instances of distal runoff vessel embolization.
Figure 2.
Filter wire in distal popliteal artery prior to popliteal artery atherectomy. Patient had critical limb ischemia with tandem stenosis in the superficial femoral artery and popliteal arteries, and single-vessel runoff via a diseased anterior tibial artery.
When treating total occlusions, we start with the combination of a Quick-Cross catheter (Spectranetics Corporation, Colorado Springs, CO) and 0.014-inch Whisper wire (Guidant Medical, St. Paul, MN), and then switch to the filter wire. We often begin atherectomy with a device sized to the nondiseased vessel. If the device will not pass, as can occur with total occlusions or stenosis > 90%, we begin with a MX or even an SS device to create a channel through the lesion as opposed to angioplasty to create a working channel. Our belief is that there is less vessel trauma. We can then come back with the larger device to obtain the final desired result. If this is unsuccessful, we then perform angioplasty, but this is rarely a problem.
It is important to evaluate the vessel in more than one view. We often perform orthogonal views to confirm adequate plaque excision. We have used intravascular ultrasound (IVUS) to evaluate residual plaque in the vessels as well. The use of IVUS allows those beginning atherectomy to better evaluate the residual plaque and direct the atherectomy toward the residual plaque.
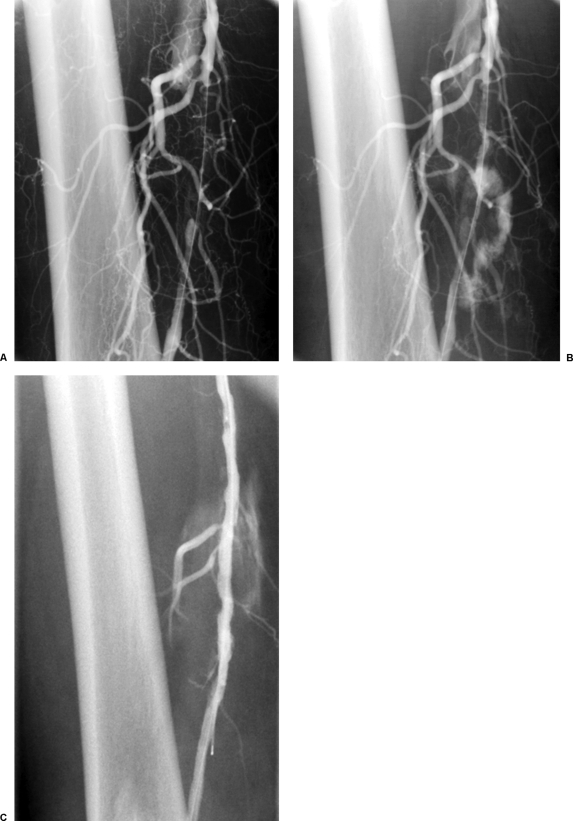
The adductor canal is a location in which great care must be taken not to perforate the vessel. Due to the course of the vessel one must take care when performing atherectomy of the medial portion of the vessel (Figs. 3A, B). It is in this location that we have experienced perforation of the vessel, although in very few patients. In most cases this was successfully treated with prolonged balloon inflation (5 to 10 minutes) (Fig. 3C), although covered stent placement was performed in one instance.
Figure 3.
(A) Superficial femoral artery (SFA) occlusion after crossing with 0.014-inch Whisper wire (Abbott Vascular, Abbott Park, IL) and Quick-Cross catheter (Spectranetics Corporation, Colorado Springs, CO). (B) SFA perforation occluded superficial femoral artery after atherectomy with SilverHawk LS device. (C) Successful tamponade or SFA perforation after prolonged low-pressure angioplasty with 5 mm × 8 cm Sailor Balloon (EV3, Plymouth, MN).
Below the Knee: Popliteal and Tibial Lesions
For popliteal and tibial lesions, we prefer the antegrade approach. Although the devices are long enough to reach the distal vessels from a retrograde approach, it is more difficult to direct the cutting window toward the desired location in the vessel. In these cases we usually use a 6F sheath.
The tibial vessels are too small to use a filter device. Using the Quick-Cross catheter and the Whisper wire, we advance the wire as distally as possible, to the dorsalis pedis or pedal arch. If there is distal embolization, this facilitates advancing a Rinspiration catheter for removal of atheroma or thrombus. Embolization in the runoff vessels occurs very infrequently. We believe this is due to the relative size of the device with respect to the vessel and prevents the device from cutting more plaque when it is full, thus decreasing the risk of distal embolization of large atheroma.
At times, we have noted poor flow after treating total occlusions as well as some stenosis in these small vessels. Believing that this may be from distal embolization of very fine debris, we have used the Rinspiration system and have removed fine sandlike flakes after atherectomy of these vessels. We have found that the 6F catheter designed for use in the coronary arteries works well in these vessels. The key, though, is to have a guidewire already in the foot to facilitate passage of the catheter.
We believe that antiplatelet agents are very important for ATK and even more so for BTK interventions. In addition to receiving aspirin and Plavix, patients with BTK lesions are frequently treated with ReoPro as a single bolus dose at the completion of the case, unless there is a contraindication (i.e., low platelet count).
Clinical Outcomes
The midterm results from the TALON registry were recently published.32 This multicenter registry enrolled 601 patients in which 748 limbs were treated by plaque excision. Approximately 50% of the patients had diabetes, and nearly a third of the patients had Rutherford ischemia category ≥ 4. Of the lesions treated, 87% were de novo. The procedural success was 97.6%. Nearly three quarters (73.3%) of the lesions did not require adjunctive therapy after plaque excision. Stent placement was required in only 6.3% of lesions. The 6- and 12-month rates of target lesion revascularization (TLR) were 10% and 20%, respectively. The rates of TLR were similar among patients with diabetes (11%) and without diabetes (9%).
The European experience is similar to that in the United States.29 Eighty-four patients with Rutherford ischemia category 2 to 5 were followed for 18 months using ultrasound to evaluate for restenosis. Of the 100 limbs treated, there was a nearly equal distribution of de novo lesions (34%), native vessel restenosis (33%), and in-stent restenosis (33%). The technical success rate was 100%. Additional angioplasty was required in 59% of the cases with stent placement in 6% of the lesions. At 18 months, de novo lesions had the better primary patency (73%) than restenotic lesions (42%) or in-stent restenosis (49%). TLR at 12 and 18 months was 16% and 22%, respectively, for de novo lesions, 44% and 56%, respectively, for restenotic lesions, and 47% and 49%, respectively, for in-stent restenosis.
At the Transcatheter Cardiovascular Therapies (TCT) meeting in 2005,33 we reported our results in a subset of patients normally excluded from clinic studies; those with endstage renal disease (ESRD) on dialysis. These patients are typically excluded from clinical studies because of the underlying morbidity and mortality associated with ESRD. The study group consisted of 48 patients with diabetes and ESRD on dialysis. Plaque excision was performed in 74 limbs. All patients had Rutherford ischemia category ≥ 4. These patients were poor surgical candidates, many of whom had been referred for amputation. These patients were followed for 12 months postintervention. After plaque excision, medical therapy was optimized with regular dialysis, strict control of diabetes and hypertension, treatment for hypercholesterolemia with statins, Plavix and aspirin as indicated, and smoking cessation. Of the 74 limbs treated, 65 (88%) were saved from amputation. Only 9 limbs (12%) underwent amputation at some level. All patients who underwent amputation were noncompliant with medical therapy and risk factor modification.
In November 2006, FoxHollow announced the PROOF trial. The PROOF trial is a noninferiority trial comparing SilverHawk plaque excision with open bypass for the treatment of critical limb ischemia. The primary endpoint is amputation-free survival assessed at 6, 12, 24, 36, and 60 months. Secondary endpoints include mortality, clinical improvement, clinical patency, procedural success, and wound healing. The study will also assess quality of life, reintervention rates, and costs. The trial is targeting 200 patients per arm and will involve ~40 international centers.
Economics of Limb Ischemia
Our patient population provides us a unique environment with respect to other regions in the United States. According to the Centers for Disease Control and Prevention (CDC), the rate of diabetes is nearly 50% higher along the U.S.-Mexico border.34 The risk of lower extremity amputation has been estimated at 15 to 40 times higher among persons with diabetes than among persons without diabetes.35 In 2003, a total of 7325 lower extremity amputations were performed on patients with diabetes in the state of Texas.36 Studies have shown that the in-hospital cost of even a digital amputation is approximately $22,000,37 and the total costs of a major amputation are between $40,000 and $70,000.38 This does not include nursing home costs or other costs outside the hospital for modification required to make a home accessible for individuals after a major amputation.
Several studies have also shown that endovascular treatment for critical limb ischemia (CLI) reduces the costs associated with treatment of CLI with respect to bypass39,40 without significant differences in outcome. In a 2001 study by Muradin and Myriam Hunink41 it was estimated that a $3000 device would be cost effective if compared with femoral popliteal bypass surgery for critical limb ischemia if the 5-year patency rate is 29 to 46%. In addition, use of the same device would be cost effective compared with angioplasty for disabling claudication and stenosis if the 5-year patency rate was 69 to 86%. This type of economic analysis would likely favor endovascular treatment over below-knee bypass because the patency rates of femoral below-knee and femoral tibial bypass are even lower than the patency rates for femoral above-knee bypass.42
The current TASC II recommendations advocate angioplasty for TASC A and B lesions in the femoral popliteal region. However, for TASC C and D lesions, bypass surgery is currently advocated. Yet these are typically the patients who are the poorest surgical candidates due to multiple comorbidities (e.g., diabetes, coronary artery disease, hypertension, hypercholesterolemia, renal insufficiency and failure) and would most benefit from a minimally invasive procedure. In infrapopliteal occlusive disease, angioplasty is only recommended for CLI and limb salvage.43 In the diabetic population, which is at higher risk of CLI and amputation, and typically presents with infrapopliteal disease, amputation could be prevented if a durable, minimally invasive treatment is performed prior to the onset of CLI, when the only symptoms are intermittent claudication.
Future Directions
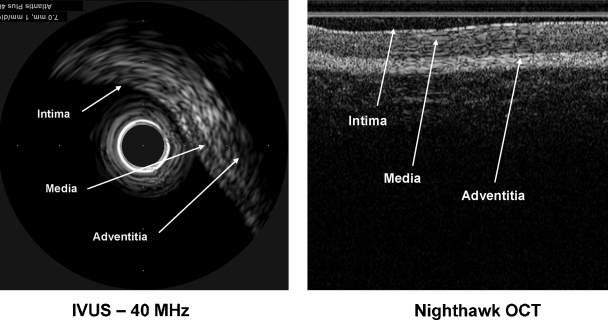
The next generation catheters will contain imaging sensors (Fig. 4) that will allow the operator to visualize the vessel wall to better direct the atherectomy. (Fig. 5). The results of the first clinical trials of these devices, the NightHawk, were presented at TCT 2006.44 The operators confirmed the findings of the preclinical studies; real-time plaque visualization improved the results of the procedure through increased operator confidence with regard to location of residual plaque. This enhancement of the atherectomy catheter is expected to increase the safety and efficacy of this procedure.
Figure 4.
Optical coherence tomography imaging integrated into NightHawk catheter. (Image courtesy of FoxHollow Corporation, Redwood City, CA.)
Figure 5.
Conventional intravascular ultrasound image (Atlantis Plus; Boston Scientific, Natick, MA) and optical coherence tomography (OCT) image obtained with NightHawk catheter. (Images courtesy of FoxHollow Corporation, Redwood City, CA.)
CONCLUSION
The SilverHawk atherectomy catheters provide another minimally invasive tool in the endovascular treatment of peripheral vascular disease. The short-term and midterm results are promising,45 and as more studies are published in peer-reviewed journals we will be better able to determine which patients can benefit from this device.46,47 At this point it appears that vessel segments that are inhospitable to stenting, such as the common femoral artery, distal SFA, popliteal, and runoff vessels, are particularly suitable for plaque excision with the FoxHollow device.
REFERENCES
- Clark T W, Groffsky J L, Soulen M C. Predictors of long-term patency after femoropopliteal angioplasty: results from the STAR registry. J Vasc Interv Radiol. 2001;12:923–933. doi: 10.1016/s1051-0443(07)61570-x. [DOI] [PubMed] [Google Scholar]
- Bolia A, Miles K A, Brennan J, Bell P RF. Percutaneous transluminal angioplasty of occlusions of the femoral and popliteal arteries by subintimal dissection. Cardiovasc Intervent Radiol. 1990;13:357–363. doi: 10.1007/BF02578675. [DOI] [PubMed] [Google Scholar]
- Tefera G, Hoch J, Turnipseed W D. Limb-salvage angioplasty in vascular surgery practice. J Vasc Surg. 2005;41:988–993. doi: 10.1016/j.jvs.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Soder H K, Manninen H I, Jaakkola P, et al. Prospective trial of infrapopliteal artery balloon angioplasty for critical limb ischemia: angiographic and clinical results. J Vasc Interv Radiol. 2000;11:1021–1031. doi: 10.1016/s1051-0443(07)61332-3. [DOI] [PubMed] [Google Scholar]
- Laird J R, Biamino G, McNamara T, et al. Cryoplasty for the treatment of femoropopliteal arterial disease: extended follow-up results. J Endovasc Ther. 2006;13(suppl 2):II52–II59. doi: 10.1177/15266028060130S209. [DOI] [PubMed] [Google Scholar]
- Laird J, Jaff M R, Biamino G, McNamara T, et al. Cryoplasty for the treatment of femoropopliteal arterial disease: results of a prospective, multicenter registry. J Vasc Interv Radiol. 2005;16:1067–1073. doi: 10.1097/01.RVI.0000167866.86201.4E. [DOI] [PubMed] [Google Scholar]
- Saxon R R, Coffman J M, Gooding J M, et al. Long-term results of ePTFE stent-graft versus angioplasty in the femoropopliteal artery: single center experience from a prospective, randomized trial. J Vasc Interv Radiol. 2003;14:303–311. doi: 10.1097/01.rvi.0000058425.01661.d0. [DOI] [PubMed] [Google Scholar]
- Rosenthal D, Martin J D, Schubart P J, et al. Remote superficial femoral artery endarterectomy and distal aSpire stenting: multicenter medium-term results. J Vasc Surg. 2004;40:67–72. doi: 10.1016/j.jvs.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Geraghty P J. Covered stenting of the superficial femoral artery using the Viabahn stent-graft. Perspect Vasc Surg Endovasc Ther. 2006;18:39–43. doi: 10.1177/153100350601800117. [DOI] [PubMed] [Google Scholar]
- Rubin B G, Sicard G A. The Hemobahn endoprosthesis: a self-expanding polytetrafluoroethylene-covered endoprosthesis for the treatment of peripheral arterial occlusive disease after balloon angioplasty. J Vasc Surg. 2001;33(suppl):S124–S128. doi: 10.1067/mva.2001.111674. [DOI] [PubMed] [Google Scholar]
- Wensing P J, Scholten G, Buijs P C, et al. Arterial tortuosity in the femoropopliteal region during knee flexion: a magnetic resonance angiography study. J Anat. 1995;187:133–139. [PMC free article] [PubMed] [Google Scholar]
- Diaz J A, Villegas M, Tamashiro G, et al. Flexions of the popliteal artery: dynamic angiography. J Invasive Cardiol. 2004;16:712–715. [PubMed] [Google Scholar]
- Scheinert D, Scheinert S, Sax J, et al. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J Am Coll Cardiol. 2005;45:312–315. doi: 10.1016/j.jacc.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Arena F J. Arterial kink and damage in normal segments of the superficial femoral and popliteal arteries abutting nitinol stents: a common cause of late occlusion and restenosis? A single-center experience. J Invasive Cardiol. 2005;17:482–486. [PubMed] [Google Scholar]
- Sanborn T A, Greenfield A J, Guben J K, et al. Human percutaneous and intraoperative laser thermal angioplasty: initial clinical results as an adjunct to balloon angioplasty. J Vasc Surg. 1987;5:83–90. [PubMed] [Google Scholar]
- Litvack F, Grundfest W S, Adler L, et al. Percutaneous excimer-laser and excimer-laser-assisted angioplasty of the lower extremities: results of initial clinical trial. Radiology. 1989;172:331–335. doi: 10.1148/radiology.172.2.2526348. [DOI] [PubMed] [Google Scholar]
- Snyder S O, Wheeler J R, Gregory R T, et al. The Kensey catheter: preliminary results with a transluminal atherectomy tool. J Vasc Surg. 1988;8:541–543. doi: 10.1067/mva.1988.avs0080541. [DOI] [PubMed] [Google Scholar]
- Myers K A, Denton M J, Devine T J. Infrainguinal atherectomy using the transluminal endarterectomy catheter: patency rates and clinical success for 144 procedures. J Endovasc Surg. 1994;1:61–70. doi: 10.1583/1074-6218(1994)001<0061:IAUTTE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Myers K A, Denton M J. Infrainguinal atherectomy using the Auth Rotablator: patency rates and clinical success for 36 procedures. J Endovasc Surg. 1995;2:67–73. doi: 10.1583/1074-6218(1995)002<0067:IAUTAR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- The Collaborative Rotablator Atherectomy Group (CRAG) Peripheral atherectomy with the rotablator: a multicenter report. J Vasc Surg. 1994;19:509–515. doi: 10.1016/s0741-5214(94)70079-6. [DOI] [PubMed] [Google Scholar]
- White C J, and the PAC Investigators Peripheral atherectomy with the Pullback atherectomy catheter: procedural safety and efficacy in a multicenter trial. J Endovasc Surg. 1998;5:9–17. doi: 10.1583/1074-6218(1998)005<0009:PAWTPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Jahnke T, Link J, Muller-Hulsbeck S, et al. Treatment of infrapopliteal occlusive disease by high-speed rotational atherectomy: initial and mid-term results. J Vasc Interv Radiol. 2001;12:221–226. doi: 10.1016/s1051-0443(07)61829-6. [DOI] [PubMed] [Google Scholar]
- Vroegindeweij D, Tielbeek A V, Buth J, et al. Directional atherectomy versus balloon angioplasty in segmental femoropopliteal artery disease: two-year follow-up with color-flow duplex scanning. J Vasc Surg. 1995;21:255–269. doi: 10.1016/s0741-5214(95)70267-9. [DOI] [PubMed] [Google Scholar]
- Tielbeek A V, Vroegindeweij D, Buth J, et al. Comparison of balloon angioplasty and Simpson atherectomy for lesions in femoropopliteal artery: angiographic and clinical results of a prospective randomized trial. J Vasc Interv Radiol. 1996;7:837–844. doi: 10.1016/s1051-0443(96)70857-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Okamura T, Rajyaguru V. Laser arterial disobstructive procedures in 148 lower extremities. J Vasc Surg. 1989;10:169–177. [PubMed] [Google Scholar]
- Zeller T, Frank U, Burgelin K, et al. Initial clinical experience with percutaneous atherectomy in the infragenicular arteries. J Endovasc Ther. 2003;10:987–993. doi: 10.1177/152660280301000523. [DOI] [PubMed] [Google Scholar]
- Zeller T, Rastan A, Schwarzwalder U, et al. Percutaneous peripheral atherectomy of femoropopliteal stenoses using a new-generation device: six-month results from a single center experience. J Endovasc Ther. 2004;11:676–685. doi: 10.1583/04-1316R.1. [DOI] [PubMed] [Google Scholar]
- Zeller T, Rastan A, Schwarzwalder U, et al. Midterm results after atherectomy-assisted angioplasty of below-knee arteries with use of the SilverHawk device. J Vasc Interv Radiol. 2004;15:1391–1397. doi: 10.1097/01.RVI.0000138060.05915.9D. [DOI] [PubMed] [Google Scholar]
- Zeller T, Rastan A, Sixt A, et al. Long-term results after directional atherectomy of femoro-popliteal lesions. J Am Coll Cardiol. 2006;48:1573–1578. doi: 10.1016/j.jacc.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Keeling W B, Shames M L, Stone P A, et al. Plaque excision with the SilverHawk catheter: early results in patients with claudication or critical limb ischemia. J Vasc Surg. 2007;45:25–31. doi: 10.1016/j.jvs.2006.08.080. [DOI] [PubMed] [Google Scholar]
- Wholey M H, Toursarkissian B, Postoak D, et al. Early experience in the application of distal protection devices in treatment of peripheral vascular disease of the lower extremities. Catheter Cardiovasc Interv. 2005;64:227–235. doi: 10.1002/ccd.20254. [DOI] [PubMed] [Google Scholar]
- Ramaiah V, Gammon R, Kiesz R S, et al. Midterm outcomes from the TALON registry: treating peripherals with SilverHawk: outcomes collection. J Endovasc Ther. 2006;13:592–602. doi: 10.1583/05-1780MR.1. [DOI] [PubMed] [Google Scholar]
- Kiesz R S. The dialysis patient and end-stage CLI. Washington, DC: Abstract presented at: Transcatheter Cardiovascular Therapies; October 22–27, 2006.
- Ingram M, Gallegos G, Elenes J. Diabetes is a community issue: the critical elements of a successful outreach and education model on the U.S.-Mexico border. Prev Chronic Dis. 2005;2:A15. [PMC free article] [PubMed] [Google Scholar]
- Reiber G E, Boyko E J, Smith D G. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennett PH, editor. Diabetes in America. 2nd ed. Washington, DC: National Institutes of Health; 2002. Lower extremity foot ulcers and amputations in diabetes. Publication no. 95-1468.
- Centers for Disease Control and Prevention (CDC) Geographic disparities in diabetes-related amputations—Texas-Mexico border, 2003. MMWR Morb Mortal Wkly Rep. 2006;55(46):1251–1253. [PubMed] [Google Scholar]
- Eckman M H, Greenfield S, Mackey W C, et al. Foot infections in diabetic patients: decisions and cost-effective analysis. JAMA. 1995;273:712–720. [PubMed] [Google Scholar]
- VHA PACT program directive. May 2001. 2001–030.
- Dosluoglu H H, O'Brien-Irr M S, Lukan J. Does preferential use of endovascular interventions by vascular surgeons improve limb salvage, control of symptoms, and survival of patients with critical limb ischemia? Am J Surg. 2006;192:572–576. doi: 10.1016/j.amjsurg.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Adam D J, Beard J D, Cleveland T, Bell J, et al. Bypass versus angioplasty in severe ischemia of the leg (BASIL): multicentre, randomized controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- Muradin G S, Myriam Hunink M G. Cost and patency rate targets for the development of endovascular devices to treat femoropopliteal arterial disease. Radiology. 2001;218:464–469. doi: 10.1148/radiology.218.2.r01ja09464. [DOI] [PubMed] [Google Scholar]
- Faries P L, Logerfo F W, Arora S, et al. A comparative study of alternative conduits for lower extremity revascularization: all-autogenous conduit versus prosthetic grafts. J Vasc Surg. 2000;32:1080–1090. doi: 10.1067/mva.2000.111279. [DOI] [PubMed] [Google Scholar]
- Norgren L, Hiatt W R, Dormandy J A, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(suppl):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Kiesz R S, Buszman P E, Simpson J B, et al. Integrated optical coherence tomography guided peripheral atherectomy system. Washington, DC: Abstract presented at: Transcatheter Cardiovascular Therapies; October 16–21, 2005.
- Kandzari D E, Kiesz R S, Allie D, et al. Procedural and clinical outcomes with catheter-based plaque excision in critical limb ischemia. J Endovasc Ther. 2006;13:12–22. doi: 10.1583/05-1634.1. [DOI] [PubMed] [Google Scholar]
- Das T S, Beregi J P, Garcia L A, et al. Infrainguinal lesion-specific device choices: round-table discussion. J Endovasc Ther. 2006;13(suppl 2):II60–II71. doi: 10.1177/15266028060130S210. [DOI] [PubMed] [Google Scholar]
- Muhs B E, Gagne P J, Maldonado T, et al. Minimally invasive revascularization strategies for chronic lower limb ischemia. Int J Low Extrem Wounds. 2006;5:35–39. doi: 10.1177/1534734606286473. [DOI] [PubMed] [Google Scholar]