ABSTRACT
Atherosclerotic occlusive lesions of the common carotid artery (CCA), the internal carotid artery (ICA), and the intracranial branches are amenable to angioplasty and stenting. Non atheromatous occlusive lesions caused by fibromuscular dysplasia, arteritis, or trauma may also be treated by image guided intervention in selected patients. Aneurysmal lesions of the CCA, ICA and the intracranial branches of degenerative, mycotic or traumatic etiologies, as well as carotid cavernous fistulae are mostly best treated by embolization. Technological developments continuously expand the indications of interventional treatment in these vascular territories.
Keywords: Common carotid artery, internal carotid, stenosis, aneurysms, stents, embolization
CAROTID INTERVENTIONS BELOW THE BULB
Common Carotid Artery Atherosclerotic Occlusive Disease
Atherosclerosis is the most common pathology of the extracranial carotid arteries. Most stenotic lesions are in the internal carotid bulb. Common carotid lesions are much less frequent, occurring in 1 to 2% of the patients with symptomatic carotid artery disease.1 Less than 5% of the patients with carotid bifurcation atherosclerotic lesions have concurrent common carotid artery (CCA) ostial lesions; 5% have significant stenoses of the intracranial internal carotid artery (ICA).2 Lesions of the right CCA are seen less frequently than those of the left CCA, and usually in combination with innominate artery lesions.
The patient may be asymptomatic or have symptoms similar to those of bifurcation disease: amaurosis fugax, other transient ischemic attacks (TIAs), and stroke. Physical examination may reveal diminished carotid pulses or a proximal neck bruit.3 Noninvasive imaging is vital to the detection of cerebrovascular disease in these territories: gray-scale and color Doppler ultrasonography is not recommended for the evaluation of the CCA ostium because of its deep location. Contrast-enhanced magnetic resonance angiography (CE-MRA) has a sensitivity of > 90% and a specificity of > 90% for the detection of significant stenoses. Similarly high (> 90%) sensitivity and specificity are reported for computed tomographic angiography (CTA).
Surgery offers intrathoracic and cervical (extrathoracic) open procedures. Intrathoracic operations involve ascending aorta to common carotid bypass using synthetic grafts or endarterectomy. These operations have high morbidity and mortality. However, they are indicated when multivessel aortic arch disease is present.4 Extra-anatomic operations include carotid-subclavian bypass and carotid-subclavian transposition, the former being much more frequently used. These operations are said to have zero mortality and significantly fewer complications. Primary 5-year patency rates are in the range of 94%.5
The interventional treatment for aortic arch branches emerged in the 1980s and proved to be successful. Today, stenting has gained widespread acceptance in the management of bifurcation and proximal ICA lesions in selected patients.6 The CCA occlusive lesions are similarly treated by interventional techniques, but in the literature they are reported (because of their relative rarity) in the context of brachiocephalic arteries (innominate, left carotid, left subclavian) or aortic arch branches.
Current guidelines suggest that carotid angioplasty and stenting (CAS) with embolic-protection devices (EPDs) is reasonable and appropriate for symptomatic patients with carotid stenosis of ≥ 70% at high risk for surgery.7 If performed within trials, the procedure may be performed in high-risk symptomatic patients with 50 to 70% stenosis and in high-risk asymptomatic patients with stenosis of ≥ 80%. Criteria for high risk for surgery include cardiac failure and unstable angina, contralateral carotid occlusion, recent myocardial infarction, recurrent stenosis post carotid endarterectomy, and radiation-induced stenosis. However, these guidelines apply mostly for bifurcation and proximal ICA disease, for which surgery is excellent.
Proximal CCA lesions should be treated only when a patient is symptomatic with a stenosis > 50%.8 Exceptions are be made for high-grade (> 80%) stenoses in asymptomatic patients in whom the potential for stroke is high.
The indications for treatment include the following:
Amaurosis fugax and TIAs
Nondevastating stroke with good recovery
Critical > 80% asymptomatic stenosis
Relative contraindications include the following:
Disabling stroke or dementia
Intracranial tumor
Intracranial hemorrhage
Acute thrombus at the CCA
TECHNIQUE
Lesions in the left CCA can be negotiated with a selective catheter and a 0.035-inch hydrophilic guidewire. After crossing the left CCA lesion with the guidewire, the catheter is advanced and placed into the external carotid artery (ECA) so an exchange stiffer guidewire (0.035-inch Amplatz; Boston Scientific, Natick, MA) can be inserted. An 80-cm-long 8F sheath is inserted over the Amplatz guidewire with its tip just proximal to the CCA lesion (Figs. 1 and 2). Predilation of the lesion with an undersized balloon (5 mm × 2 cm) is usually performed to facilitate the passage of the stent and also helps determine the vessel diameter. Subsequently the stent is inserted and deployed across the lesion while precision is facilitated by digital subtraction angiography (DSA) or road mapping performed through the sheath. Nitinol self-expanding stents are easier to insert and adapt to the vessel tortuosity. However, balloon expandable stents are more accurately positioned in ostial lesions (Figs. 1 and 2). For right CCA lesions, one initially would selectively catheterize the right subclavian artery and place the stiff guidewire with its tip into the distal right subclavian artery. With this maneuver the 8F 80-cm sheath can be securely inserted with its tip into the innominate artery. Crossing of the CCA lesion can be then performed with a 0.035-inch hydrophilic guidewire or a 0.014- to 0.018-inch platinum-tip guidewire. The subsequent steps are similar to those previously described.8
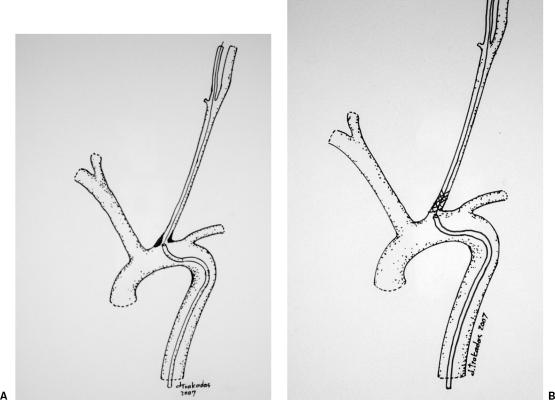
Figure 1.
Interventional treatment of an ostial left common carotid artery lesion. (A) An 80-cm-long sheath is inserted over a stiff guidewire and positioned proximal to the lesion. (B) A balloon-expanded stent is deployed across the lesion.
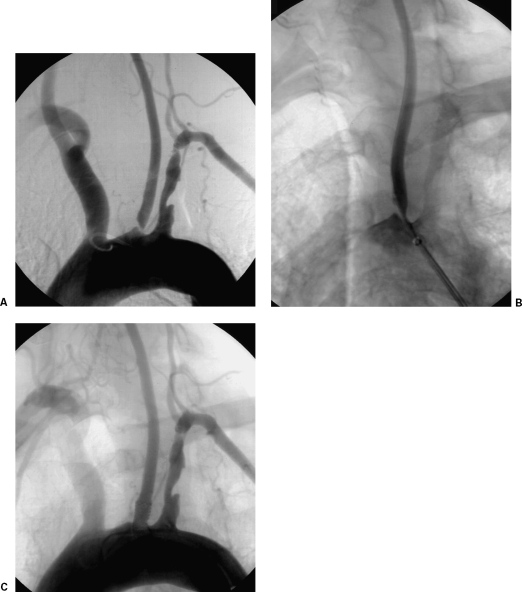
Figure 2.
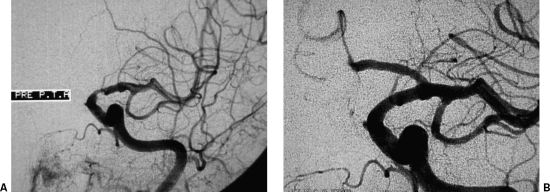
Interventional treatment of a left common carotid artery (CCA) ostial lesion. (A) Arch digital subtraction angiography (DSA) using a pigtail catheter depicts a high-grade left CCA ostial lesion. (B) DSA via a long sheath placed in the aortic arch is used to place the stent accurately; note the guidewire already placed across the lesion. (C) Completion DSA depicts successful recanalization with the use of a balloon-expandable stent. (Images courtesy of Bryan T. Petersen, M.D., Dotter Interventional Institute, Health and Sciences University, Oregon.)
Some operators have described cervical CCA access either percutaneously or via surgical cutdown. The CCA lesion is crossed with the guidewire in a retrograde fashion. Protection against cerebral embolism can be established with proximal CCA control (Fig. 3). These are the indications for this approach4,9,10:
Figure 3.
Cervical access for the interventional management of a right common carotid artery lesion: A surgical cutdown has been used to expose the artery and allow for cerebral embolic protection with the use of a sling.
Extremely tortuous arch anatomy
Aortoiliac-femoral occlusions precluding femoral access
Failed femoral approach because of “flush” ostial CCA lesion
Tandem CCA and bifurcation lesion treated with endarterectomy
Presence of fresh thrombus at the CCA
Most operators do not use EPDs during CCA interventions, although it would be logical to believe that brain embolization may occur during these procedures despite the low periprocedural stroke rates. There are no available EPDs for use in the CCA, so smaller diameter devices designed for the ICA can be used if necessary. An additional drawback is that the 0.014-inch guidewire carrying the EPD may not be strong enough to support a CCA intervention, especially in the case of tortuous anatomy. Antiplatelet medications are routinely administered before the intervention, and usually they are prescribed for life after it. Atropine is given in case of bradycardia development during dilation, although this should not happen because baroreceptors are mainly located in the bulb.
The intervention has a high success rate (95%) and low complication rates even without protection devices. In a relatively large series of 37 patients with 42 CCA lesions, two patients (4.7%) experienced minor stroke that resolved completely within 30 days; no patient had major stroke. Neurological event-free survival at 2 years was 96 ± 4%.8
Fibromuscular Dysplasia
The ICA is the second most common site for fibromuscular dysplasia (FMD), with the renal arteries the first. The most common pathological type is medial fibroplasia, which angiographically has the typical “string of beads” appearance.2,11 FMD is a rare cause of cerebrovascular symptoms. More commonly it is an incidental finding or occurs together with atheromatous bifurcation disease. When the patient is symptomatic, treatment should be considered. Surgery consists of resection of the diseased segment or intraoperative dilation with balloons or rigid dilators. The lesions respond very well to percutaneous balloon angioplasty.12 Endovascular treatment by PTA is the first-line option for FMD of the ICA.
Arteritis
The most common vasculitis affecting the carotid arteries is Takayasu's arteritis. This giant-cell arteritis can affect the CCA, the subclavian arteries, the aorta, the splanchnic and renal arteries, and the pulmonary arteries. The typical patient is a young woman; characteristic lesions are long smooth stenoses of the CCA and subclavian arteries. Successful interventional treatment with percutaneous angioplasty (PTA) has been reported during the inactive phase of the disease; however the lesions are frequently less compliant to dilation and high pressures are required.13,14 Recently the use of cutting balloon for difficult CCA arteritis has been reported.
Carotid Dissection
Carotid artery dissection (CAD) may occur as a consequence of a congenital problem (Marfan's syndrome, Ehlers-Danlos syndrome, FMD), after trauma, or in apparently healthy vessels. CAD, although a rare case of stroke, is a common cause for stroke in young patients. The symptoms may be misleading or delayed. Symptoms include ipsilateral headache, loss of superficial temporal artery pulse, Horner's syndrome (ptosis, miosis, unilateral anhydrosis), TIAs, or stroke.2 Strokes secondary to CAD may have a benign course. Most dissections eventually resolve; about two thirds of the occlusions recanalize and one third of the resulting aneurysms decrease in size within 2 to 3 months or longer. However, it may lead to hemorrhagic or ischemic stroke with resulting significant disability.15
Diagnosis can be established with color Doppler ultrasonography, CTA, MRA, and DSA. The mechanism of stroke development in CAD is embolization of thrombi that develop on the intimal flap. Another mechanism is brain hypoperfusion caused by critical stenosis of the true lumen. The standard treatment is anticoagulant therapy with a target international normalized ratio of 2.0 to 3.0 for 3 to 6 months after CAD. Surgical treatment consists of ligation of the carotid artery with bypass and is reserved for patients unresponsive to anticoagulation.16 Stent placement in the true lumen with restoration of antegrade flow has been reported in selected patients: Selective microcatheterization of the true lumen is required. The arterial segment to be stented is long and requires multiple stents in a telescoping fashion to avoid dissection between stents if a part of the artery is not stented.15
Trauma
Trauma to the carotid arteries is most often due to penetrating or blunt injury, neck hyperextension, and blast injuries. Injury may present as spasm, intimal tear, dissection, thrombosis, transection, pseudoaneurysm, arteriovenous fistulas, or active extravasation.
Vascular injuries are more common with penetrating neck injuries than with blunt injuries: 25% of the patients with penetrating neck injury have a traumatic vascular lesion, with an overall mortality of 5%. The incidence of carotid trauma in blunt neck injury is 0.33%.17
The neck is divided into three vascular zones: below cricoid cartilage to clavicles, cricoid cartilage to mandibular angle, and above mandibular angle to the skull base.
Zone 2 injuries are more common (60 to 70%), but they are accessible to surgery. Zone 1 and 3 require extensive surgical procedure. The mortality rate of zone 1 injuries is 12%. It is not unusual for trauma to involve more than one artery (vertebral, branches of the external carotid artery) and the veins. Symptoms develop immediately or may be delayed by 24 hours to many months after the injury. The causes of delayed symptoms are probably emboli originating from the dissection.
Imaging can be performed, in the stable patient, with ultrasound or CTA, but DSA remains the standard imaging modality for vascular injuries to the neck. Conservative treatment with anticoagulation is advocated for most blunt carotid injuries.18 Many (62%) carotid dissections reverted to normal on follow-up. However, more severe forms of injury warrant surgical repair if it is technically feasible. Carotid ligation with or without extracranial-intracranial bypass may be performed if direct reconstruction is not possible.19
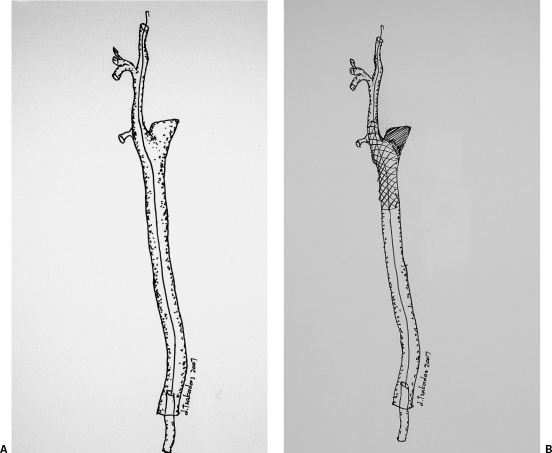
Interventional treatment is increasingly seen as first-line treatment, however. Carotid pseudoaneurysms (PAs) can be successfully treated with bare stent placement20 or in combination with coil embolization; the bare stent securely keeps the coils within the pseudoaneurysm21 (Fig. 4A). More recently stent grafts have been used successfully to exclude CCA PAs and carotid-jugular fistulae.17,19,22 Self-expanding stent grafts have been used in the majority of the patients (Fig. 4B).
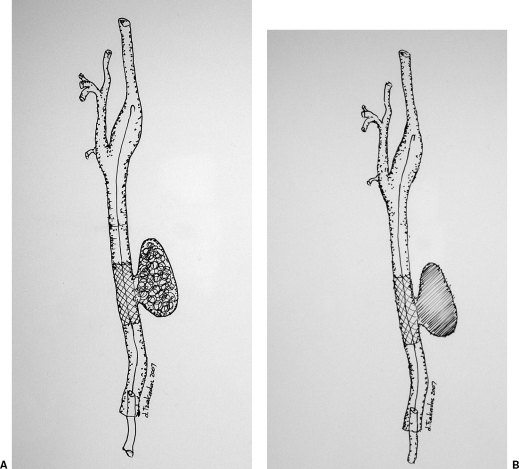
Figure 4.
Interventional techniques for the treatment of a common carotid pseudoaneurysm. (A) A bare stent is deployed across the pseudoaneurysm neck to “trap” the coils within its sac. (B) A stent graft is deployed across the pseudoaneurysm neck to exclude it form the circulation.
Carotid Blowout Syndrome
Bleeding from the carotid artery or its branches is a well-recognized complication following treatment or recurrence of head and neck malignancy (Fig. 5A–C). The majority of these patients are treated conservatively with end-of-life support measures. Interventional treatment options include embolization of tumoral neovascularity derived from ECA branches, occlusion of a major branch (CCA), or stent grafting of a bleeding PA.23 Before proceeding with total CCA occlusion, the interventional radiologist should test the tolerance of CCA occlusion with angiographic and clinical confirmation of the cerebral collateral supply through the circle of Willis.
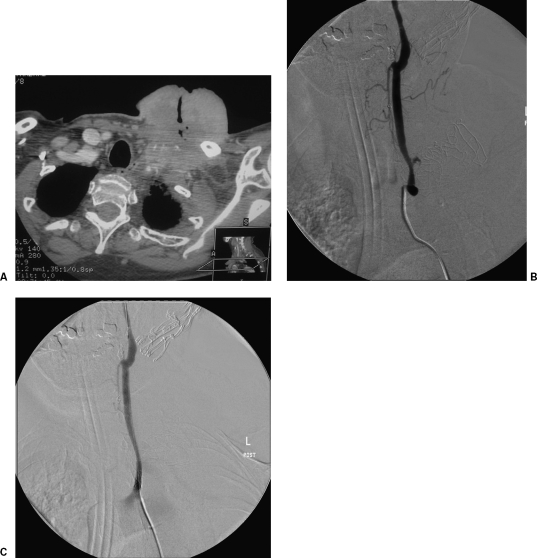
Figure 5.
A computed tomography (CT) scan of the superior thorax and neck in a patient with advanced breast sarcoma. The tumor is invasive and surrounding the great vessels on the left (A). This caused low-grade bleeding and required external artery embolization. The patient was later admitted with a large hemorrhage that at angiography was coming from the left common carotid artery (B). After discussion with the patient and her family, the bleeding was stopped by inserting a stent graft (C).
Carotid Stump Syndrome
Carotid stump syndrome is a rare cause of cerebrovascular events. In patients with occluded ICA, the carotid stump is a potential source of emboli that pass through the ipsilateral ECA into the middle cerebral artery circulation by way of reversed flow in the ophthalmic artery.24 The standard treatment is surgical exploration with exclusion of the ICA stump and endarterectomy of the ECA. Endovascular treatment with exclusion of the stump by bridging the CCA into the ECA with a stent graft has been described in case reports24 (Fig. 6A,B).
Figure 6.
Interventional treatment of carotid stump syndrome. (A) A guidewire is inserted into the external carotid artery. (B) A stent graft is deployed across the stump of the occluded internal carotid to exclude it from the circulation.
CAROTID INTERVENTIONS ABOVE THE BULB
Intracranial Internal Carotid Artery Occlusive Lesions
The annual risk of stroke from all causes in patients with intracranial atherosclerosis ranges from 3.6 to 13%. For symptomatic lesions the risk for stroke is higher (15 to 18%).25,26,27 Cavernous ICA lesions are more frequent, followed by petrous and supraclinoid lesions. Treatment of these patients is difficult. Medical therapy for symptomatic intracranial arterial stenoses is not satisfactory; neither warfarin nor aspirin offer acceptable protection from stroke25,26. Surgery, however, is not much better. A randomized study comparing extracranial-intracranial bypass to medical therapy showed failure of surgery in reducing the risk of stroke.27
By the late 1990s, the development of improved microballoon catheters and smaller balloon expandable stents, initially for coronary applications, led to an increasing number of reports on the revascularization of intracranial atherosclerotic lesions. Currently intracranial revascularization is indicated for symptomatic stenosis > 50% failing best medical treatment.28 Technical success rates exceed 90% and complication rates ranging from 0 to 20% have been reported.29,30,31
At least 4 days before the procedure, the patient is premedicated with aspirin, 325 mg/day, and clopidogrel, 75 mg/day. The procedure is performed under general anesthesia and systemic heparinization (5000 IU bolus and 1000 IU/h continuous infusion) to maintain an activated clotting time of 200 to 300 s throughout the procedure. Adjunctive drugs such as IIb/IIIa inhibitors are used only in patients at very high risk for thromboembolic complications.
With the use of a coaxial catheter system, a flexible micro guidewire with a microcatheter is advanced through the stenosis and the microcatheter is positioned distally. Then an exchange length 0.014-inch micro guidewire is placed through the microcatheter. A balloon expandable stent is then advanced over the stiff micro guidewire and positioned across the stenosis (Fig. 7A,B). The balloon inflation should be done very slowly to avoid vessel dissection. Accurate sizing of the stent is essential. A long-term antiplatelet regimen is recommended.
Figure 7.
Interventional treatment of an occlusive lesion of the cavernous internal carotid artery (ICA). (A) Selective left ICA digital subtraction angiography (DSA) in a symptomatic patient depicts high-grade stenosis of the cavernous part of the ICA. (B) Successful recanalization with a stent. The symptoms resolved.
Wojak et al treated 71 symptomatic intracranial lesions. Periprocedural events were 4.8%. At follow-up annual stroke rate in the treated territory was only 1.8%.31 Similarly the results of the Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA) trial were encouraging, with 95% technical success rate and no mortality. Thirty days and 1-year stroke rates were 6.6% and 7.3%, respectively.32 More recently good results were reported with the use of a new self-expanding stent specifically designed for the intracranial vasculature.33
Traumatic Carotid Cavernous Fistulae
Traumatic carotid cavernous fistulae (TCCFs) are secondary to rupture of the ICA wall toward the cavernous sinus caused by direct or indirect trauma. In some patients there will be no history of trauma or only minor trauma, and one may suspect a traumatic rupture of a preexisting aneurysm or angiodysplasia.34 TCCFs result in reversal of the venous drainage from the cavernous sinus. The symptomatology depends on the route of venous drainage. Reversed flow into the ophthalmic vein system produces exophthalmus, chemosis, dilated conjunctival veins, lid engorgement, venous retinopathy, increased intraocular pressure with secondary glaucoma, and visual loss. Drainage to the sphenoparietal sinus with reflux to the superficial or deep Sylvian venous system may produce venous hypertension with potential risk of intracranial hemorrhage. Drainage into the pterygoid venous plexus may result in epistaxis.34
The diagnosis of the TCCF is confirmed by computed tomography and/or magnetic resonance imaging. Evaluation of the extent of cranial injury can be made as well as the degree of proptosis and assessment of ophthalmic veins, lateral bulging of the cavernous sinus, and dilation of its tributaries. DSA determines the exact site of ICA wall laceration and evaluates the venous drainage of the fistula.
The endovascular approach to TCCFs is the treatment of choice. For traumatic laceration of the ICA siphon, the use of detachable balloons is the best available method of treatment. The balloon enters the cavernous sinus and with progressive inflation occludes the fistula while preserving carotid flow (Fig. 8). In the largest reported series, the fistulae were cured in 85 to 98% of cases, with 1.3 to 9% recurrence rate, which usually responded well to a second endovascular treatment.35 If the preservation of the ICA flow is technically impossible, sacrifice of the ICA at the exact point of the fistula after a tolerance test will prevent collateral filling or recanalization of the TCCF. The venous approach is very useful in the subacute and chronic stages when the acquired venous wall thickening allows for safer catheterization. The approach to the sinus is feasible through the inferior petrosal sinus or even through retrograde ophthalmic vein catheterization.
Figure 8.
Detachable balloon occlusion technique for the treatment of a traumatic carotid cavernous fistula (TCCF) in a patient with diplopia and headaches following head injury. (A) Lateral projection of left internal carotid artery (ICA) digital subtraction angiography (DSA) depicts the TCCF. The laceration is located at the C5 segment. (B) Nonsubtracted images depict two detachable balloons deployed into the left cavernous sinus. (C) Completion DSA depicts complete sealing of the TCCF with good ICA flow.
Extradural Carotid Artery Aneurysms
The most commonly affected area is the cavernous segment of the ICA followed by the petrous and the cervical segment. Aneurysms of the CCA are most frequently traumatic. Aneurysms involving the cervical portion of the ICA mostly are congenital or developmental, followed by traumatic, atherosclerotic, and mycotic pseudoaneurysms. At the level of the petrous segment of the ICA, the cause is most often traumatic, secondary to blunt trauma or iatrogenic following biopsy, and rarely dysplastic in origin. Finally, at the level of the cavernous ICA, they are frequently congenital in origin. Large aneurysms that are partially thrombosed are usually atherosclerotic or developmental in origin.36
Giant Cavernous ICA Aneurysms
Two major types are encountered: saccular and fusiform. All are thought to result from aberrant vascular remodeling and secondary healing responses to vessel wall injury by chronic hemodynamic stress. Abnormal vessel healing from a chronic atherosclerotic dissection or other entities such as Ehlers-Danlos syndrome, Marfan's syndrome, polycystic kidney disease, and osteogenesis imperfecta are associated with fusiform giant aneurysms and partially thrombosed aneurysms of the cavernous ICA.36
The most common presentation is mass effect on adjacent structures in 39 to 75% of patients. Subarachnoid hemorrhage (SAH) may occur in 25 to 70% of patients.37 The 5-year cumulative rupture rates for patients with no history of SAH with giant aneurysms are 40 to 50%. When a giant aneurysm presents with SAH, the cumulative frequency of rebleeding at 14 days is 18.4%.38 The objectives of treatment are protection from bleeding, reduction of mass effect, and prevention of thromboembolic complications.
The currently available techniques for interventional management of giant aneurysms are deconstructive with sacrifice of the parent artery and reconstructive with isolation of the aneurysmal lumen from the intracranial circulation.
DECONSTRUCTIVE TECHNIQUES
Deconstructive methods include parent artery occlusion and aneurysmal trapping. The endovascular approach is advantageous compared with surgical parent artery occlusion, not only because it allows better occlusion to the aneurysm, reducing the chances of leaving significant collateral flow and persistent filling and growth, but it also permits simultaneous assessment of the adequacy of the collateral circulation.39 A balloon occlusion tolerance test is required before the parent artery occlusion. Even after successful balloon occlusion test, delayed ischemic complications may occur in 4 to 15% of cases.40 When the patient cannot tolerate the balloon test occlusion, surgical bypass with the superficial temporal or occipital artery may be considered.41
RECONSTRUCTIVE TECHNIQUES
Reconstructive techniques include embolization with coils or liquid embolic materials with or without adjunctive techniques such as balloon protection or stent placement. Since the introduction of the Guglielmi detachable coil (GDC), this type of embolization has replaced balloon embolization, particularly when parent artery preservation is attempted.42 However, the rate of complete occlusion and the long-term success for large and giant aneurysms are very limited. Complete embolization of giant aneurysms using GDC coils is achieved only in 24.1%, with a 69% rate of residual necks.43 Also, recanalization due to coil compaction can be as high as 90% in giant aneurysms treated by coil embolization. Thus giant aneurysms may require either multiple embolization sessions44 or additional surgical procedures.
One of the most useful evolutions to overcome these difficulties was the introduction of the remodeling technique by Moret et al in 1997.45 A protection balloon is placed across the aneurysmal neck while a microcatheter is positioned inside the aneurysm. The coils are placed into the aneurysm while the balloon is inflated intermittently, to prevent their migration into the parent artery and to facilitate higher packing density. Also major improvements in the coil technology such as 3D coils and bioactive and hydrogel coils have widened the range of aneurysms that are regarded as suitable for endovascular therapy.
The development of intravascular stents may overcome important limitations in the treatment of giant or wide neck aneurysms.46 The stent is placed across the aneurysmal neck, and then a microcatheter is advanced through the struts and positioned inside the aneurysm. The stent works as a scaffold allowing higher packing density (Fig. 9). One of the most significant limitations of this technique is the difficulty in deployment of the stent in the parent artery, particularly in large fusiform aneurysms, as well as the necessity of antiplatelet therapy before and after the procedure, which may become a limitation in cases of ruptured aneurysms. Thromboembolic complications related to stent placement may also be a problem.46
Figure 9.
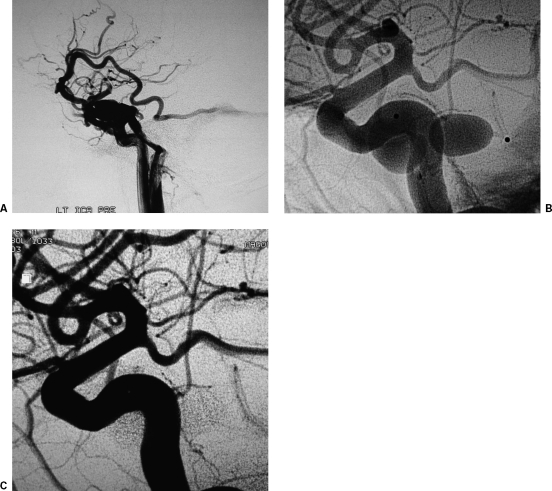
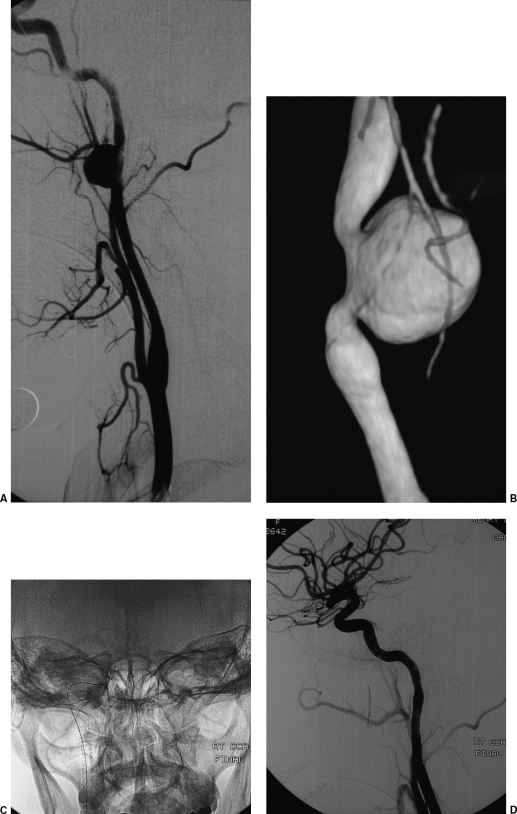
Treatment of an internal carotid artery (ICA) aneurysm using stent and coils. (A) Lateral projection of the left ICA digital subtraction angiography (DSA) depicts wide neck aneurysm arising from the C5 segment of the ICA. (B) Lateral digital spot view depicts a self-expandable stent placed across the aneurysm neck, allowing for secure aneurysmal sac coil occlusion through the stent struts. (C) Lateral projection of the completion DSA depicts occlusion of the aneurysm.
As an aneurysm enlarges, the neck frequently occupies an increasing proportion of the circumference of the parent vessel wall. This makes closure of the neck and healing reconstruction of the vessel wall more problematic. Considering the limitations of balloons and even coils to succeed complete occlusion of these aneurysms, liquid embolic materials (Onyx; Micro Therapeutics, Irvine, CA) seem to be an alternative treatment in very dysplastic vessels at the level of the wide neck.47 The adjunctive use of a stent may improve the morphologic results. Also, covered stents placed across the aneurysm neck may be considered in the treatment of giant fusiform aneurysms, although further studies and technical developments are needed to accurately define the best treatment strategy.
Traumatic ICA Pseudoaneurysms
Traumatic PA of the ICA is a rare but serious complication following blunt or penetrating trauma.48 Patients with PAs are at risk of distal thromboembolism, continued enlargement with vessel occlusion, and rebleeding with intracranial or extracranial hemorrhage.49 Aggressive management of these lesions is generally advocated to prevent life-threatening hemorrhage and thromboembolic phenomena.50 Historically, treatment of ICA PAs after penetrating injuries has been primarily surgical.51 The currently preferred surgical method is excision of the damaged arterial segment with reconstruction of the ICA. For inaccessible Pas, anticoagulation and proximal ligation with or without ECA-ICA bypass have been proposed.52 Except for bypass procedures, several cases reports of using bare stents and secondary coiling of ICA pseudoaneurysms have been published in recent years with promising results.53 Indications for stent placement include enlarging pseudoaneurysms and dissections that progress despite full anticoagulation. The use of stent grafts seems very appealing because it secures immediately the incompetent arterial wall segment while preserving the parent artery19 (Fig. 10A–D). The stent graft has advantages, especially in cases of pseudoaneurysms that lack surrounding support. There are some reports on the preliminary use of stent grafts in the intracranial carotid arteries using coronary stent grafts.53,54,55 However, the currently available stent grafts are not optimized for neuroendovascular use because their limited flexibility is a drawback while their usable diameter is too small for wide ICAs. At present, the use of stent grafts is an individual decision; stent grafts that are designed and developed for intracranial vessels are necessary before their use can be recommended on a broader basis.
Figure 10.
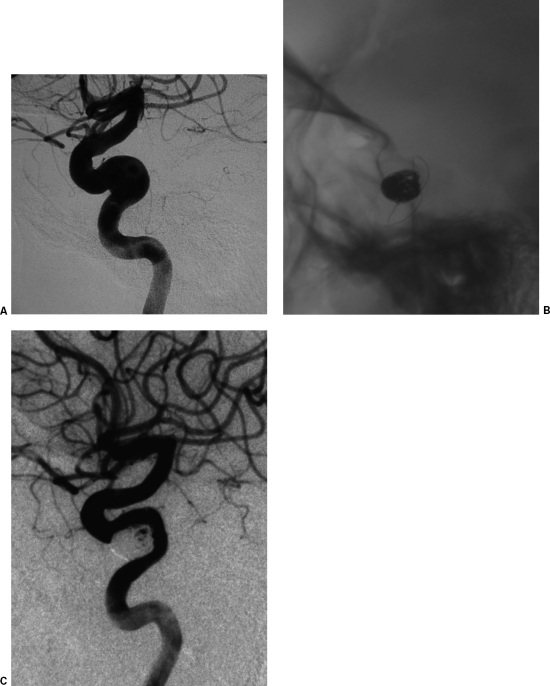
Stent-graft treatment of a traumatic internal carotid artery (ICA) pseudoaneurysm (PA) following biopsy of the right parapharyngeal space. (A,B) Lateral projection and 3D reconstruction of a right common carotid artery (CCA) digital subtraction angiography (DSA) depicts a wide-neck high cervical segment ICA PA. (C) Anteroposterior digital radiograph depicts the stent graft. (D) Lateral projection of the completion DSA depicts exclusion of the PA.
ACKNOWLEDGMENTS
The authors thank Dimitrios A. Trakadas, M.Sc., for his artwork.
REFERENCES
- Riles T S, Imparato A M, Posner M P, Eikelboom B C. Common carotid occlusion: assessment of distal vessels. Ann Surg. 1984;199:363–366. doi: 10.1097/00000658-198403000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J A, Nesbit G M. In: Kaufman JA, Lee MJ, editor. The Requisites: Vascular and Interventional Radiology. Philadelphia: Mosby; 2004. Carotid and vertebral arteries. pp. 119–141.
- Cherry K J., Jr In: Rutherford RB, editor. Vascular Surgery. 5th ed. Vol. 1. Philadelphia: WB Saunders Company; 2000. Atherosclerotic occlusive disease of brachiocephalic arteries. pp. 1140–1162.
- Criado F J, Abul-Khoudoud O. Interventional techniques to facilitate supraaortic angioplasty and stenting. Vasc Endovascular Surg. 2006;40:141–147. doi: 10.1177/153857440604000209. [DOI] [PubMed] [Google Scholar]
- Law M M, Colburn M D, Moore W S, et al. Carotid-subclavian bypass for brachiocephalic occlusive disease: choice of conduit and long-term follow-up. Stroke. 1995;26:1565–1571. doi: 10.1161/01.str.26.9.1565. [DOI] [PubMed] [Google Scholar]
- Roffi M, Yadav J S. Carotid stenting. Circulation. 2006;114:e1–e4. doi: 10.1161/CIRCULATIONAHA.106.624379. [DOI] [PubMed] [Google Scholar]
- CMS decision memo for carotid artery stenting (CAG-00085R) Available at: http://www.cms.hhs.gov/med/viewdecisionmemo.asp?id=157. Accessed December 12, 2005. Available at: http://www.cms.hhs.gov/med/viewdecisionmemo.asp?id=157
- Chio F L, Jr, Liu M W, Khan M A, Iyer S S, Roubin G S. Effectiveness of elective stenting of common carotid artery lesions in preventing stroke. Am J Cardiol. 2003;92:1135–1137. doi: 10.1016/j.amjcard.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Diethrich E B, Marx P, Wrasper R, Reid D B. Percutaneous techniques for endoluminal carotid interventions. J Endovasc Surg. 1996;3:182–202. doi: 10.1583/1074-6218(1996)003<0182:PTFECI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Allie D E, Hebert C J, Lirtzman M D, et al. Intraoperative innominate and common carotid intervention combined with carotid endarterectomy: a “true” endovascular surgical approach. J Endovasc Ther. 2004;11:258–262. doi: 10.1583/03-1119.1. [DOI] [PubMed] [Google Scholar]
- Schneider P A, Rutherford R B. In: Rutherford RB, editor. Vascular Surgery. 5th ed. Vol. 1. Philadelphia: WB Saunders Company; 2000. Extracranial fibromuscular arterial dysplasia. pp. 1837–1842.
- Hasso A N, Bird C R, Zinke D E, Thompson J R. Fibromuscular dysplasia of the internal carotid artery: percutaneous transluminal angioplasty. AJR Am J Roentgenol. 1981;136:955–960. doi: 10.2214/ajr.136.5.955. [DOI] [PubMed] [Google Scholar]
- Kumar S, Mandalam K R, Rao V R, et al. Percutaneous transluminal angioplasty in nonspecific aortoarteritis (Takayasu's disease): experience in 16 cases. Cardiovasc Intervent Radiol. 1989;12:321–325. doi: 10.1007/BF02575430. [DOI] [PubMed] [Google Scholar]
- Tyagi S, Verma P K, Gambhir D S, et al. Early and long-term results of subclavian angioplasty in aortoarteritis (Takayasu disease): comparison with atherosclerosis. Cardiovasc Intervent Radiol. 1998;21:219–224. doi: 10.1007/s002709900248. [DOI] [PubMed] [Google Scholar]
- Cohen J E, Leker R R, Cotkine M, et al. Emergent stenting to treat patients with carotid artery dissection: clinically and radiologically directed therapeutic decision making. Stroke. 2003;34:e254–e257. doi: 10.1161/01.STR.0000101915.11128.3D. [DOI] [PubMed] [Google Scholar]
- Schievink W I. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344:898–906. doi: 10.1056/NEJM200103223441206. [DOI] [PubMed] [Google Scholar]
- Fusonie G E, Edwards J D, Reed A B. Covered stent exclusion of blunt traumatic carotid artery pseudoaneurysm: case report and review of the literature. Ann Vasc Surg. 2004;18:376–379. doi: 10.1007/s10016-004-0037-2. [DOI] [PubMed] [Google Scholar]
- Fabian T C, Patton J H, Croce M A, et al. Blunt carotid injury: importance of early diagnosis and anticoagulant therapy. Ann Surg. 1996;223:513–525. doi: 10.1097/00000658-199605000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J V, Rossbach M M, Cleveland T J, Gaines P A, Beard J D. Endovascular stent-graft repair of traumatic carotid artery pseudoaneurysm. Clin Radiol. 2002;57:308–311. doi: 10.1053/crad.2001.0808. [DOI] [PubMed] [Google Scholar]
- Coldwell D M, Novak Z, Ryu R K, et al. Treatment of posttraumatic internal carotid artery pseudoaneurysms with endovascular stents. J Trauma. 2000;48:470–472. doi: 10.1097/00005373-200003000-00016. [DOI] [PubMed] [Google Scholar]
- Diaz-Daza O, Arraiza F J, Barkley J M, Whigham C J. Endovascular therapy of traumatic vascular lesions of the head and neck. Cardiovasc Intervent Radiol. 2003;26:213–221. doi: 10.1007/s00270-002-2619-0. [DOI] [PubMed] [Google Scholar]
- Ramsay D W, McAuliffe W. Traumatic pseudoaneurysm and high flow arteriovenous fistula involving internal jugular vein and common carotid artery: treatment with covered stent and embolization. Australas Radiol. 2003;47:177–180. doi: 10.1046/j.0004-8461.2003.01147.x. [DOI] [PubMed] [Google Scholar]
- Chaloupka J C, Putman C M, Citardi M J, et al. Endovascular therapy for carotid blowout syndrome in head and neck surgical patients: diagnostic and managerial considerations. AJNR Am J Neuroradiol. 1996;17:843–852. [PMC free article] [PubMed] [Google Scholar]
- Nano G, Dalainas I, Casana R, et al. Endovascular treatment of the carotid stump syndrome. Cardiovasc Intervent Radiol. 2006;29:140–142. doi: 10.1007/s00270-005-0098-9. [DOI] [PubMed] [Google Scholar]
- Chimowitz M I, Kokkinos J, Strong J, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45:1488–1493. doi: 10.1212/wnl.45.8.1488. [DOI] [PubMed] [Google Scholar]
- Chimowitz M I, Lynn Ch B MJ, Howlett-Smith H, et al. Comparison of Warfarin and Aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke: results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med. 1985;313:1191–1200. doi: 10.1056/NEJM198511073131904. [DOI] [PubMed] [Google Scholar]
- Higashida R T, Meyers P M, Connors J J, III, et al. Intracranial angioplasty and stenting for cerebral atherosclerosis: a position statement of the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, and the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2005;26:2323–2327. [PMC free article] [PubMed] [Google Scholar]
- Marks M P, Marcellus M, Norbash A M, Steinberg G K, Tong D, Albers G W. Outcome of angioplasty for atherosclerotic intracranial stenosis. Stroke. 1999;30:1065–1069. doi: 10.1161/01.str.30.5.1065. [DOI] [PubMed] [Google Scholar]
- Marks M P, Marcellus M L, Do H M, et al. Intracranial angioplasty without stenting for symptomatic atherosclerotic stenosis: long-term follow up. AJNR Am J Neuroradiol. 2005;26:525–530. [PMC free article] [PubMed] [Google Scholar]
- Wojak J C, Dunlap D C, Hargrave K R, DeAlvare L A, Culbertson H S, Connors J J., III Intracranial angioplasty and stenting: long term results from a single center. AJNR Am J Neuroradiol. 2006;27:1882–1892. [PMC free article] [PubMed] [Google Scholar]
- SSYLVIA Study Investigators Stenting of symptomatic atherosclerotic stenosis in the vertebral and intracranial arteries (SSYLVIA): study results. Stroke. 2004;35:1388–1392. doi: 10.1161/01.STR.0000128708.86762.d6. [DOI] [PubMed] [Google Scholar]
- Henkes H, Miloslavski E, Lowens S, Reinartz J, Leibig T, Kuhne D. Treatment of intracranial atherosclerotic stenoses with balloon dilatation and shelf-expanding stent deployment (WingSpan) Neuroradiology. 2005;47:222–228. doi: 10.1007/s00234-005-1351-2. [DOI] [PubMed] [Google Scholar]
- In: Berenstein A, Lasjaunias P, Ter Brugge KG, editor. Surgical Neuroangiography, 2.1. New York: Springer: 2004. Traumatic carotid cavernous fistulae. pp. 279–333.
- Wu Z, Wang C, Yang X, et al. Endovascular embolization of traumatic carotid cavernous fistulas. Chin Med J (Engl) 1999;112:433–437. [PubMed] [Google Scholar]
- Mizutani T, Miki Y, Kojima H, et al. Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery. 1999;45:253–259. doi: 10.1097/00006123-199908000-00010. [DOI] [PubMed] [Google Scholar]
- Symon L. Management of giant intracranial aneurysms. Acta Neurochir (Wien) 1992;116:107–118. doi: 10.1007/BF01540863. [DOI] [PubMed] [Google Scholar]
- Wiebers D O, Whisnant J P, Huston J, III, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- Leibowitz R, Do H M, Marcellus M L, Chang S D, Steinberg G K, Marks M P. Parent vessel occlusion for vertebrobasilar fusiform and dissecting aneurysms. AJNR Am J Neuroradiol. 2003;24:902–907. [PMC free article] [PubMed] [Google Scholar]
- Chaloupka J C, Awad I A. In: Awad IA, Barrow DL, editor. Giant Intracranial Aneurysms. Park Ridge, IL: American Association of Neurological Surgeons; 1995. Therapeutic strategies and armamentarium of treatment options. pp. 91–116.
- Hoh B L, Putman C M, Budzik R F, Carter B S, Ogilvy C S. Combined surgical and endovascular techniques of flow alteration to treat fusiform and complex wide necked intracranial aneurysms that are unsuitable for clipping or coil embolization. J Neurosurg. 2001;95:24–35. doi: 10.3171/jns.2001.95.1.0024. [DOI] [PubMed] [Google Scholar]
- Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach: Part 2. Preliminary clinical experience. J Neurosurg. 1991;75:8–14. doi: 10.3171/jns.1991.75.1.0008. [DOI] [PubMed] [Google Scholar]
- Gonzalez N, Murayama Y, Nien Y L, et al. Treatment of unruptured aneurysms with GDCs: clinical experience with 247 aneurysms. AJNR Am J Neuroradiol. 2004;25:577–583. [PMC free article] [PubMed] [Google Scholar]
- Kuether T A, Nesbit G M, Barnwell S L. Clinical and angiographic outcomes, with treatment data, for patients with cerebral aneurysms treated with GDCs: a single center experience. Neurosurgery. 1998;43:1016–1025. doi: 10.1097/00006123-199811000-00007. [DOI] [PubMed] [Google Scholar]
- Moret J, Cognard C, Weil A, Castaigns L, Rey A. Reconstruction technique in the treatment of wide neck intracranial aneurysms: long term angiographic and clinical results—a propos of 56 cases. J Neuroradiol. 1997;24:30–44. [PubMed] [Google Scholar]
- Benitez R P, Silva M T, Klem J, Veznedaroglou E, Rosenwasser R H. Endovascular occlusion of wide necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery. 2004;54:1359–1368. doi: 10.1227/01.neu.0000124484.87635.cd. [DOI] [PubMed] [Google Scholar]
- Molyneux A J, Cerkirge S, Saatci I, Gal G. Cerebral aneurysm multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol. 2004;25:39–51. [PMC free article] [PubMed] [Google Scholar]
- Cogbill T H, Moore E E, Meissner M, et al. The spectrum of blunt injury to the carotid artery: a multicenter experience. J Trauma. 1994;37:473–479. doi: 10.1097/00005373-199409000-00024. [DOI] [PubMed] [Google Scholar]
- Mokri B. Traumatic and spontaneous extracranial internal carotid artery dissections. J Neurol. 1990;237:356–361. doi: 10.1007/BF00315659. [DOI] [PubMed] [Google Scholar]
- Redekop G, Marotta T, Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg. 2001;95:412–419. doi: 10.3171/jns.2001.95.3.0412. [DOI] [PubMed] [Google Scholar]
- Ramadan F, Rutledge R, Oller D, et al. Carotid artery trauma: a review of contemporary trauma center experiences. J Vasc Surg. 1995;21:46–55. doi: 10.1016/s0741-5214(95)70243-1. [DOI] [PubMed] [Google Scholar]
- D'Alise M D, Vardiman A B, Kopitnik T A, Jr, et al. External carotid to middle cerebral by pass in the treatment of complex internal carotid injury. J Trauma. 1996;40:452–455. doi: 10.1097/00005373-199603000-00023. [DOI] [PubMed] [Google Scholar]
- Bush R L, Lin P H, Dodson T F, et al. Endoluminal stent placement and coil embolization for the management of carotid artery pseudoaneurysms. J Endovasc Ther. 2001;8:53–61. doi: 10.1177/152660280100800109. [DOI] [PubMed] [Google Scholar]
- Magoufis G L, Vrachliotis T G, Stringaris K A. Covered stents to treat partial recanalization of Onyx-occluded giant intracavernous carotid aneurysm. J Endovasc Ther. 2004;11:742–746. doi: 10.1583/03-1195R.1. [DOI] [PubMed] [Google Scholar]
- Saatci I, Cekirge H S, Ozturk M H, et al. Treatment of internal carotid aneurysms with a covered stent: experience in 24 patients with mid-term follow up results. AJNR Am J Neuroradiol. 2004;25:1742–1749. [PMC free article] [PubMed] [Google Scholar]