ABSTRACT
Development of the aorta takes place during the third week of gestation. It is a complex process that can lead to a variety of congenital variants and pathological anomalies. In diagnostic and interventional radiology, knowledge of aortic abnormalities and variant branching sequence is crucially important. This article gives a systematic overview of anatomical variability of the aorta.
Keywords: Thoracic aorta, embryology, anatomical variants
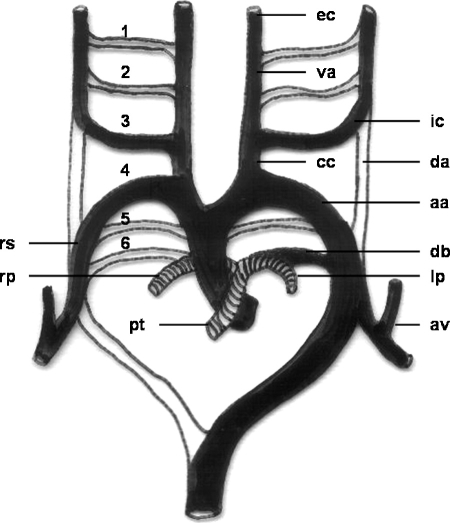
Development of the aorta takes place during the third week of gestation.1 It is a complex process associated with the formation of the endocardial tube (day 21), which lends itself to a variety of congenital variants. Each primitive aorta consists of a ventral and a dorsal segment that are continuous through the first aortic arch. The two ventral aortae fuse to form the aortic sac. The dorsal aortae fuse to form the midline descending aorta. Six paired aortic arches, the so-called branchial arch arteries, develop between the ventral and dorsal aortae. In addition, the dorsal aorta gives off several intersegmental arteries (Fig. 1).
Figure 1.
Schematic drawing of the development of the aortic arch and its branches. 1, first aortic arch; 2, second aortic arch; 3, third aortic arch; 4, fourth aortic arch; 5, fifth aortic arch; 6, sixth aortic arch; aa, aortic arch; va, ventral aorta; da, dorsal aorta; cc, common carotid artery; ic, internal carotid artery; ec, external carotid artery; rs, right subclavian artery; av, vertebral artery; pt, pulmonary trunk; rp, right pulmonary artery; lp, left pulmonary artery; db, ductus arteriosus Botalli.
The vessels derived from each arch are as follows: The first pair contributes to formation of the maxillary and external carotid arteries. The second pair contributes to formation of the stapedial arteries. The third aortic arch constitutes the commencement of the internal carotid artery and is therefore named the carotid arch. Proximal segments of the third pair form the common carotid arteries. Together with segments of the dorsal aortae, the distal portions contribute to formation of the internal carotid arteries. The left arch of the fourth pair forms the segment of normal left aortic arch between the left common carotid and subclavian arteries. The right fourth arch forms the proximal right subclavian artery. The distal right subclavian artery is derived from a portion of the right dorsal aorta and the right seventh intersegmental artery. Rudimentary vessels that regress early develop out of the fifth pair. The left arch of the sixth pair contributes to the formation of the main and left pulmonary arteries and ductus arteriosus; this duct obliterates a few days after birth. The right sixth arch contributes to formation of the right pulmonary artery.2 With the caudad migration of the heart in the second fetal month, the seventh intersegmental arteries enlarge and migrate cephalad to form the distal subclavian arteries. The left subclavian artery is derived entirely from the left seventh intersegmental artery, whereas the portions of the right are derived from the right fourth arch and the right dorsal aorta.2 Malformations of the aortic arch system can be explained by persistence of segments of the aortic arches that normally regress or disappearance of segments that normally remain, or both.
NORMAL ANATOMY
Regression of the right dorsal aortic root (between the right subclavian artery and the descending aorta) and the right ductus arteriosus leaves the normal left aortic arch. The classic left aortic arch and descending thoracic aorta are seen in ~70% of individuals. The three main branches of the aortic arch are the brachiocephalic (innominate) artery (dividing into the right subclavian and common carotid arteries), the left common carotid artery, and the left subclavian artery.2
VARIANT OR ABNORMAL ANATOMY OF THE AORTIC ARCH
Magnetic resonance imaging is widely accepted to be the gold standard for imaging the aortic arch with the ability to demonstrate not only the arterial branching pattern but also the relationship of aorta and its branches to the trachea and bronchi.3
Hypoplastic Ascending Aorta
Hypoplasia of the ascending aorta usually occurs concomitant with hypoplastic left heart syndrome (HLHS). HLHS comprises a wide spectrum of cardiac malformations, including hypoplasia or atresia of the aortic and mitral valves and hypoplasia of the left ventricle and ascending aorta. The great vessels are normally related in this congenital anomaly. HLHS has a reported prevalence of 0.2 per 1000 live births and occurs twice as often in boys as in girls. Left untreated, HLHS is invariably lethal. After surgical intervention infants are now surviving into childhood.4 Interruption of the aortic arch and HLHS in the same patient is exceptional.5
Coarctation of the Aorta
Coarctation of the aorta accounts for ~5 to 7% of all congenital heart disease. It is defined as a discrete stenosis in the proximal descending thoracic aorta (Fig. 2). The traditional classification into infantile (preductal) and adult (postductal) types is now regarded as too simplistic because many patients with preductal lesions do not present until adulthood (Fig. 3). It is only those with the most severe obstruction (e.g., aortic arch atresia or interruption) or associated cardiac defects who invariably present in infancy.6 Most other cases are identified because of a murmur or hypertension found on routine examination (Fig. 4). Delayed or absent femoral pulses and an arm/leg systolic blood pressure difference of 20 mm Hg or more in favor of the arms may be considered as evidence for aortic coarctation.7 Otherwise, age at presentation is related to the severity rather than the site of obstruction, as a result of cardiac failure or occasionally cerebrovascular accident, aortic dissection, or endocarditis.6 Aortic coarctation may be subclassified into isolated coarctation, coarctation with ventricular septal defect, and coarctation with complex intracardiac anomalies.8 An exceedingly rare congenital anomaly is coarctation of a right aortic arch.9
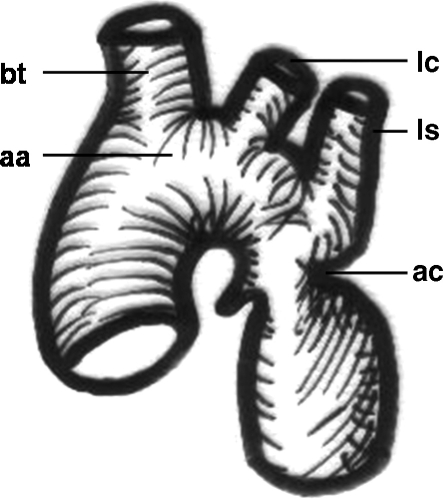
Figure 2.
Schematic drawing of an aortic coarctation. aa, aortic arch; bt, brachiocephalic trunk; lc, left carotid artery; ls, left subclavian artery; ac, coarctation of the aorta.
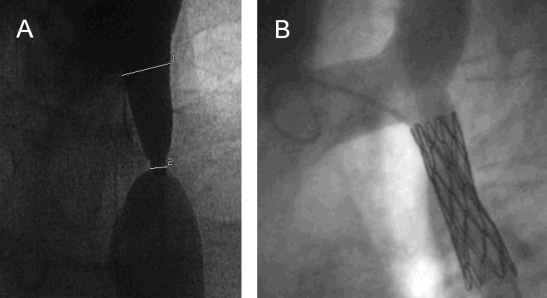
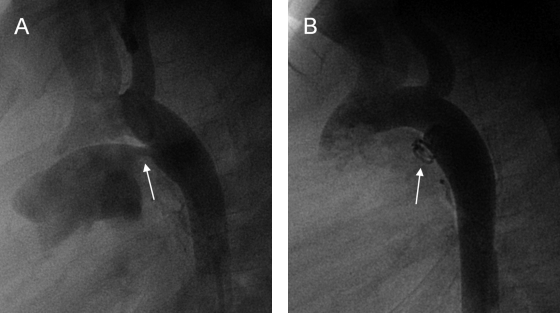
Figure 3.
Angiogram in a 35-year-old patient showing (A) postductal coarctation of the aorta with upstream segmental hypoplasia. (B) Stenting reduced the systolic blood pressure gradient from 50 mm Hg to below 20 mm Hg. (Images courtesy of A. Gamillscheg, Department of Pediatric Cardiology, Medical University of Graz, Graz, Austria.)
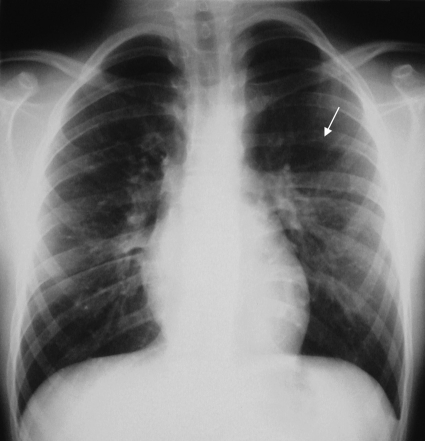
Figure 4.
Plain chest radiograph in a 12-year-old boy with proven coarctation of the aorta. Rib notching (arrow) due to pressure erosion indicates enlargement and tortuosity of intercostal arteries that provide collateral blood flow to circumvent the stenotic aortic segment.
Interrupted Aortic Arch
Interrupted aortic arch is defined as the loss of luminal continuity between the ascending and descending aorta. It is associated with a multitude of lesions ranging from isolated ventricular septal defects to complex ones. An interrupted aortic arch may be subclassified into anatomical types based on the location of the interruption.8 Although results have improved, repair of this abnormality is associated with a significant mortality and morbidity.10
Patent Ductus Arteriosus
In intrauterine life, the ductus arteriosus Botalli permits blood flow between the aorta (distal to the left subclavian artery) and the pulmonary artery (Fig. 5). In a full-term infant, the ductus usually closes within the first 2 days of life. Persistent patency beyond that point is generally permanent, being two to three times as common in girls as in boys (Figs. 6 and 7). Most of the cases occur as isolated defects. Typical concomitant findings are left ventricle hypertrophy and pulmonary artery dilation. Persistent ductus arteriosus may also be associated with coarctation of the aorta, transposition of the great vessels, and ventricular septal defect.11
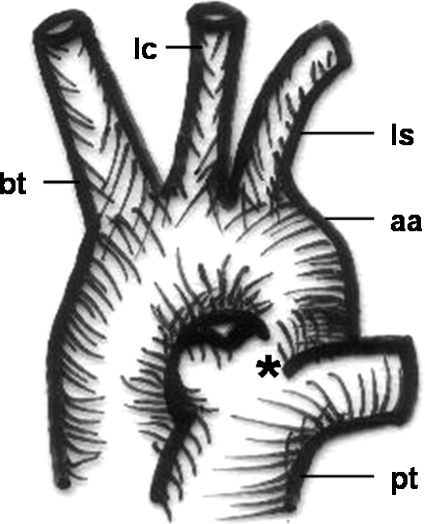
Figure 5.
Schematic drawing of a patent ductus arteriosus Botalli. bt, brachiocephalic trunk; lc, left carotid artery; ls, left subclavian artery; aa, aortic arch; pt, pulmonary trunk; *, ductus arteriosus.
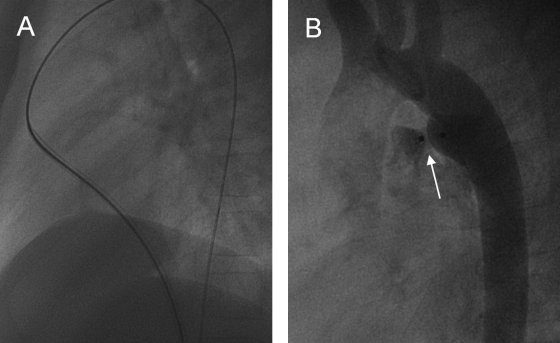
Figure 6.
(A) Aortogram (lateral view) depicting a patent ductus arteriosus (arrow). (B) Repeat angiogram showing endovascular coiling (arrow) was successful in this young adult patient. (Images courtesy of A. Gamillscheg, Department of Pediatric Cardiology, Medical University of Graz, Graz, Austria.)
Figure 7.
Occlusion of a patent ductus arteriosus in an adolescent with the use of Amplatzer (AGA, Golden Valley, MN) duct occluder. (A) During this intervention fluoroscopic images show the sheath (here with guidewire) taking the following course: inferior vena cava—right atrium—right ventricle—pulmonary trunk—patent ductus arteriosus—descending aorta. (B) Postintervention aortography shows minor residual shunt. (Images courtesy of A. Gamillscheg, Department of Pediatric Cardiology, Medical University of Graz, Graz, Austria.)
Common Brachiocephalic Trunk
A common brachiocephalic trunk, in which both common carotid arteries and the right subclavian artery arise from a single trunk off the arch, is the most frequent normal variant of aortic arch branching (Fig. 8).2 This so-called bovine trunk occurs in ~10 to 22% of individuals according to the literature and accounts for more than two thirds of all arch vessel anomalies.12,13 Very recently, a descriptive aortic arch naming scheme has been postulated because the human aortic branching pattern to which the term bovine aortic arch is ascribed is not commonly found in cattle.14 The remaining anomalies of the main branches account for < 3% of arch vessel anomalies.2
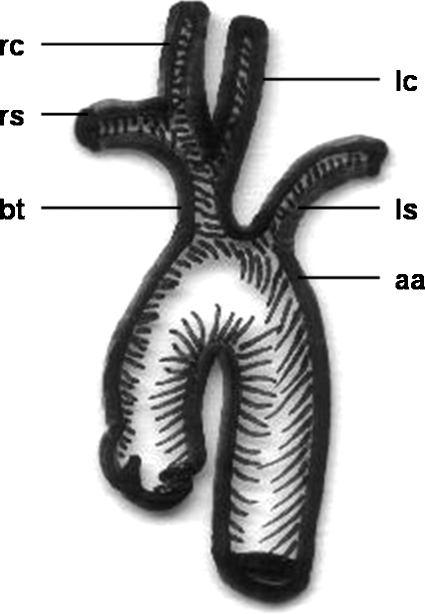
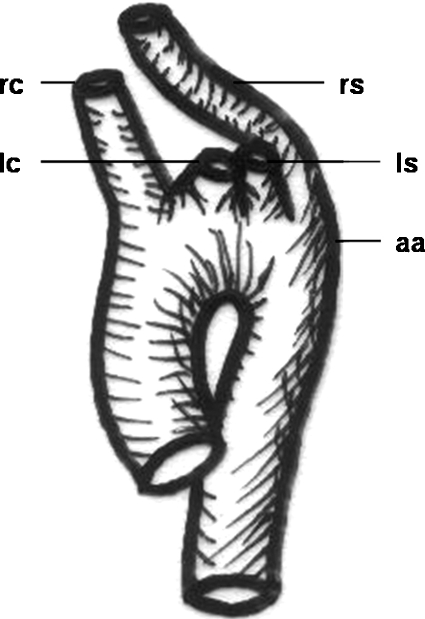
Figure 8.
Schematic drawing of a “bovine trunk.” rc, right carotid artery, rs, right subclavian artery; bt, “bovine trunk” (common trunk of right subclavian and both carotid arteries); lc, left carotid artery; ls, left subclavian artery; aa, aortic arch.
Variant Origin of Vertebral Arteries
Unusual vertebral artery origins are various.15,16 Not infrequently the left vertebral artery arises from the aortic arch, with reported prevalences of 2.4 to 5.8% (Fig. 9).17 The most frequent location is between the left common carotid and subclavian arteries.2 Rarely, the proximal left vertebral artery is duplicated. In this case, one part arises from the arch and the other from the left subclavian, or both originate from the aortic arch. Occasionally the left vertebral artery is the last branch of the aortic arch, which is rarely true for both vertebral arteries.18 So far, a single case of bilateral arch origin of the vertebral arteries has been reported in the literature.19
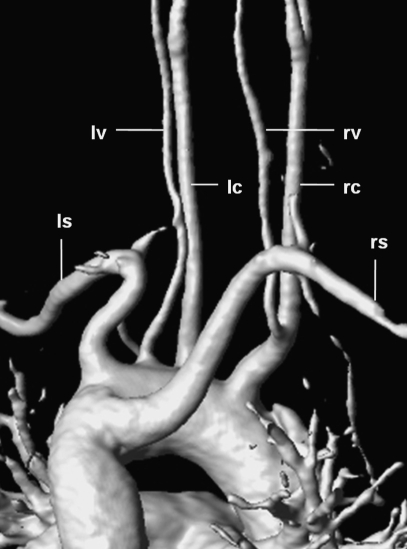
Figure 9.
3D-reconstructed magnetic resonance angiography in a 38-year-old woman showing a rare branching pattern of the aortic arch (view from posterior). The right common carotid artery (rc) gives rise to the right vertebral artery (rv); the left vertebral artery (lv) arises directly from the aortic arch between the left common carotid artery (lc) and the left subclavian artery (ls); the right subclavian artery (rs) originates as the last branch and takes a retroesophageal course (“arteria lusoria”).
Thyroid Ima Artery
The thyroidea ima artery is a collateral vessel feeding the thyroid gland20 According to literature, this vessel occurs in up to 16.9%.21 It may be a branch of the aortic arch between the brachiocephalic and left subclavian arteries. However, more frequently it is a branch of the brachiocephalic artery. A further variant of origin is from the right common carotid artery. In the remaining cases it may originate from the internal mammary, subclavian, or inferior thyroid arteries.2
Aberrant Right Subclavian or Brachiocephalic Artery
The right subclavian artery is the last branch of the aortic arch in ~l% of individuals (Figs. 9 and 10).22 It courses to the right behind the esophagus in ~80% of these cases, between the esophagus and trachea in 15%, and anterior to the trachea or mainstem bronchus in 5%.2 A retroesophageal course may be the cause of so-called dysphagia lusoria (Fig. 11).23,24 The aberrant right brachiocephalic artery is rare.
Figure 10.
Schematic drawing of a variant branching sequence of supra-aortal arteries with the right subclavian artery originating distal to the left one. rc, right carotid artery; lc, left carotid artery; ls, left subclavian artery; rs, right subclavian artery; aa, aortic arch.
Figure 11.
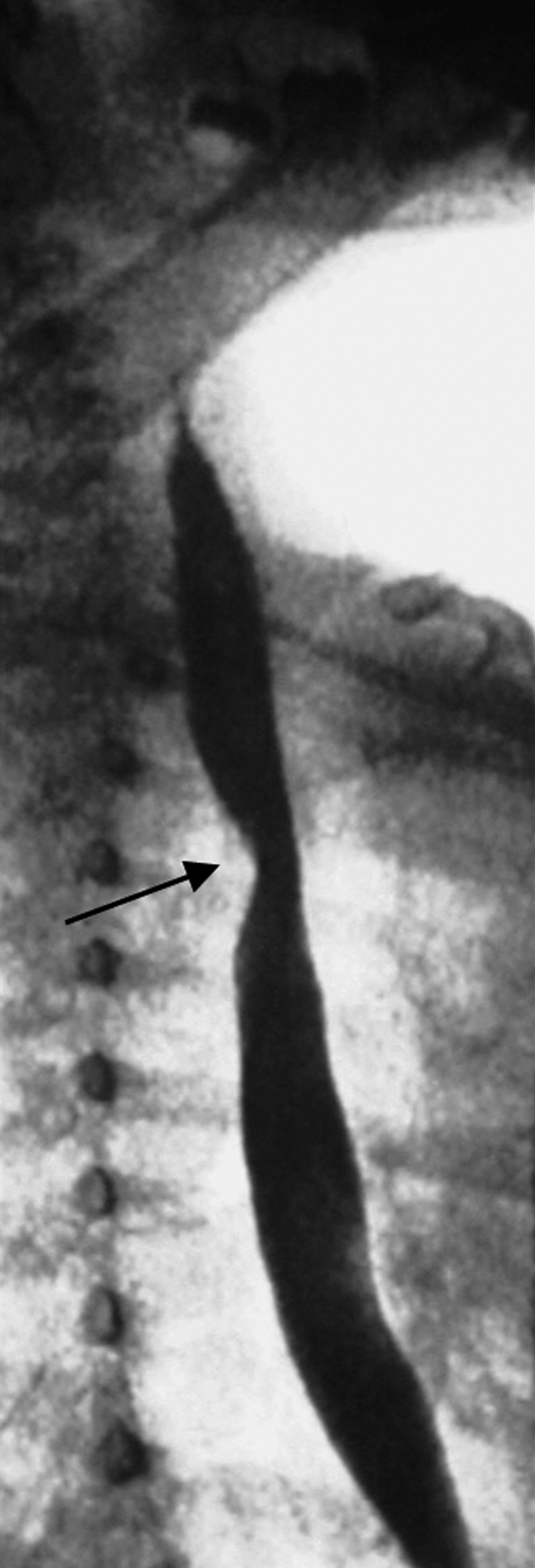
Image of a barium swallow test in a 6-year-old boy showing a suggestive posterior notch of the esophagus (arrow). “Arteria lusoria” (retroesophageal course of the right subclavian artery) has been confirmed by computed tomography (not shown).
Right Aortic Arch
Right aortic arch is an uncommon anatomical anomaly that occurs in < 0.1% of the population (Figs. 12 and 13).25 It results from the persistence of the right fourth branchial arch.2 The most common type is the right aortic arch with an aberrant left subclavian artery (Fig. 14). The vessels originate in the following order: left common carotid, right common carotid, right subclavian, and left subclavian artery. This type is rarely associated with congenital heart disease. However, symptoms may arise from vascular ring formation.26 The mirror-image type (left brachiocephalic trunk, right common carotid and subclavian arteries) is almost always associated with congenital heart disease, especially the cyanotic type.27
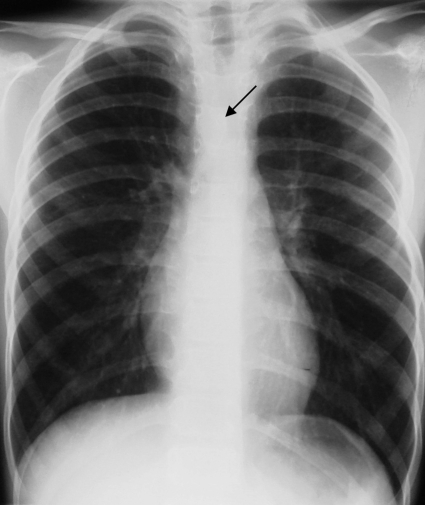
Figure 12.
Plain chest radiograph in a 7-year-old boy depicting a right aortic arch (arrow). Absence of a left-sided aortic knob is the even more apparent sign.
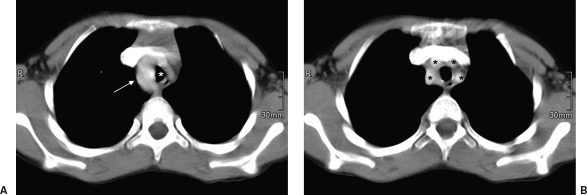
Figure 13.
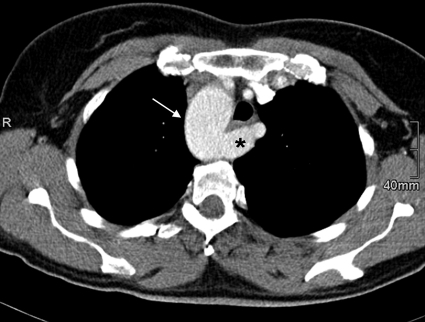
(A) Contrast-enhanced computed tomography in a 7-year-old boy with a right aortic arch (arrow) narrowing the trachea (white asterisk). (B) As another variation, the four supra-aortal arteries (black asterisks) originate separately.
Figure 14.
Contrast-enhanced computed tomography in a 42-year-old woman with a right aortic arch (arrow) showing the pattern of a so-called arteria pseudolusoria. The left subclavian artery (asterisk) is crossing posterior to the esophagus and trachea.
Ductus Diverticulum
The aortic isthmus in adults has a variable appearance. Its configuration may show a concavity, a straightening or slight convexity, or a discrete focal bulge. The latter finding represents a ductus diverticulum, present in ~9% of individuals. Representing the most distal segment of the embryonic right arch, the ductus diverticulum is a fusiform dilation of the ventromedial portion of the proximal descending thoracic aorta. At times a prominent ductus diverticulum may resemble a traumatic pseudoaneurysm of the aortic isthmus.28
Double Aortic Arch
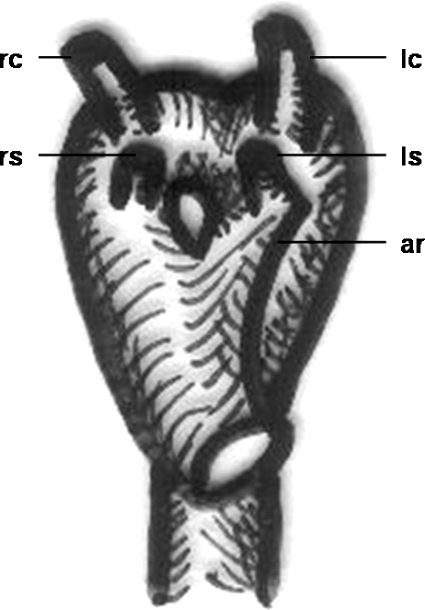
The double aortic arch is a rare anomaly caused by persistence (to varying degrees) of the fetal double aortic arch system.2 The ascending aorta divides into two arches that pass to either side of the esophagus and trachea and reunite to form the descending aorta (Fig. 15). Therefore, it is a form of complete vascular ring, resulting in noncardiac morbidity, but rarely associated with intracardiac defects.29 The descending aorta is usually on the left side. Most commonly, one arch is dominant, whereas the other may be of small caliber or represented by a fibrous band.
Figure 15.
Schematic drawing of a double aortic arch with aortic ring formation. rs, right subclavian artery; rc, right carotid artery; lc, left carotid artery; ls, left subclavian artery; ar, aortic ring.
Cervical Aortic Arch
The cervical aortic arch refers to an unusually high location of the aortic arch in the low or midneck region.2 This rare type of aortic arch anomaly is presumed to result from persistence of the third aortic arch and regression of the normal fourth arch. Abnormalities of brachiocephalic arterial branching and arch laterality are common in patients with a cervical aortic arch.30 There is no association with congenital heart disease, and the anomaly occurs most frequently in association with a right aortic arch. Most of the patients with this anomaly are asymptomatic, but symptoms of dysphagia and respiratory distress due to the compression by the vascular ring have been reported.31 It should be considered in the differential diagnosis of pulsatile masses in the neck.32
Other Variant Branching Sequence
Variations in the sequence of branching of the major arch vessels also occur rarely (< 0.5%).2 For example, the left subclavian artery may be the second branch (before the left common carotid), or the internal and external carotid arteries may originate independently from the aortic arch. There are manifold case reports as well as reviews in literature on various branching patterns.33
DESCENDING THORACIC AORTA
Intercostal and Subcostal Arteries
The anterior intercostal arteries arise from the internal mammary artery. In 95% of cases, the first, second, and third posterior intercostal arteries originate from the superior intercostal artery, which is a branch of the costocervical trunk. There are several variations in the origin of the first through third intercostal arteries. The remaining intercostal and subcostal spaces are supplied by paired dorsal branches of the thoracic aorta. In ~5% of cases all posterior intercostal arteries arise from the aorta. The origins of the upper intercostal arteries lie closer together and are more commonly involved in the formation of common trunks supplying two or more intercostal spaces.2
Bronchial Arteries
The bronchial arteries arise from the third through seventh intercostal spaces. In addition to supplying the major bronchi, the bronchial arteries provide blood supply to the larger bronchopulmonary lymph nodes and have direct anastomoses with the pulmonary and occasionally the coronary arteries.2
In ~60% of individuals, there is a single right bronchial artery. Overall, a common origin of a right bronchial and intercostal arteries occurs in > 70% of individuals. Multiple bronchial arteries are more common on the left side (~70%). A left intercostal bronchial trunk, usually with a right intercostal artery, occurs infrequently (4%). A common bronchial trunk of left and right bronchial arteries occurs in ~45% of individuals.2
Anomalous origin of the bronchial arteries may be from the internal mammary, superior intercostal, subclavian, brachiocephalic, or inferior thyroid arteries. A left bronchial artery may originate from the concavity of the aortic arch in ~15% of individuals. A left bronchointercostal trunk occurs in only 4% of individuals.2
Spinal Arteries
The anterior 70 to 80% of the spinal cord is supplied by the anterior spinal artery (ASA). The posterior 20 to 30% is supplied by the paired posterior spinal arteries (PSA).34
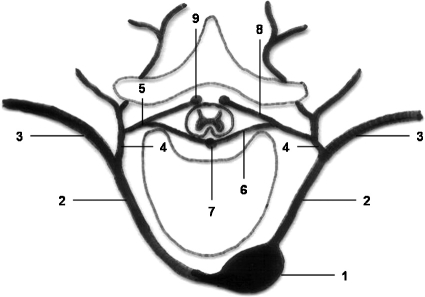
The ASA is formed near the vertebrobasilar junction from branches of the vertebral arteries. The posterior spinal arteries are formed by posterior branches of the vertebral or posterior inferior cerebellar arteries. Both the PSAs and ASA receive branches from extraspinal arteries (e.g., vertebral, subclavian, intercostal, bronchial, lumbar, internal iliac). Those branches that supply the spinal cord are termed radiculomedullary arteries (Fig. 16).2
Figure 16.
Schematic drawing of the posterior intercostal artery and its dorsal branch. 1, thoracic aorta; 2, posterior intercostal artery; 3, ventral branch; 4, dorsal branch; 5, spinal branch; 6, anterior radicular branch; 7, anterior spinal artery; 8, posterior radicular branch; 9, posterior spinal artery. (Modified from Leonhardt.34)
Tributaries contributing to the formation of the anterior spinal artery are the following: anterior spinal branches of the vertebral arteries; radiculomedullary branches from the vertebral artery (C2-C3); radiculomedullary branches from the costal, cervical, or thyrocervical trunk (C5-C6); radiculomedullary branch from the first intercostal or the costocervical trunk (C8); radiculomedullary branch from a posterior intercostal artery (T4-T5); arteria radicularis magna (T9-T12); lumbar or lateral sacral branches.2
The most important arterial feeding vessel of the thoracolumbar spinal cord is the great anterior radiculomedullary artery (artery of Adamkiewicz) (Fig. 17).35,36 This artery supplies the lower third of the spinal cord, originating in the left intercostal or lumbar artery in 68 to 73% of cases and at the level of the 9th to 12th intercostal artery in 62 to 75%.2 Magnetic resonance (MR) and computed tomographic (CT) angiography are noninvasive procedures that may allow clear visualization of the artery of Adamkiewicz. MR angiography has been reported to provide detection rates as high as 93% and 80% with the morphological “hairpin turn” criterion and the anatomical “continuity” criterion, respectively. Sixteen-detector row CT angiography provided detection rates as high as 83% and 60%, respectively, in this recent study. Use of both MR angiography and CT angiography provided higher detection rates of 97% and 90%, respectively.37
Figure 17.
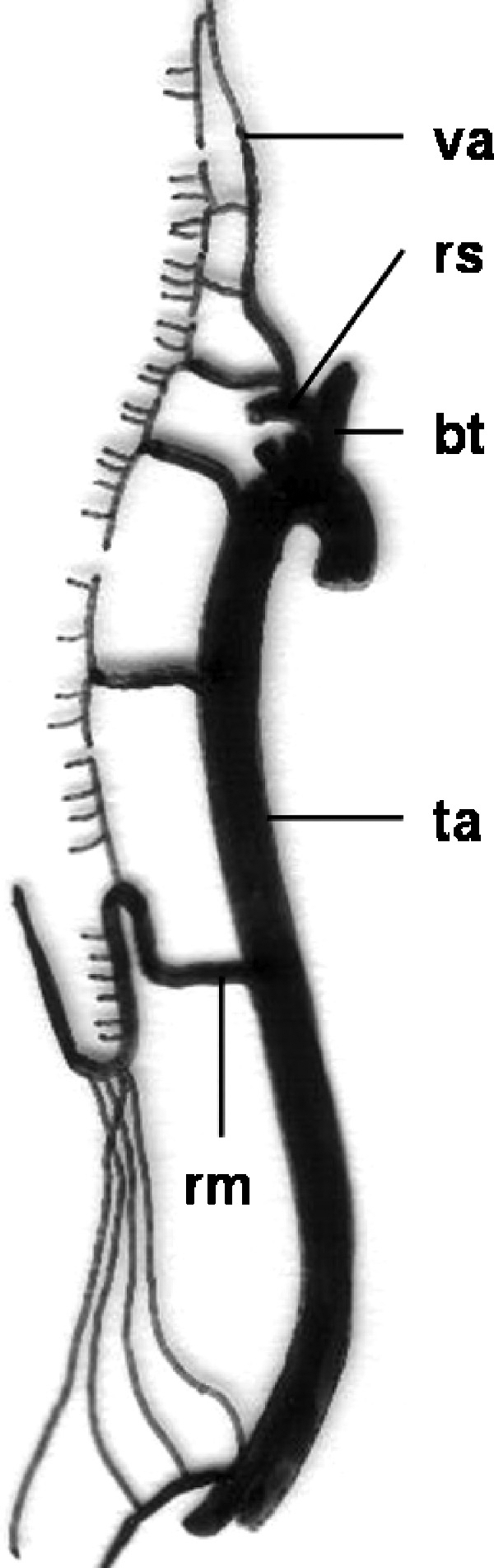
Schematic drawing of blood supply of the spinal cord. va, vertebral artery; rs, right subclavian artery; bt, brachiocephalic trunk; ta, thoracic aorta; rm, arteria radicularis magna (Adamkiewicz). (Modified from Leonhardt.34)
Esophageal Arteries
The esophageal arteries are approximately four to five in number and form a longitudinal vascular chain along the esophagus. The esophageal vascular supply can be divided into three segments: a cervical one (from the inferior thyroid artery, mostly from the right, or from the subclavian and common carotid arteries), a thoracic segment (from bronchial and intercostal arteries or directly from the thoracic aorta), and an abdominal segment (from two to three branches of the left gastric artery or accessory left hepatic artery or left inferior phrenic arteries).2
Other Arteries
Further branches of the descending thoracic aorta are variably developed pericardiac branches, mediastinal branches, and weakly developed superior phrenic arteries.2
Pulmonary Sequestration
Pulmonary sequestration is an uncommon anomaly. It refers to the situation whereby a portion of lung tissue receives its blood supply from an anomalous systemic artery originating from the aorta or one of its branches. Three main variants exist: intralobar, extralobar, and communicating bronchopulmonary foregut malformations. Early embryologic development of the accessory lung bud results in formation of the sequestration within normal lung tissue and is encased within the same pleural covering. This is the intrapulmonary variant. In contrast, later development of the accessory lung bud results in the extrapulmonary type. Venous drainage is typically via a systemic vein, although drainage into the pulmonary veins is well documented.38
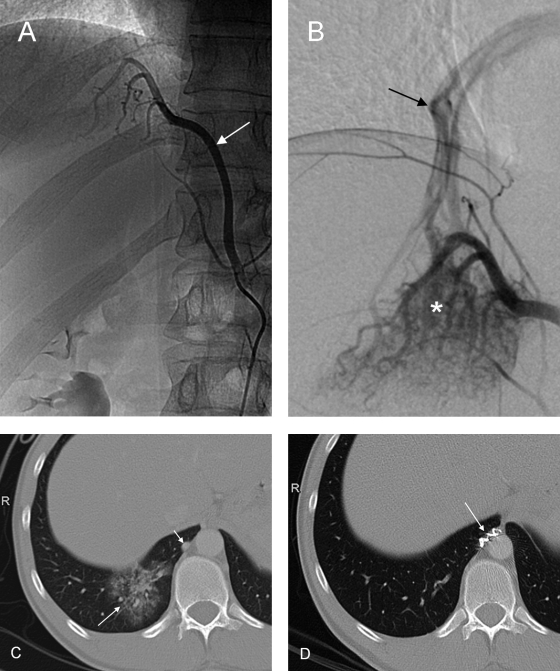
Anomalous arterial supply to the normal basal segments of the lower lobe without sequestration is a rare congenital abnormality, and whether it belongs to the broad spectrum of sequestration disorders remains controversial (Fig. 18).
Figure 18.
Angiography in a 27-year-old patient presenting with recurrent hemoptysis shows anomalous systemic arterial supply to the posterobasal segment of the right lower pulmonary lobe. (A) The feeding artery (arrow) arises from the celiac trunk. (B) Selective angiography depicts racemose vessels (asterisk) in the lung base and a draining pulmonary vein (arrow). (C) Computed tomography shows the area of “pseudo-sequestration” (long arrow) and the abnormal artery (short arrow). (D) After embolization with microspheres and coiling (arrow) of the feeding artery, the posterobasal segment of the right lower lobe has normalized in computed tomography.
In conclusion, this article gives a systematic overview of aortic anomalies on the basis of embryologic development. The different types and entities are considered with respect to their frequency of occurrence. Under clinical aspects, anatomical variants may be differentiated from potentially or invariably pathological abnormalities. Diagnostic and interventional radiology certainly require knowledge of both.
REFERENCES
- Schleich J M. Images in cardiology—development of the human heart: days 15–21. Heart. 2002;87:487. doi: 10.1136/heart.87.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadir S. In: Kadir S, editor. Atlas of Normal and Variant Angiographic Anatomy. Philadelphia: WB Saunders; 1991. Regional anatomy of the thoracic aorta. pp. 19–54.
- Weinberg P M. Aortic arch anomalies. J Cardiovasc Magn Reson. 2006;8:633–643. doi: 10.1080/10976640600713756. [DOI] [PubMed] [Google Scholar]
- Bardo D M, Frankel D G, Applegate K E, Murphy D J, Saneto R P. Hypoplastic left heart syndrome. Radiographics. 2001;21:705–717. doi: 10.1148/radiographics.21.3.g01ma09705. [DOI] [PubMed] [Google Scholar]
- Devloo-Blancquaert A, Titus J L, Edwards J E, Vallaeys J H, De Gezelle H R, Coppens M. Interruption of aortic arch and hypoplastic left heart syndrome. Pediatr Cardiol. 1995;16:304–308. doi: 10.1007/BF00798068. [DOI] [PubMed] [Google Scholar]
- Jenkins N P, Ward C. Coarctation of the aorta: natural history and outcome after surgical treatment. QJM. 1999;92:365–371. doi: 10.1093/qjmed/92.7.365. [DOI] [PubMed] [Google Scholar]
- Rao P S. Coarctation of the aorta. Curr Cardiol Rep. 2005;7:425–434. doi: 10.1007/s11886-005-0060-0. [DOI] [PubMed] [Google Scholar]
- Backer C L, Mavroudis C. Congenital Heart Surgery Nomenclature and Database Project: vascular rings, tracheal stenosis, pectus excavatum. Ann Thorac Surg. 2000;69:S308–S318. doi: 10.1016/s0003-4975(99)01279-5. [DOI] [PubMed] [Google Scholar]
- Maxey T S, Bradner M W, Reece T B, Keeling W B, Kron I L. Coarctation of a right aortic arch. J Card Surg. 2006;21:261–263. doi: 10.1111/j.1540-8191.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Tchervenkov C I, Jacobs J P, Sharma K, Ungerleider R M. Interrupted aortic arch: surgical decision making. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2005:92–102. doi: 10.1053/j.pcsu.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Campbell M. Natural history of persistent ductus arteriosus. Br Heart J. 1968;30:4–13. doi: 10.1136/hrt.30.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azakie A, McElhinney D B, Messina L M, Stoney R J. Common brachiocephalic trunk: strategies for revascularization. Ann Thorac Surg. 1999;67:657–660. doi: 10.1016/s0003-4975(98)01322-8. [DOI] [PubMed] [Google Scholar]
- Lamers L J, Rowland D G, Seguin J H, Rosenberg E M, Reber K M. The effect of common origin of the carotid arteries in neurologic outcome after neonatal ECMO. J Pediatr Surg. 2004;39:532–536. doi: 10.1016/j.jpedsurg.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Layton K F, Kallmes D F, Cloft H J, Lindell E P, Cox V S. Bovine aortic arch variant in humans: clarification of a common misnomer. AJNR Am J Neuroradiol. 2006;27:1541–1542. [PMC free article] [PubMed] [Google Scholar]
- Yamaki K, Saga T, Hirata T, et al. Anatomical study of the vertebral artery in Japanese adults. Anat Sci Int. 2006;81:100–106. doi: 10.1111/j.1447-073x.2006.00133.x. [DOI] [PubMed] [Google Scholar]
- Koenigsberg R A, Pereira L, Nair B, McCormick D, Schwartzman R. Unusual vertebral artery origins: examples and related pathology. Catheter Cardiovasc Interv. 2003;59:244–250. doi: 10.1002/ccd.10503. [DOI] [PubMed] [Google Scholar]
- Lemke A J, Benndorf G, Liebig T, Felix R. Anomalous origin of the right vertebral artery: review of the literature and case report of right vertebral artery origin distal to the left subclavian artery. AJNR Am J Neuroradiol. 1999;20:1318–1321. [PMC free article] [PubMed] [Google Scholar]
- Goray V B, Joshi A R, Garg A, Merchant S, Yadav B, Maheshwari P. Aortic arch variation: a unique case with anomalous origin of both vertebral arteries as additional branches of the aortic arch distal to left subclavian artery. AJNR Am J Neuroradiol. 2005;26:93–95. [PMC free article] [PubMed] [Google Scholar]
- Albayram S, Gailloud P, Wasserman B A. Bilateral arch origin of the vertebral arteries. AJNR Am J Neuroradiol. 2002;23:455–458. [PMC free article] [PubMed] [Google Scholar]
- Wolpert S M. The thyroidea ima artery: an unusual collateral vessel. Radiology. 1969;92:333–334. doi: 10.1148/92.2.333. [DOI] [PubMed] [Google Scholar]
- Vasovic L, Arsic S, Vlajkovic S, Zdravkovic D. Morphological aspect of the thyroid ima artery in human fetuses. Ital J Anat Embryol. 2004;109:189–197. [PubMed] [Google Scholar]
- Richardson J V, Doty D B, Rossi N P, Ehrenhaft J L. Operation for aortic arch anomalies. Ann Thorac Surg. 1981;31:426–432. doi: 10.1016/s0003-4975(10)60994-0. [DOI] [PubMed] [Google Scholar]
- Bayford D. An account of a singular case of obstructed deglutition. Memoirs Med Soc London. 1794;2:275–286. [Google Scholar]
- Edwards J E. In: Gould SE, editor. Pathology of the Heart. 2nd ed. Springfield: Charles C Thomas; 1960. Congenital malformations of the heart and great vessels. Section H: malformations of the thoracic aorta. pp. 391–462.
- Cina C S, Althani H, Pasenau J, Abouzahr L. Kommerell's diverticulum and right-sided aortic arch: a cohort study and review of the literature. J Vasc Surg. 2004;39:131–139. doi: 10.1016/j.jvs.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Son J A van, Bossert T, Mohr F W. Surgical treatment of vascular ring including right cervical aortic arch. J Card Surg. 1999;14:98–102. doi: 10.1111/j.1540-8191.1999.tb00957.x. [DOI] [PubMed] [Google Scholar]
- McElhinney D B, Hoydu A K, Gaynor J W, Spray T L, Goldmuntz E, Weinberg P M. Patterns of right aortic arch and mirror-image branching of the brachiocephalic vessels without associated anomalies. Pediatr Cardiol. 2001;22:285–291. doi: 10.1007/s002460010231. [DOI] [PubMed] [Google Scholar]
- Goodman P C, Jeffrey R B, Minagi H, Federle M P, Thomas A N. Angiographic evaluation of the ductus diverticulum. Cardiovasc Intervent Radiol. 1982;5:1–4. doi: 10.1007/BF02552093. [DOI] [PubMed] [Google Scholar]
- Alsenaidi K, Gurofsky R, Karamlou T, Williams W G, McCrindle B W. Management and outcomes of double aortic arch in 81 patients. Pediatrics. 2006;118:e1336–e1341. doi: 10.1542/peds.2006-1097. [DOI] [PubMed] [Google Scholar]
- McElhinney D B, Thompson L D, Weinberg P M, Jue K L, Hanley F L. Surgical approach to complicated cervical aortic arch: anatomic, developmental, and surgical considerations. Cardiol Young. 2000;10:212–219. doi: 10.1017/s1047951100009136. [DOI] [PubMed] [Google Scholar]
- Acikel U, Ugurlu B, Hazan E, Salman E. Cervical aortic arch: a case report. Angiology. 1997;48:659–662. doi: 10.1177/000331979704800715. [DOI] [PubMed] [Google Scholar]
- Hyman R A, Stein H L. The cervical aortic arch anomaly. Angiology. 1975;26:749–758. doi: 10.1177/000331977502601007. [DOI] [PubMed] [Google Scholar]
- Nelson M L, Sparks C D. Unusual aortic arch variation: distal origin of common carotid arteries. Clin Anat. 2001;14:62–65. doi: 10.1002/1098-2353(200101)14:1<62::AID-CA1012>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Leonhardt H. In: Leonhardt H, Tillmann B, Zilles K, editor. Rauber/Kopsch—Anatomie des Menschen, Lehrbuch und Atlas: Topographie der Organsysteme, Systematik der peripheren Leitungsbahnen. Stuttgart: Thieme; 1988. Rumpfwand. Systematik der peripheren Leitungsbahnen der Rumpfwand. p. 243.
- Adamkiewicz A. Die Blutgefasse des menschlichen Ruckenmarkes. I: Die Gefasse der Ruckenmarksubstanz. Sitz Akad Wiss Wien Math Natur Klass. 1882;84:469–502. [Google Scholar]
- Alleyne C H, Jr, Cawley C M, Shengelaia G G, Barrow D L. Microsurgical anatomy of the artery of Adamkiewicz and its segmental artery. J Neurosurg. 1998;89:791–795. doi: 10.3171/jns.1998.89.5.0791. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Niinuma H, Ehara S, Nakajima T, Nakamura M, Kawazoe K. MR angiography and CT angiography of the artery of Adamkiewicz: state of the art. Radiographics. 2006;26:S63–S73. doi: 10.1148/rg.26si065506. [DOI] [PubMed] [Google Scholar]
- Corbett H J, Humphrey G M. Pulmonary sequestration. Paediatr Respir Rev. 2004;5:59–68. doi: 10.1016/j.prrv.2003.09.009. [DOI] [PubMed] [Google Scholar]