ABSTRACT
Interventional radiologists (IRs) now play a major role in the management of thoracic aortic and great vessel trauma. The recent availability of a wide range of stent grafts able to treat vessels from 3 to 46 mm in diameter is clearly a significant contributor to this change. Stent grafts can now treat the majority of incomplete aortic injuries with much lower morbidity and mortality than open surgery. Short- to medium-term follow-up is encouraging, but the long-term durability is unknown, and close monitoring of these patients must continue. In great vessel trauma, stent grafts are a useful adjunct to balloon tamponade, embolization, and bare stents. As a result, a wide range of head neck and upper limb vascular injuries can be managed with less local trauma, blood loss, and physiological stress. The increased involvement of IR in the management of vascular trauma is not simply the result of technological advances. IRs have increasingly made themselves available to carry out these emergency procedures. IRs should assist in the development of trauma protocols and management algorithms that involve endovascular expertise early in the assessment of the major trauma patient.
Keywords: Blunt aortic trauma, aortic transection, stent graft, endoluminal repair
Recent developments in endovascular technologies, particularly the widespread availability of commercial stent grafts that can treat vessels ranging in size from the thoracic aorta to vertebral arteries, has led to a change in the treatment of thoracic aortic and great vessel trauma. Adding major iatrogenic trauma, in the form of open surgery, to a patient who has already sustained multiple injuries is a major physiological insult that leads to significant morbidity and mortality. Radiologically guided endovascular interventions allow the effective treatment of vascular injuries but with much reduced physiological stress. In thoracic aortic injury stent-graft placement has quickly evolved into the primary treatment across all Injury Severity Score profiles.1
In thoracic trauma, the thoracic aorta is the most commonly injured vessel, accounting for 84% of injuries. In 81% it is injured in isolation, and a further 3% have a combined aortic and aortic branch vessel injury. The great vessels alone are injured in 16%.2
THORACIC AORTIC INJURIES
Complete (full-thickness) aortic injury typically results in immediate exsanguination. Hospitalized patients almost invariably have an incomplete aortic injury (IAI) with intact adventitia or periadvential tissues.3
Site and Frequency
The frequency and site of injury depends on whether postmortem studies, those surviving to hospitalization, or a combination of the two is being considered and on the cohort being examined. IAIs account for ~17% of deaths due to road traffic accidents (RTAs).4 Most fatal IAIs due to RTAs occur at the aortic isthmus.5 In 1958 Parmley et al found that 85% of patients who sustain an aortic injury from blunt trauma die at the scene of the accident.6 This data also found that of the 15% who survived for 1 hour, 30% would succumb in the first 6 hours and at 24 hours half would be dead. At 4 months only 2% of patients were alive. For almost half a century this powerful data justified early surgical management but it is underappreciated that the information comes from postmortem studies of 296 victims of nonpenetrating aortic injury in the Korean War. The contribution of other injuries to death was not considered, and the percentage of longer term survivors is likely to be significantly underestimated. Although this series is widely quoted, its applicability to current civilian practice is questionable.
For hospitalized patients the incidence varies with the sensitivity of the imaging technique used. Using catheter angiography, Ahrar et al found that 96% of injuries occur at the aortic isthmus, 1% at the proximal ascending aorta, 1% at both the aortic isthmus and the proximal ascending aorta, 1% at the distal ascending aorta, and 1% in the descending aorta.7
Mechanism
The thoracic aorta is most commonly injured by a sudden and dramatic change in velocity. The most common cause is sudden deceleration from RTAs and falls from a height of > 10 to 15 feet.8,9 Acceleration injuries due to pedestrians and cyclists impacting with motor vehicles are less common but well recognized.10 Explosions may cause aortic injury by acceleration or deceleration.8 Occasionally crush injuries produce similar injuries, but in general these injure a longer length of aorta and cause extensive intramural hematoma.
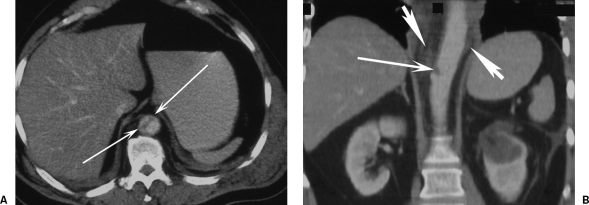
Traction, torsion, and hydrostatic forces as a consequence of differential slowing of thoracic structures have all been implicated in sudden deceleration injury.2 Horizontal shear forces result from the aortic arch being fixed by the great vessels.6 The mobile ascending and descending aorta stop later, resulting in focal stresses on the aortic root and aortic isthmus. The osseous pinch theory suggests that rupture occurs because the aorta is compressed between the spine and the bones of the anterior chest (sternum, clavicle, and upper ribs) on sudden deceleration.11 It is proposed that the aorta distal to the left subclavian is most commonly injured because the superior aortic arch is fixed by its major neck branches and the descending aorta is closely apposed to the spine by the intercostal vessels and the ligamentum arteriosum. The intervening proximal descending aorta is fixed in position and cannot move away, causing it to be pinched front to back. Support for this mechanism comes from the recognition of crush injury as a cause of IAI.12 Other mechanisms that have been proposed include (1) sudden compression of the heart, which causes a severe increase in aortic pressure with upward displacement of the aortic arch and traction on the aortic isthmus due to the descending aorta being anchored to the spine; (2) relative weakness of the aortic wall at the isthmus due to fewer elastic fibers; and (3) horizontal and/or cranial movement of the aortic arch along with a sudden increase in intrathoracic pressure that may kink the aorta at the diaphragm, leading not only to local injury at this site (Fig. 1A,B) but also a sudden increase in thoracic aortic pressure.13 It is likely that a combination of these mechanisms is causal in many patients.5
Figure 1.
Computed tomography (CT) of deceleration injury. (A) Axial CT and (B) coronal CT reformat images show a T11 focal intimal tear (thin white arrows) with minimal hemorrhage (broad white arrows) from a road traffic accident deceleration injury while wearing a seat belt. There is no contour change in the aorta.
The only penetrating injury that is commonly survived is iatrogenic trauma, most often due to misplaced central venous catheters. These are survivable when the injury is recognized before the device is removed.
Spectrum of Injuries
Pathologically, a spectrum of findings has long been recognized, with injuries ranging from minor intimal flaps through increasing depth of injury extending to “pseudoaneurysm” formation and ultimately active extravasation.13 Contour changes in the aortic wall are often described as pseudoaneurysms, but the majority should be strictly considered to be true aneurysms with at least an intact adventitia.3
With modern computed tomography (CT) scanners, all of these injuries are now evident on imaging. The spectrum of findings in thoracic aortic injury includes the following14:
Focal abrupt change in the aortic contour with an irregular outpouching, most commonly just distal to the left subclavian artery (Fig. 2C)
Circumferential abrupt change in the aortic contour with an irregular outpouching. It has been proposed that this group is at higher risk of rupture15 (Fig. 2D)
Intramural hematoma alone
Intramural hematoma plus contour changes and/or intimal flaps (Fig. 2E)
True aortic dissection (long flap indistinguishable from spontaneous dissection and managed in the same way as spontaneous dissections). This is an uncommon injury (Fig. 2F)
Pseudocoarctation (obstructive flap reducing the caliber of the descending aorta) (Fig. 2G)
Late saccular (or less commonly fusiform) focal aneurysm formation, often with peripheral calcification (Fig. 2H)
Active extravasation (rare and often an immediately antemortem finding) (Fig. 2I)
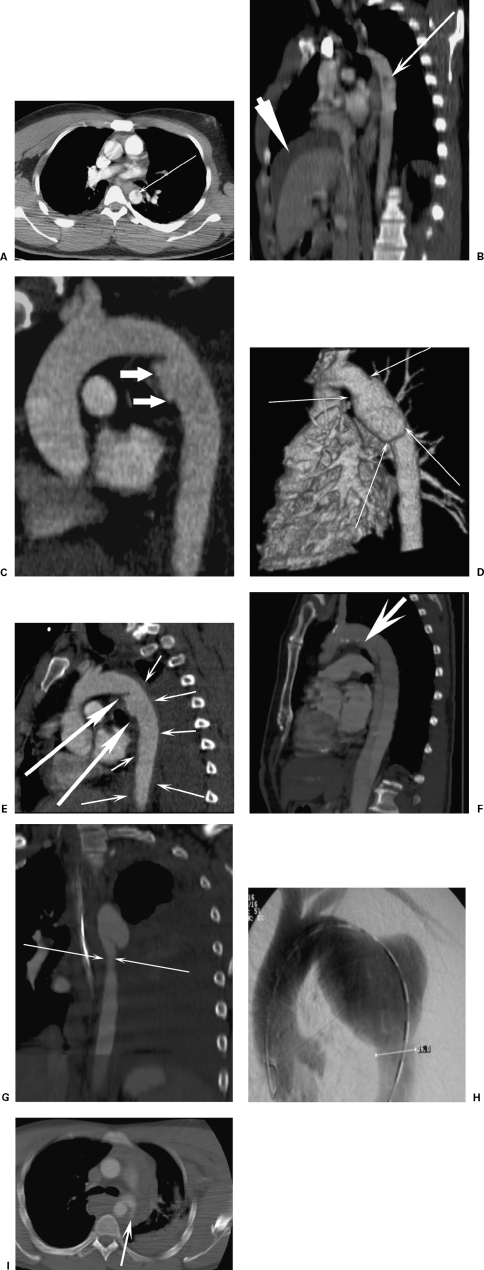
Figure 2.
The spectrum of aortic injuries. (A) Axial computed tomography (CT) and (B) sagittal oblique reformat of focal intimal flap (thin arrow, intimal flap; thick arrow, perihepatic hematoma from liver contusion). (C) CT reconstruction demonstrates a focal aortic injury at the isthmus. (D) A surface rendered CT image shows a circumferential aortic injury. (E) A 47-year-old man crushed by a steel door. CT reformat shows focal aortic injury (large arrows) with extensive intramural hematoma (small arrows). (F) A 5-year follow-up CT of a post-traumatic dissection shows the entry tear distal to the left subclavian artery (arrow). Minimal false lumen expansion had occurred over this period. (G) Coronal CT reformat shows pseudocoarctation. (H) A 36-year-old with a 6.5-cm late saccular aneurysm. The patient had had a polytrauma motorbike accident 18 years previously. A thoracic angiogram shows an aneurysm that was incidentally detected on a diving medical chest radiograph. (I) A 24-year-old male car driver. Axial CT scan shows active bleeding (arrows) from an aortic injury. The patient had a lethal head injury and died within 1 hour of this scan.
Because active extravasation is rare, this question arises: Where does the mediastinal blood commonly associated with IAI on CT come from? Although it may be that in some cases there has been a luminal leak that has spontaneously sealed, it is more plausible that because the adventitia remains intact in the majority, the blood comes from damaged vasa vasorum and small mediastinal arteries and veins. The mediastinal hemorrhage reflects the severity of the trauma rather than aortic luminal blood. The quantity of mediastinal blood seen on CT is often small, but despite this IAI is commonly implicated as the cause of hemodynamic instability. If there is no active bleeding on CT and the mediastinal hematoma is small, it follows that this is not the cause of shock, and an alternative source of hemorrhage should be sought. This life-threatening injury should be addressed before the aorta is treated. A caveat to this is that a large mediastinal hematoma can cause hemodynamic compromise without active bleeding due to compression of vascular structures such as the heart (particularly the right ventricle) and pulmonary arteries.16
Isolated anterior mediastinal hematoma is unlikely to be due to IAI. Injuries to the internal mammary artery (which may be life threatening), bronchial arteries, superior intercostals veins and rib, clavicle, and sternal fractures are all more likely culprits.16
Imaging
CHEST X-RAY (CXR)
A CXR has long been a part of the initial imaging assessment of suspected IAI with a long list of signs described. It is still widely advocated in recent publications10,17 but others concede it is unreliable.18 CXR is of limited use in determining whether there has been a vascular injury. The mediastinum commonly appears widened on a supine CXR but the subsequent CT will show no aortic injury or mediastinal hemorrhage. Conversely, and more importantly, a normal CXR does not exclude an aortic injury. The mediastinum is normal in 7.3% of patients who are subsequently proven to have an IAI.19 Supplementary signs can reduce this number further, but the fact remains that some IAIs would remain undiagnosed, resulting in avoidable deaths. A 95% negative predictive value20 is not acceptable for a lethal condition. If a patient has the correct mechanism of injury, he or she must undergo early definitive imaging assessment to exclude IAT. The fact that cardiothoracic centers still see patients with ruptured or incidental late post-traumatic aneurysms could be taken as evidence that vigilance remains suboptimal. Elderly patients can sustain an aortic injury with lower level impacts, which should lead to a heightened awareness in this group.5 Additionally, Trupka et al found CT to be superior to a CXR in detecting other thoracic injuries and that it significantly influenced management.21
CATHETER ARTERIOGRAPHY
Catheter angiography was once considered to be the reference standard for IAI, but it is invasive, often slower to organize than noninvasive tests, only provides information on vascular injuries, is expensive, and complications may rarely occur.22 A minimum of two projections are required, usually anteroposterior and 40 to 50 degree left anterior-oblique. It does not demonstrate intramural hematoma and may miss less severe injuries, such as intimal flaps. A false-positive interpretation can occur due to focal atheroma, particularly if ulcerated; an overlapping vessel; and a ductus diverticulum (Fig. 3). The latter should be easily distinguished by its smooth contour on the inner curve of the aorta just distal to the left subclavian artery with flat obtuse margins with the aorta rather than the acutely angled undercut margins of IAI, but false positives continue to be reported.23 Catheter angiography is now most commonly used to plan endovascular intervention.
Figure 3.
Ductus diverticulum. Catheter angiogram showing the characteristic smooth margins with flat (obtuse) angles with the adjacent aorta. Compare with the irregular sharp angles of the incomplete aortic transaction in Fig. 2C.
TRANSESOPHAGEAL ECHOCARDIOGRAPHY
The most common site of IAT is accessible to transesophageal echocardiography (TOE). It is reported to identify intimal tears, intramural hematoma, pseudoaneurysms, pseudocoarctation, and active bleeding with a sensitivity of 91% and a specificity of 98%.20 TOE is an operator-dependent technique, and even in experienced hands there is incomplete visualization of the entire aortic circumference in a third of patients. Patients with facial injuries, unstable cervical spine injuries, or where cervical spine injuries have not been excluded are unsuitable. There are concerns about elevating the blood pressure and shear force on a traumatized aorta by intubating the esophagus under sedation alone, and anesthetizing a severely injured patient adds to the procedural risk. TOE does not demonstrate the full spectrum of injuries that cross-sectional imaging can. In addition to its utility in diagnosis, TOE is a useful adjunctive imaging technique in thoracic stent-graft deployment where it can identify the great vessels and the stent graft simultaneously. It can be problematic to use TOE and fluoroscopy simultaneously because the TOE probe often overlies and obscures the target proximal drop zone.
COMPUTED TOMOGRAPHY
Diagnostic algorithms for IAI have evolved in the past few years, mirroring the changes in those for other aortic emergencies. Initially CT was widely used to screen for mediastinal hematoma, with positive studies proceeding to catheter angiography. This was a flawed approach because, analogous to the normal CXR rate, 9% of patients with surgically proven IAIs (pseudoaneurysms, 6%; intimal tears, 3%) have no periaortic hematoma on CT.24 The introduction of faster spiral (helical) CT scanners with quicker reformatting capability, along with equivalent negative predictive value and sensitivity, changed this position.25 Wicky et al found spiral CT to have a sensitivity of 100% and specificity of 99.8%.14 Intuitively multidetector CT (MDCT) is superior to helical CT with its increased cephalocaudal coverage speed, improved z-axis resolution, and more effective contrast utilization. This is unlikely to improve diagnostic performance significantly for IAI if published results for spiral CT translate into everyday practice, but there will still be clinical benefit from MDCT's decreased scan time and its ability to be a one-stop shop for almost all injuries, including spinal. Ideally the CT scanner should be in, or in close proximity to, trauma rooms.26
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) can certainly demonstrate significant IAI, but it is not commonly available acutely, may be contraindicated by the support or immobilization equipment required in polytrauma, and does not demonstrate other injuries as well or as quickly as CT. MRI is useful in the follow-up of patients who have been treated with nonferrous stent grafts.
Does Every Patient with Incomplete Aortic Injury Need Treating?
First with spiral CT and MRI, and even more so now with thin-section (≤ 1 mm) MDCT and TOE, very subtle injuries are being identified that were previously beyond the resolution of the available imaging. This is particularly true of small intimal flaps with no mural irregularity that catheter angiography is unlikely to have demonstrated, even with the use of multiple two-dimensional projections. This creates a dilemma. Which injuries require treatment? If not all, which injuries can be ignored or only treated medically? Some practitioners (Refs. 15, 27, and author's personal practice) withhold intervention for minor aortic injuries. These have been defined as small (1 cm) intimal flaps with no or minimal periaortic hematoma.15 In other reports it is unclear what criteria are used for adopting an observational position. Most authors who report intervention do not document which subtypes of IAI were treated, raising the possibility that at least some minor injuries are actively treated. Open surgery has been reported on such lesions.24 Complete resolution of minor aortic changes has been observed,27 but complications can also result from such injuries (Fig. 4). It is possible, if not probable, that such injuries are not usually clinically important and that the risk-benefit ratio favors observation, but the evidence to back this assumption is currently lacking. Long-term follow-up of these patients, who we are only now identifying, is required.
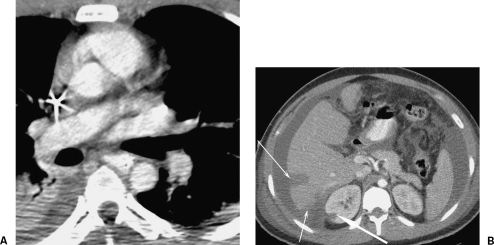
Figure 4.
A complication of an intimal flap. A 27-year-old construction worker fell 15 feet onto a scaffolding bar. His initial aortic images are shown in Fig. 2A and B. The thickness of the flap suggests there may be thrombus formation on it. His life-threatening liver contusion and active hemorrhage on computed tomography (CT) was successfully treated by coil embolization (not shown). Follow-up axial CT examination 7 days later (A) at the same level as Fig. 2A show no evidence of an intimal flap and (B) a new right renal infarction (broad arrow). This became a focal scar on follow-up. The hepatic laceration is shown (narrow arrows). If the embolus had passed into one of the mesenteric vessels, it may have had serious consequences.
Surgical Treatment Methods
The rationale for treating IAI is to prevent early rupture from the acute injury and prevent late aneurysm formation and rupture. The traditional treatment for IAI is early open surgery with a left thoracotomy and single-lung ventilation.10 Patients with pulmonary injuries may not tolerate single-lung ventilation.28 Mortality rates from open surgery have been reported to be between 5% and 28%.10 The most significant morbidity is paraplegia from spinal cord ischemia, which occurs in 2.3 to 14%.10 The repair may be a primary direct suturing, an interposition graft, or an intraluminal graft.9 There are two different approaches to open repair, each of which has its advocates.18,29
The first is the so-called clamp-and-sew technique. The aorta is clamped distal to the left subclavian if possible; if not, the arch is clamped distal to the left common carotid with a second clamp on the left subclavian. A distal clamp is then placed as proximal as possible in the descending aorta to maximize spinal cord collateral flow. Clamp-and-sew benefits the polytrauma patient by avoiding the additional risks of systemic heparinization, but this is at the price of increased paraplegia risk. Several publications have shown that cross-clamp times of > 30 minutes lead to the greatest risk of paraplegia.30,31,32 With simple cross-clamping, paraplegia rates as high as 19.2% have been reported.30
The surgical alternative is to perfuse the distal thoracic aorta and thereby the spinal cord by extracorporeal circulation. Several techniques are available with either active or passive perfusion. These include active centrifugal pump perfusion, such as left atrial to femoral bypass, and passive shunt perfusion, such as left ventricle to aorta or ascending to descending aortic shunts.31,33 These techniques decrease the risk of paraplegia and remove the need for rapid repair (< 30 minutes) to avoid paraplegia. The downside is that all require systemic heparinization. Typically the thoracic aorta is not injured in isolation; closed head injuries, solid organ and pulmonary contusions, and pelvic and long bone fractures are all adversely affected by systemic anticoagulation.
Where the aortic injury is associated with extensive hematoma, the repair must be extended to the normal aorta above and below. The graft cannot be sutured into the friable abnormal vessel with intramural hematoma. The increased length of repair increases the risk of paraplegia.
Patients who reach hospital alive after penetrating thoracic aortic injury are managed with open surgery.
Stent-Graft Treatment
A decade has elapsed since stent grafts were first used in IAI.34 Initially only used in those deemed too high risk for conventional repair, they are now widely considered first-line therapy in many hospitals.1,35 Despite higher injury severity scores, mortality and morbidity is much lower in patients treated with stent grafts.28 In the United Kingdom (UK) the National Health Service National Institute for Health and Clinical Excellence (NICE) has produced initial guidance on the use of stent grafts in the thoracic aorta but it is restricted to aneurysms and dissections.36 Despite the accumulating evidence, there is currently no UK national guidance on the use of stent grafts for aortic trauma.
Commercial stent grafts range in diameter from 26 to 46 mm. Current devices were primarily designed and developed to treat aneurysmal disease. The shortest possible length of device (typically 10 to 12 cm) is used because the injury is focal and this minimizes the risk of paraplegia. Oversizing the device diameter by 10 to 15% is recommended. This may be problematic when these devices are used in young patients. Some young patients may have aortic diameters of < 20 mm, which is too small for standard devices. In these cases, proximal cuffs for abdominal aortic stent grafts may be used, but the delivery systems can be too short for thoracic deployment (typically ~60 cm) even with the use of retroperitoneal common iliac access to maximize the usable length.10 Patients in their teens and early 20s commonly have an aortic arch with tight radius of curvature. In such cases the apposition on the inner curve of the aortic arch may be suboptimal due to insufficient device flexibility. In addition, we do not know how the devices will withstand 50 years, or more, of postimplantation stress or the normal aortic dilation with age. They require close imaging surveillance, a cost and, often, a radiation burden.
The stent-graft systems typically require access sheaths of 18 to 24F. The most widely used systems are Talent (Medtronic, Minneapolis, MN), Excluder (W.L. Gore, Flagstaff, AZ), and Zenith (Cook, Bloomington, IN). General anesthesia is usually used. Local anesthesia is feasible, but there are concerns regarding increased stresses on the injury site from pain or anxiety-induced blood pressure elevation, and TOE use is precluded. The common femoral artery may be surgically isolated for direct access or percutaneously punctured, with closure device10 or subsequent surgical repair. The common femoral or external iliac arteries may be too small (< 6 to 8 mm depending on the device being used) to insert the device. This problem is overrepresented in IAI compared with other thoracic stent-grafting procedures due to the higher percentage of young patients, and particularly young and female patients who have generally smaller vessels. This can be overcome by the use of retroperitoneal access to the common iliac artery (CIA) via a Rutherford-Morrison incision. In slim patients direct access to the vessels may be used; otherwise a conduit of an 8- to 10-mm-diameter surgical graft is sewn onto the CIA.37 Where the CIA is also too small or diseased (stenosed or calcified), the stent graft can be inserted via the abdominal aorta.
Although systemic heparinization is usually employed during routine stent-grafting procedures, it is not obligatory. Heparin is withheld in cases of polytrauma where concomitant injuries (head injury, solid organ injury [liver, kidney, spleen], and fractures [pelvic and long bone]) result in the risk of use exceeding the benefit. Heparin 5000 U is administered where other injuries do not preclude its use. Use of a contralateral groin puncture allows continuous angiographic guidance and provides the safety of rapid aortic balloon access should the feared complication of iliac rupture occur (Fig. 5). Some operators prefer a left brachial catheter to optimize imaging of the left subclavian artery origin. Use of TOE is advocated by some15 to guide the deployment, but it adds to the procedural complexity and there is no evidence that it improves outcomes. Angiography is performed in a steep left anterior oblique projection. It can be difficult to achieve sufficient angulation using mobile intensifiers in the operating room. Placing the patient in a partial right decubitus position or laterally tilting the operating table can be useful maneuvers in young patients who have very anteroposteriorly orientated aortic arches.
Figure 5.
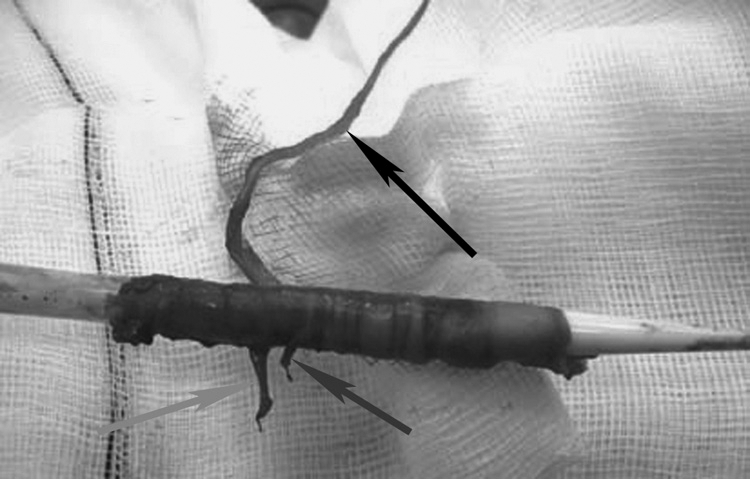
External iliac artery avulsion on attempted withdrawal of a stent-graft system. Specimen photograph shows the branch arteries (deep circumflex iliac, black; inferior epigastric, dark gray; external pudendal, light gray). Contralateral access allows balloon control if this catastrophe occurs.
There is a variable approach to the proximal sealing (or drop or landing) zone or neck. For aneurysmal disease, 15 to 20 mm is the usual recommendation. Some authors have adopted a similar approach in treating IAI.37 IAIs are by definition often closer to the left subclavian artery (LSA) than this. Different approaches to this have been adopted. The proximal sealing zone can be increased by placing the stent graft distal to the left common carotid artery and intentionally covering the LSA. This is usually well tolerated, and acute arm ischemia is rare.15 Prior revascularization of the LSA has been used,10 but experience has shown it is not necessary and increases the procedural complexity and morbidity. In IAI, unlike in aneurysmal disease, the aorta is normal apart from the injured segment. This has led to the alternative approach of placing a stent graft distal to the LSA in patients with at least 5 mm of normal aorta distal to the LSA.15,38 This approach is not associated with poorer outcomes. Where there is < 5 mm of normal aorta distal to the LSA, the LSA is covered.
Pharmacologically dropping the mean arterial blood pressure to 70 mm Hg, or less, at the time of stent-graft deployment can decrease the distal displacement forces on the stent graft and aid accurate positioning. This is particularly useful in those devices that fully open from the tip backward because it reduces the power of the windsock effect to which they are prone.
In IAI with extensive hematoma, stent grafting can treat the injury site locally, and the intramural hematoma will resolve over the next few weeks to months (Fig. 6).
Figure 6.
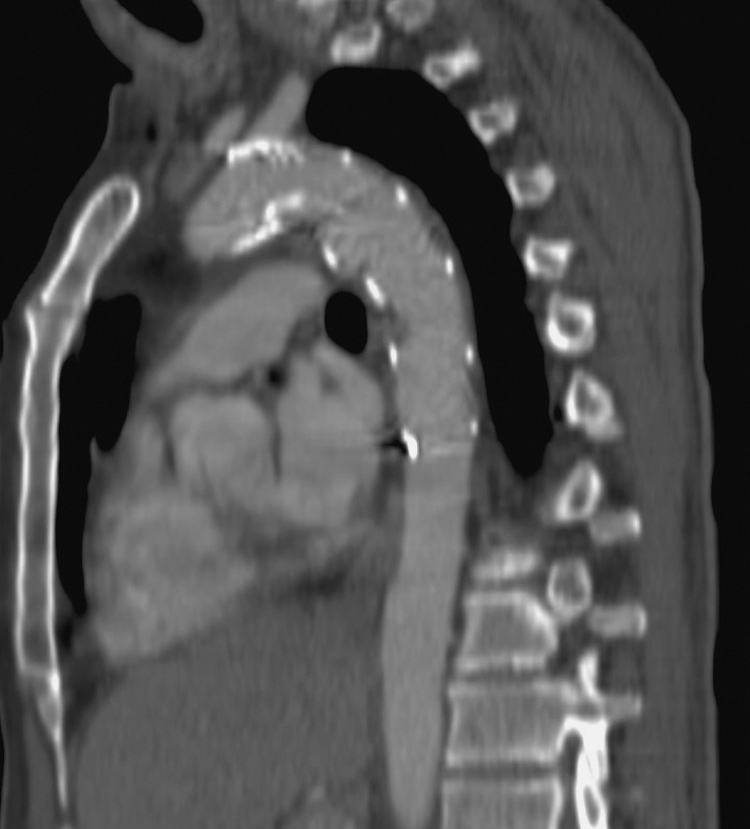
A 47-year-old man crushed by a steel door. Same patient as Fig. 2E. Four weeks post–stent grafting, computed tomography reformat shows no evidence of the aortic tear and the intramural hematoma has resolved. The appearances were unchanged at the most recent follow-up (2 years).
Delayed Repair
The observation by Parmley of a 50% mortality in the first 24 hours posthospitalization (the majority in the first 6 hours) had a major impact on surgical care.6 For many years the standard treatment for IAI was emergency thoracic surgery, irrespective of the severity of other injuries. This ignored the fact that many of the deaths would have been due to associated injuries. The mortality from this conventional surgical approach was 21.3% in a 20 year meta-analysis.30
In the mid-1990s, several groups, extrapolating from the medical management of Stamford type B aortic dissection, adopted a conservative approach to IAI patients with multiple trauma. Vasodilators and β-blockers were used. Pate et al found that out of a cohort of 112 patients with acute isthmic IAI, none of the 46 patients with formal systolic blood pressure control (systolic arterial pressure < 140 mm Hg with therapy including β-blockers) died of aortic rupture.39 Six patients in the group who did not receive pharmacological therapy died of aortic exsanguination, all within 4 hours of injury. Beta blockade is essential; by altering left ventricular systolic ejection dynamics they reduce aortic wall shear stresses, thereby reducing the risk of rupture.40 The target mean arterial pressure is < 80 mm Hg, but this must be achieved immediately after diagnosis if it is to prevent rupture.
A DELAYED MANAGEMENT APPROACH WITH AGGRESSIVE BLOOD PRESSURE CONTROL
Rousseau et al compared 28 patients treated by early surgery (< 48 hours) and 29 treated by stent-graft repair with a mortality and paraplegia rate of 21% and 7%, respectively, for surgery compared with no deaths or paraplegia in the stent-graft group.15 However, the 7 patients in this series who were treated with β-blockers and who all had major associated injuries treated surgically before delayed IAI surgery at between 5 and 257 days postinjury also had 0% mortality and paraplegia. Only 2 patients in the stent-graft group were treated within 24 hours, and 21 were treated > 14 days post-IAI. Although the results for stent grafting in terms of mortality and morbidity are impressive, it must be conceded that in many published series the data are not directly comparable, with the surgical patients coming from an older cohort and/or the stent-grafted group including acute and chronic injuries.15,35,38 Stent grafting has undoubtedly benefited from the coincidence of its development with the introduction of selective aggressive blood pressure control allowing initial conservative management.
The concept that IAI should always take priority over other injuries has been proven to be flawed, but some patients do still require urgent intervention. Delayed repair is not appropriate where there is a significant hemothorax or pseudocoarctation syndrome and in patients who have head injuries that require pharmacological elevation of blood pressure to maintain cerebral perfusion. Under these circumstances the thoracic aortic injury should be treated as soon as any significant active hemorrhage is ameliorated.
Outcomes and Complications
A literature search in January 2007 revealed no reports of paraplegia after stent grafting, but most series are small with even the largest not exceeding 30 patients.15 Iliac artery injuries are recognized but are usually treatable without long-term consequences.15 Increasing awareness of the potential for iliac artery damage on the introduction or withdrawal of these large delivery systems has lowered most experienced operators' threshold for moving to direct or conduit access via the common iliac arteries or the distal abdominal aorta in patients with borderline groin or external iliac vessels.
Mortality is generally much lower after stent grafting than open surgery and usually related to other injuries sustained. Several larger series (Rousseau, 29 patients;15 Fattori, 19 patients;38 Dunham, 16 patients;41 Peterson, 11 patients;10 England, 11 patients)42 have reported zero mortality with complete exclusion of the site of injury and no secondary interventions. Although Reed et al had no intraoperative deaths in 13 patients, three deaths (23%) occurred in the postoperative period, but two of these three deaths were due to multiorgan failure.43 It is predictable that some patients will inevitably die from associated injuries and their physiological consequences. Other reported causes of fatal outcomes include cerebrovascular accidents,44 iliac artery complications,43 and stent-graft occlusion of the thoracic aorta due to excessive oversizing, poor wall apposition, and acute proximal graft infolding.45
Stent-Graft Follow-up
Clinical and imaging follow-up is required. The short- and medium-term data (up to 10 years) for stent grafting in IAI is very encouraging (Fig. 7). Despite this, vigilance should be maintained. In an 18-year-old, the device will likely have > 50 years of stress to endure. Fatigue fractures should be sought with annual plain films, at least initially at this frequency. Physicians and their patients are rightly concerned about the long-term risks of irradiation, particularly in the young. This makes annual CT follow-up difficult. MRI is an alternative where MR-compatible devices have been used, although its poorer spatial resolution could lead to a lowered sensitivity for subtle complications and will not detect stent-graft migration. In the vast majority of patients, the injury site is apparently completely healed, or perhaps just sealed, on the early follow-up CTs with a small bulge in the stent graft at the site of the IAI but no thrombus or wall thickening. Under these circumstances there is no potential for endoleak, so is CT follow-up necessary for these patients? Stent-graft migration could potentially occur as a late complication, but at least some of these will be detected if standardized centering and projections are used for the plain films. We currently do not know if there remains a late risk of chronic pseudoaneurysm formation, although intuitively this seems unlikely. CTs every 3 to 5 years for this majority, along with plain films (at least initially annually), would probably give us the natural history data we currently lack. This would detect most complications that may occur at a lower radiation burden. It may fail, however, to detect complications in some individuals. There is no current guidance on follow-up. Each unit treating IAIs must decide where they view the risk-benefit ratio based on current evidence and discuss the follow-up with individual patients to maximize compliance.
Figure 7.
Aortic injury treated by stent grafting. (A) Catheter angiogram at the time of injury. (B) Follow-up computed tomography at 7 years shows no residual abnormality with the usual bulge of the stent graft at the site of previous injury.
Management Strategies
Endovascular repair has clear advantages over open surgery in patients with severe concomitant injuries or medical comorbidities and should be the treatment of choice for the majority.18 Those with hemodynamic instability for which no other cause can be found on CT, evidence of active bleeding, large mediastinal hematomas, or pseudocoarctation require emergency treatment. In the remainder, stent grafting can be delayed until the next working day if other injuries, most commonly head injuries, do not preclude the use of aggressive blood pressure control. Although stent-graft placement has become the primary treatment across all Injury Severity Score profiles in some centers,1 there remains some doubt whether the patient with isolated IAI is better served by emergency thoracotomy or endografting.18 Stent grafts have been placed in contaminated fields following trauma with no adverse short- to medium-term sequelae.46
Chronic aneurysms post untreated IAI can be treated by stent grafting or open surgery (Fig. 8). The short-term gain of decreased morbidity and mortality with stent grafting must, as in the acute situation, be balanced against long-term imaging follow-up and unknown durability.
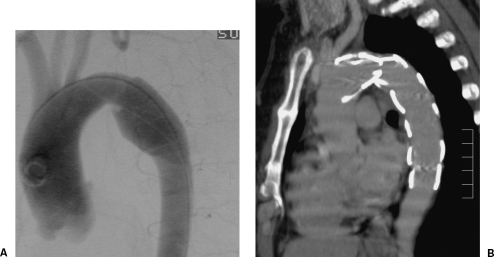
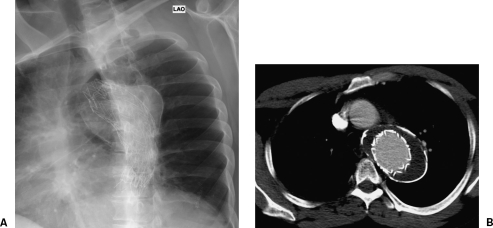
Figure 8.
Chronic (18 years) post-traumatic pseudoaneurysm treated with a W.L. Gore (Flagstaff, AZ) stent graft. (A) Chest x-ray. (B) Six-year follow-up computed tomography shows no endoleak with a stable aneurysm sac. Heavily calcified aneurysm sacs such as this are unlikely to shrink.
GREAT VESSEL INJURIES
The great vessels may be injured by narrow impact (e.g., a horse kick) or wide impact (e.g., deceleration injury [Fig. 9]) blunt trauma. Blunt trauma may result in complete or incomplete vessel transection, dissection (with or without hematoma), and rarely an arteriovenous fistula (AVF). Penetrating injuries are more common than in the thoracic aorta, a large percentage of these being iatrogenic. Gunshot wounds are rarer in most parts of Europe than the United States. Penetrating injuries may result in hemorrhage, false aneurysm, or AVF. When iatrogenic injuries are considered, vessel thrombosis can be added to this list (Fig. 10).
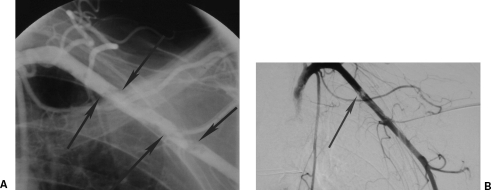
Figure 9.
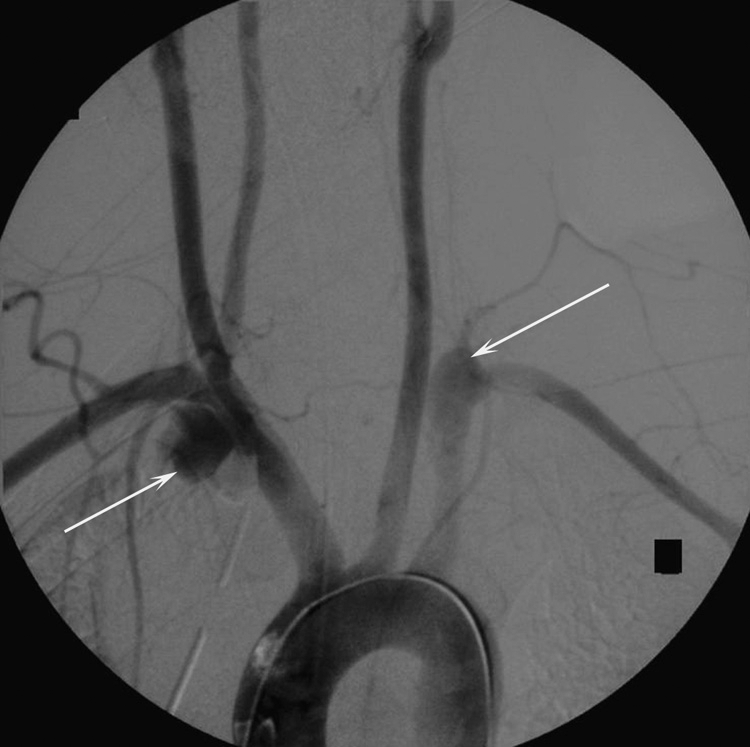
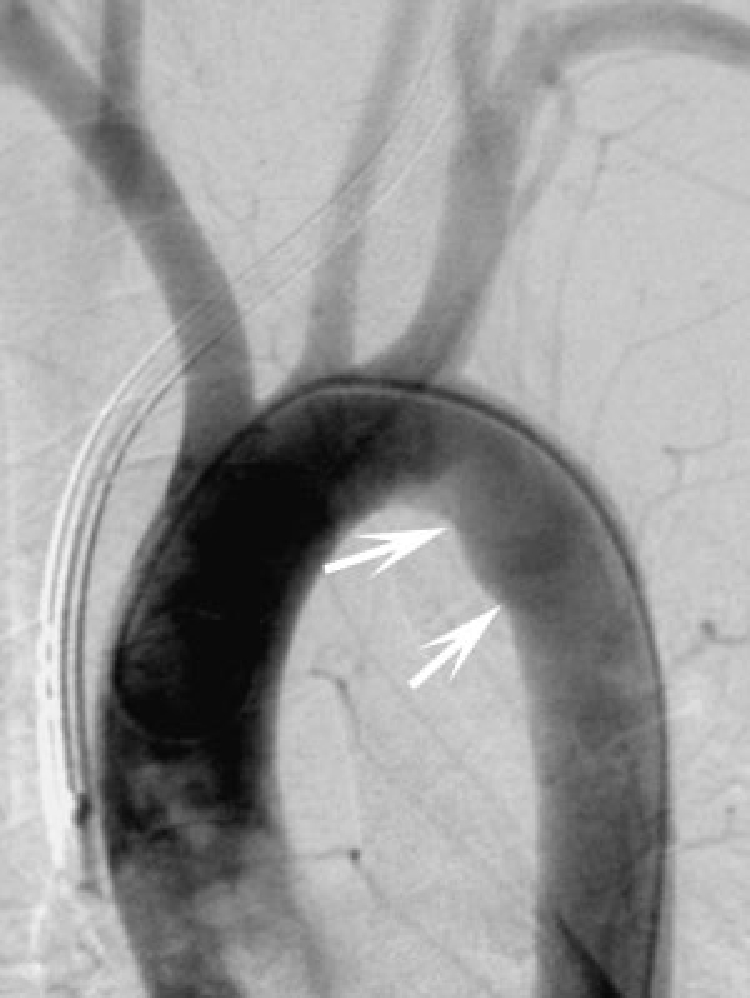
A 22-year-old parachutist suffered extensive polytrauma including bilateral subclavian artery tears shown on aortic arch angiography (arrow) with large hemothoraces after his main parachute failed to deploy and the reserve only partially deployed. The patient died minutes after this angiogram.
Figure 10.
Acute left arm ischemia after a left axillary venous line was misplaced in the left axillary artery. (A) Subclavian angiogram shows the line with surrounding near occlusive thrombus (arrows). (B) Following overnight thrombolysis via the catheter, repeat subclavian angiogram shows a small amount of residual nonocclusive thrombus (arrow). The line was repositioned in the vein using a wire via the proximal side hole and the patient heparinized. Follow-up ultrasound showed no arteriovenous fistula, false aneurysm, or arterial occlusion.
Treatment Options
As with vascular injuries elsewhere in the body, there are three possible approaches to great vessel trauma: balloon occlusion, embolization, and stenting or stent grafting. Depending on the vascular territory, some, or all, of these may be inappropriate. Referring physicians, and where possible their patients, should be aware that the primary intention of acute endovascular treatment is to stop life-threatening hemorrhage or restore perfusion to an at-risk vascular territory. Secondary procedures, including open surgery, may be necessary. Endovascular treatment has the greatest impact when employed early, but unfortunately it does not currently feature early-in-trauma management algorithms.25 When endovascular treatment is delayed it commonly controls the bleeding, but the effects of large-volume transfusions with consequent multiorgan failure may already be established.25 Such deaths should be avoidable with better integration of diagnostic CT and interventional radiology into trauma care pathways. Radiologists should be proactive in their development.
BALLOON OCCLUSION
Manual compression at the site of bleeding or proximal to it can be lifesaving. Unfortunately, many of the great vessels cannot be adequately compressed. These include the subclavian and axillary arteries and the carotid vessels above the angle of the jaw. Balloon occlusion of the subclavian artery was first described in 197347 and allows rapid hemostasis with minimal risk. Further evidence of its effectiveness is provided by the series of six cases of penetrating injuries reported by Scalea.48 In the carotid territory the risk of cerebrovascular accident (from hypoperfusion or embolism) must be considered. In the conscious patient, the immediate response to balloon occlusion may be satisfactory, but late intolerance (> 20 to 30 minutes) is well recognized, particularly if volume replacement is inadequate. The increasing availability of a broad range of on-the-shelf stent grafts means that this is less commonly a clinical problem.
EMBOLIZATION
A sound knowledge of embolic agents and devices and of the arterial anatomy and collateral connections of the territory to be treated is a prerequisite. Embolization is usually restricted to the branches of the subclavian and axillary arteries and the external carotid artery. If particles are used, a secure catheter position and slow injection, with continuous high-resolution screening, is important to avoid proximal reflux and nontarget embolization.49
STENTS AND STENT GRAFTS
Stent grafts were first used to treat trauma in the mid-1990s.50 The initial devices were bare stents that were covered by vein or synthetic graft material by the operators. A wide range of sizes of balloon mounted and self-expanding stent-grafts is now available and should be on-the-shelf products in any center managing major trauma.
Anatomical Considerations
CAROTID ARTERIES
In the carotid territory, angiography is commonly used to assess any wound that penetrates the platysma. It is particularly useful in level III carotid injuries (those between the angle of the mandible and the skull base) that cannot be assessed by ultrasound.
Maras et al reviewed the use of stent grafts to treat internal carotid artery pseudoaneurysms since 1990.51 Twenty patients with penetrating injury or skull base fractures were successfully treated. Publication bias may mean that failed procedures and deaths have not been reported. Marked variability in postprocedure antiplatelet and/or anticoagulation was observed. On follow-up, 15% of the internal carotid arteries (ICAs) had thrombosed (at 1 week, 6 weeks, and 23 months), and one had developed a 50% in stent stenosis at 10 months. It is unclear whether any of these were symptomatic. No patient on aspirin and clopidogrel developed an occlusion. Dual antiplatelet therapy is advised. In the ICA, embolization is reserved for those surgically inaccessible cases (e.g., at the skull base) where a stent graft cannot be advanced and there is an intact circle of Willis.49 In the alternative scenario of a proximal ICA false aneurysm, but with occlusion of the more distal ICA, embolization or common to external carotid stent graft are alternatives to surgical ligation.
The external carotid artery is less commonly injured than the internal carotid or vertebral arteries.52 Craniofacial injuries result in life-threatening injuries in ~1% of cases, most commonly due to Le Fort II or III fractures. In oronasal hemorrhage following trauma, the bleeding will be bilateral in 50% of cases and there will commonly be multiple bleeding points. Bilateral carotid angiography is mandatory for hemorrhage. Gelfoam and coils are the commonest embolic agents, but polyvinyl alcohol particles can be useful. Before any particulate agent is used, any potentially dangerous external to internal carotid anastomoses must be identified.
VERTEBRAL ARTERIES
In vertebral artery hemorrhage, false aneurysm or AVF embolization may be an option, particularly in injuries of parts III or IV. The suitability for embolization depends on a thorough angiographic assessment of the posterior circulation. If the contralateral vertebral artery is absent, hypoplastic, or terminates in the posterior inferior cerebellar artery, occlusion of the vertebral artery will result in a hindbrain stroke (unless there is sufficient posterior communicating artery supply). If the contralateral vertebral and vertebrobasilar system are normal, embolization is a low-risk procedure.53 Coils are the commonest agent because of their controllability and speed of deployment. The usual principle of distal and then proximal embolization applies where vessels are damaged proximally or where there is a neuronal tissue supply by the damaged vessel.
SUBCLAVIAN AND AXILLARY ARTERIES
White et al reported registry data on the use of the Wallgraft (Boston Scientific, Natick, MA) to treat 18 subclavian injuries.54 Half of the patients had acute pseudoaneurysms with the remainder accounted for by perforations/ruptures (5 patients), AVF (2), and dissections (2). The majority of the injuries were iatrogenic. One-year primary patency and exclusion rates were 86% and 90%, respectively. Using a review of the surgical repair literature as an Objective Performance Criteria, complications were much less severe than with surgical repair, with stenoses and occlusions predominating. No surgical bypasses were required at 1 year. The costoclavicular complex will exert extrinsic forces on subclavian stents and stent grafts and may reduce long-term patency rates.
Injury Types
ARTERIAL WALL INJURY
A spectrum of injuries is observed ranging from intimal flaps that may require no active treatment through to long segments of dissection (which often appear as apparent long occlusions). Vascular injuries may be secondary to fractures, particularly of the clavicle and scapula.
Arterial spasm secondary to trauma should not be mistaken for dissection or incomplete transection. The former is usually reversible with the use of vasodilators, but if doubt persists, gentle balloon dilation will distinguish (in the event that this results in active hemorrhage reinflation of the balloon will allow time for definitive treatment). Long vessel occlusions due to dissection can be usually be traversed with a straight-tipped hydrophilic guide wire. A decision needs to be made whether to treat with a bare, usually self-expanding, stent or whether to use a stent graft. The authors practice is to use the latter where there is a large associated hematoma clinically or on imaging (Fig. 11). The presumption is there has been a breach of the main vessel or disruption of side branches. A bare stent is used where there is minimal hematoma.
Figure 11.
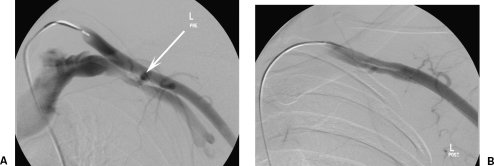
A 55-year-old pedestrian hit by car with acute arm ischemia. The patient was hemodynamically unstable with an expanding shoulder/chest wall hematoma and hemothorax. (A) Arm angiogram shows 10-cm-long occlusion of part III of the subclavian artery and the axillary artery. (B) Angiogram following placement of two overlapping Hemobahn stent grafts (W.L. Gore, Flagstaff, AZ), and balloon dilation of the distal stent graft shows exclusion of the injury. The stent graft was patent at 3 months follow-up.
HEMORRHAGE AND FALSE ANEURYSMS
Hemorrhage from a proximal subclavian or common carotid artery injury requires a thoracotomy to achieve surgical control. Identifying the site of injury can be difficult at open surgery. In 79 patients who underwent thoracotomy for a penetrating injury of the subclavian or axillary artery, Demetraides et al reported a mortality rate of 42% (22% on the table) and an arm amputation rate of 15% (for ischemia or infection).55 It is often much easier to both identify and treat an injury angiographically (Fig. 12). It is worth reiterating that the primary aim of endovascular treatment is damage control (stopping the patient's bleeding) and that secondary procedures may be required in some patients. On occasions, the presence of one injury that requires urgent open repair, such as a brachial plexus injury, may lead to a synchronous open operation for a vascular injury.25 The alternative strategy of controlling blood loss (or restoring perfusion) endovascularly is less stressful for the patient and allows surgical focus on the brachial plexus repair.
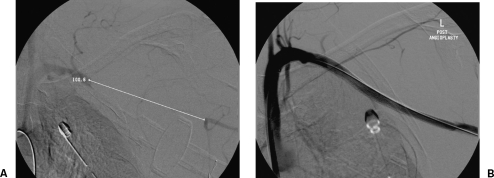
Figure 12.
Low knife wound to the right neck with a large hematoma and initial loss of consciousness. (A) Digital subtraction angiography of the common carotid artery shows a small proximal common carotid pseudoaneurysm with no evidence of dissection. (B) Follow-up carotid angiogram shows exclusion of the false aneurysm by a Wallgraft (Boston Scientific, Natick, MA). (Images courtesy of Dr. David Kessel.)
ARTERIOVENOUS FISTULA
An AVF may be created by blunt or penetrating injury, although the latter is more common. The identification of a traumatic AVF is of utmost importance, not necessarily because all have to be attended to immediately: Some will close spontaneously, and others may be left alone until the patient has recovered from other injuries. The importance of recognizing an AVF lies in the long-term progress of the patient. The window of an AVF tends to enlarge with time, more rapidly in young people. This increases the rate of shunting, which is a square function of the window's diameter.13 Neglected AVFs are not only more difficult to repair but also result in heart failure and irreversible venous stasis changes. Stent grafts are a major addition to the therapeutic armamentarium (Figs. 13 and 14). Surgical repair of great vessel AVFs involve difficult dissections, sometimes in a contaminated field. Where the arterial side of an AVF cannot be accessed or where there is no favorable position for placing a stent graft (e.g., at the origin of the right carotid artery), consideration should be given to sealing the AVF from the venous side with a stent graft.56
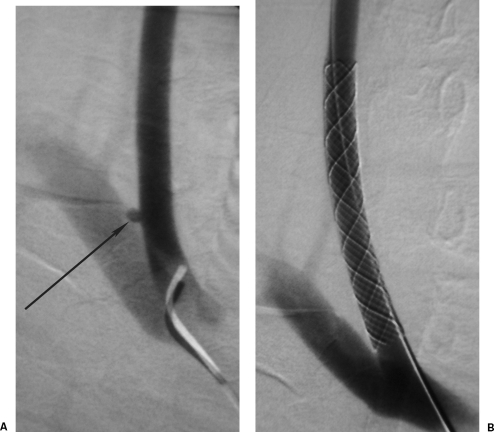
Figure 13.
A 26-year-old club doorman shot by a “pocket” crossbow. (A) Initial computed tomography showed a left axillary artery to vein arteriovenous fistula (AVF) confirmed on angiography. The left arm became markedly swollen. (B) An axillary artery Hemobahn stent graft (Gore, Flagstaff, AZ) occluded the AVF. Contrary to medical advice the patient returned to body building, and the stent graft occluded at 3 months but with minimal arm claudication.
Figure 14.
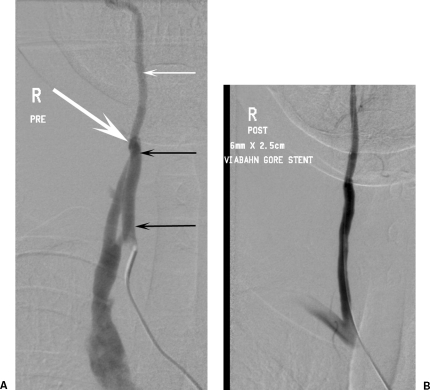
A 46-year-old woman had reconstructive breast surgery as part of the treatment for breast carcinoma. She awoke with a pulsing high-pitched buzz in her right ear. Nine months of sleep disturbance ensued until the association between an abandoned right internal jugular line at the time of surgery and her symptoms was made. (A) Digital subtraction right vertebral artery angiogram shows arteriovenous fistula (AVF) (large arrow). Note the hypertrophy of the vertebral artery proximal to the AVF (small black arrows) relative to the normal distal vertebral artery (small white arrow). (B) Angiogram was repeated after occlusion of the AVF with a 6 mm × 2.5 cm vertebral artery Viabahn stent graft (Gore, Flagstaff, AZ). At 2-year follow-up the stent graft is patent with no in-stent stenosis on duplex ultrasound, and the patient remains asymptomatic.
Central Lines
Misplaced central lines are a recurring problem despite the increased use of both ultrasound and fluoroscopy in the placement of these devices. When lines are inadvertently placed intra-arterially, they may result in hemorrhage or false aneurysms once removed. Dissection or vessel thrombosis may be the result of the insertion (Fig. 10). Fortunately, this is one area where the advantages of endovascular management are widely accepted. The IR should usually be consulted when the problem is first recognized (and before the line is removed). As with conventional trauma, stent grafts are an option, but the presence of a line with a guide-wire lumen provides an alternative approach. Arterial closure devices, such as the Angio-Seal (St. Jude Medical, St. Paul, MN) can seal the arterial puncture site. It is possible to close holes larger than the target size of the device, provided there is the ability to use supplementary balloon tamponade and stent grafts as bailout at that site (Fig. 15). In the carotid territory, the risk of emboli from the intra-arterial component would be a concern. It is not known whether a stent graft may carry less, the same, or more risk.
Figure 15.
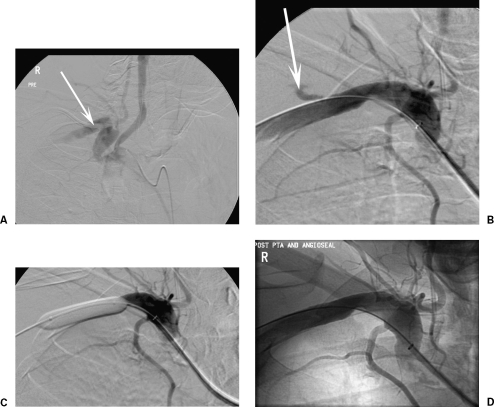
(A) An 11.5F dialysis line is misplaced in the left subclavian artery. Note the distortion of the artery. (B) The line was replaced by an 8F Angio-Seal (St. Jude Medical, St. Paul, MN) with a small leak (arrow) seen. (C) Balloon dilation across the leak (5 minutes). (D) No residual abnormality.
Conclusion
Improvements in car safety and out-of-hospital emergency care are likely to result in more patients reaching the hospital alive. Diagnosing IAI and great vessel injuries requires a high index of suspicion, rapid use of high-quality CT imaging as first-line imaging, and an integrated multidisciplinary approach to care. Endovascular treatment is lifesaving with lower mortality and morbidity than conventional surgery, but its long-term durability is uncertain, and some patients will require secondary procedures. Early endovascular expertise should be available to all patients in major trauma centers.
REFERENCES
- Lebl D R, Dicker R A, Spain D A, Brundage S I. Dramatic shift in the primary management of traumatic thoracic aortic rupture. Arch Surg. 2006;141:177–180. doi: 10.1001/archsurg.141.2.177. [DOI] [PubMed] [Google Scholar]
- Macura K J, Corl F M, Fishman E K, Bleumke D A. Pathogenesis in acute aortic syndromes: aortic aneurysm leak and rupture and traumatic aortic transaction. AJR Am J Roentgenol. 2003;181:303–397. doi: 10.2214/ajr.181.2.1810303. [DOI] [PubMed] [Google Scholar]
- Lundevall J. The mechanism of traumatic rupture of the aorta. Acta Pathol Microbiol Scand. 1964;62:34–46. doi: 10.1111/apm.1964.62.1.34. [DOI] [PubMed] [Google Scholar]
- Williams J S, Graff J A, Uku J M, Steinig J P. Aortic injury in vehicular trauma. Ann Thorac Surg. 1994;57:726–730. doi: 10.1016/0003-4975(94)90576-2. [DOI] [PubMed] [Google Scholar]
- Shkrum M J, McClafferty K J, Green R N, Nowak E S, Young J G. Mechanisms of aortic injury in fatalities occurring in motor vehicle accidents. J Forensic Sci. 1999;44:44–56. [PubMed] [Google Scholar]
- Parmley L F, Mattingly T W, Manion W C, Jahnke E J. Nonpenetrating traumatic injury of the aorta. Circulation. 1958;17:1086–1101. doi: 10.1161/01.cir.17.6.1086. [DOI] [PubMed] [Google Scholar]
- Ahrar K, Smith D C, Bansal R C, Razzouk A, Catalano R D. Angiography in blunt thoracic aortic injury. J Trauma. 1997;42:665–669. doi: 10.1097/00005373-199704000-00014. [DOI] [PubMed] [Google Scholar]
- Hunt J P, Baker C C, Lentz C W. Thoracic aortic injuries: management and outcome of 144 patients. J Trauma. 1996;40:547–556. doi: 10.1097/00005373-199604000-00005. [DOI] [PubMed] [Google Scholar]
- Guvendik L, Davis N R, Starr A. Repair of traumatic aortic dissection: a management protocol and review of twenty-one patients. Thorac Cardiovasc Surg. 1988;36:198–201. doi: 10.1055/s-2007-1020077. [DOI] [PubMed] [Google Scholar]
- Peterson B G, Matsumura J S, Morasch M D, West M A, Eskandari M K. Percutaneous endovascular repair of blunt thoracic aortic transection. J Trauma. 2005;59:1062–1065. doi: 10.1097/01.ta.0000188634.72008.d5. [DOI] [PubMed] [Google Scholar]
- Crass J R, Cohen A M, Motta A O, Tomashefski J F, Wiesen E J. A proposed new mechanism of traumatic aortic injury: the osseous pinch. Radiology. 1990;176:645–649. doi: 10.1148/radiology.176.3.2389022. [DOI] [PubMed] [Google Scholar]
- Javadpour H, O'Toole J J, McEniff J N, Luke D A, Young V K. Traumatic aortic transection: evidence for the osseous pinch mechanism. Ann Thorac Surg. 2002;73:951–953. doi: 10.1016/s0003-4975(01)03184-8. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem Y. In: Ben-Menachem Y, editor. Angiography in Trauma: A Work Atlas. Philadelphia: WB Saunders; 1981. The mechanism of injury. pp. 25–47.
- Wicky S, Capasso P, Meuli R, Fisher A, von-Segesser L, Schnyder P. Spiral CT aortography: an efficient technique for the diagnosis of traumatic aortic injury. Eur Radiol. 1998;8:828–833. doi: 10.1007/s003300050480. [DOI] [PubMed] [Google Scholar]
- Rousseau H, Dambtin C, Marcheix B, et al. Acute traumatic aortic rupture: a comparison of surgical and stent-graft repair. J Thorac Cardiovasc Surg. 2005;129:1050–1055. doi: 10.1016/j.jtcvs.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Braatz T, Mirvis S E, Killeen K, Lightman N I. CT diagnosis of internal mammary artery injury caused by blunt trauma. Clin Radiol. 2001;56:120–123. doi: 10.1053/crad.2000.0572. [DOI] [PubMed] [Google Scholar]
- Nicholson A A, Patel J V. In: Adam A, Dixon AK, Grainger RG, Allison DJ, editor. Grainger & Allison's Diagnostic Radiology. 5th ed. Oxford: Churchill Livingstone; 2007. Air-space diseases. In press.
- Nzewi O, Slight R D, Zamvar V. Management of blunt aortic injury. Eur J Vasc Endovasc Surg. 2006;31:18–27. doi: 10.1016/j.ejvs.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Woodring J H. The normal mediastinum in blunt traumatic rupture of the thoracic aorta and brachiocephalic arteries. J Emerg Med. 1990;8:467–476. doi: 10.1016/0736-4679(90)90178-x. [DOI] [PubMed] [Google Scholar]
- Wintermark M, Wicky S, Schnyder P. Imaging of acute traumatic injuries of the thoracic aorta. Eur Radiol. 2002;12:431–442. doi: 10.1007/s003300100971. [DOI] [PubMed] [Google Scholar]
- Trupka A, Waydas C, Hallfeldt K KJ, Nast-Kolb D, Pfeifer K J, Schweiberer L. Value of thoracic computed tomography in the first assessment of severely injured patients with blunt chest trauma: results of a prospective study. J Trauma. 1997;43:405–412. doi: 10.1097/00005373-199709000-00003. [DOI] [PubMed] [Google Scholar]
- Downing S W, Sperling J S, Mirvis S E, et al. Experience with spiral computed tomography as the sole diagnostic method for traumatic aortic rupture. Ann Thorac Surg. 2001;72:495–501. doi: 10.1016/s0003-4975(01)02827-2. [DOI] [PubMed] [Google Scholar]
- Uflacker R, Phillips G, Selby J B. Intravascular sonography in the assessment of traumatic injury of the thoracic aorta. AJR Am J Roentgenol. 1999;173:665–670. doi: 10.2214/ajr.173.3.10470899. [DOI] [PubMed] [Google Scholar]
- Cleverley J R, Barrie J R, Raymond G S, Primack S L, Mayo J R. Direct findings of aortic injury on contrast-enhanced CT in surgically proven traumatic aortic injury: a multi-centre review. Clin Radiol. 2002;57:281–286. doi: 10.1053/crad.2001.0908. [DOI] [PubMed] [Google Scholar]
- Parker M S, Matheson T L, Rao A V, et al. Making the transition: the role of helical CT in the evaluation of potentially acute thoracic aortic injuries. AJR Am J Roentgenol. 2001;176:1267–1272. doi: 10.2214/ajr.176.5.1761267. [DOI] [PubMed] [Google Scholar]
- Nicholson A A. Vascular radiology in trauma. Cardiovasc Intervent Radiol. 2004;27:105–120. doi: 10.1007/s00270-003-0031-z. [DOI] [PubMed] [Google Scholar]
- Hirose H, Inderjit S, Malangoni M. Nonoperative management of traumatic aortic injury. J Trauma. 2006;60:597–601. doi: 10.1097/01.ta.0000205044.99771.44. [DOI] [PubMed] [Google Scholar]
- Kasirajan K, Heffernan D, Langfield M. Acute thoracic aortic trauma: a comparison of endoluminal stent grafts with open repair and non-operative management. Ann Vasc Surg. 2003;17:589–595. doi: 10.1007/s10016-003-0066-2. [DOI] [PubMed] [Google Scholar]
- Sweeney M S, Young D J, Frazier O H, Adams P R, Kapusta M O, Macris M P. Traumatic aortic transactions: eight year experience with clamp-sew technique. Ann Thorac Surg. 1997;64:384–387. doi: 10.1016/S0003-4975(97)00561-4. [DOI] [PubMed] [Google Scholar]
- von Oppell U O, Dunne T T, De Groot M K, Zilla P. Traumatic aortic rupture: twenty year meta-analysis of mortality and risk for paraplegia. Ann Thorac Surg. 1994;58:585–593. doi: 10.1016/0003-4975(94)92270-5. [DOI] [PubMed] [Google Scholar]
- Katz N M, Blackstone E H, Kirklin J W, Karp R B. Incremental risk factors for spinal cord injury following operation for acute traumatic aortic transection. J Thorac Cardiovasc Surg. 1981;81:669–674. [PubMed] [Google Scholar]
- Degiannis E, Boffard K. Critical decisions in trauma of the thoracic aorta. Injury. 2002;33:317–322. doi: 10.1016/s0020-1383(01)00207-8. [DOI] [PubMed] [Google Scholar]
- Marvasti M A, Meyer J A, Ford B E, Parker F B., Jr Spinal cord ischaemia following operation for traumatic aortic transaction. Ann Thorac Surg. 1986;42:425–428. doi: 10.1016/s0003-4975(10)60550-4. [DOI] [PubMed] [Google Scholar]
- Kato N, Dake M D, Semba C P, et al. Traumatic thoracic aortic aneurysm: treatment with endovascular stent-grafts. Radiology. 1997;205:657–662. doi: 10.1148/radiology.205.3.9393517. [DOI] [PubMed] [Google Scholar]
- Ott M C, Stewart T C, Lawlor D K, Gray D K, Forbes T L. The management of blunt thoracic aortic injuries: endovascular stents versus open repair. J Trauma. 2004;56:565–570. doi: 10.1097/01.ta.0000114061.69699.a3. [DOI] [PubMed] [Google Scholar]
- Endovascular stent-graft placement in thoracic aortic aneurysms and dissections. London: National Institute for Health and Clinical Excellence; June 2005. ISBN 1-84629-038-4.
- Wellons E D, Milner R, Solis M, Levitt A, Rosenthal D. Stent-graft repair of traumatic thoracic aortic disruptions. J Vasc Surg. 2004;40:1095–1100. doi: 10.1016/j.jvs.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Fattori R, Napoli G, Lovato L, et al. Indications for, timing of and results of catheter-based treatment of traumatic injury to the aorta. AJR Am J Roentgenol. 2002;179:603–609. doi: 10.2214/ajr.179.3.1790603. [DOI] [PubMed] [Google Scholar]
- Pate J W, Fabian T C, Walker W. Traumatic rupture of the aortic isthmus: an emergency? World J Surg. 1995;19:119–126. doi: 10.1007/BF00316994. [DOI] [PubMed] [Google Scholar]
- Rousseau H, Suola P, Perreault P, et al. Delayed treatment of traumatic rupture of the thoracic aorta with endoluminal covered stent. Circulation. 1999;99:498–504. doi: 10.1161/01.cir.99.4.498. [DOI] [PubMed] [Google Scholar]
- Dunham M B, Zygun D, Petrasek P, Kortbeek J B, Karmy-Jones R, Moore R D. Endovascular stent grafts for acute blunt aortic injury. J Trauma. 2004;56:1173–1178. doi: 10.1097/01.ta.0000123039.92225.e5. [DOI] [PubMed] [Google Scholar]
- England A, McPherson S J, Butterfield J S, Ashleigh R J. Endovascular repair following traumatic aortic transaction: experience from two centres. UK Radiological Congress; Birmingham, United Kingdom; June 2006.
- Reed A B, Thompson J K, Crafton C J, Delvecchio C, Giglia J S. Timing of endovascular repair of blunt traumatic aortic transactions. J Vasc Surg. 2006;43:684–688. doi: 10.1016/j.jvs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Orford V P, Atkinson N R, Thomson K, et al. Blunt traumatic aortic transaction: endovascular experience. Ann Thorac Surg. 2003;75:106–111. doi: 10.1016/s0003-4975(02)04331-x. [DOI] [PubMed] [Google Scholar]
- Idu M M, Reekers J A, Balm R, Ponsen K J, de Mol B A, Legemate D A. Collapse of a stent-graft following treatment of a traumatic thoracic aortic rupture. J Endovasc Ther. 2005;12:503–507. doi: 10.1583/04-1515R.1. [DOI] [PubMed] [Google Scholar]
- Kramer S, Pamler R, Siefarth H, Brams H-J, Sunder-Plassman L, Gorich J. Endovascular grafting of traumatic aortic aneurysms in contaminated fields. J Endovasc Ther. 2001;8:262–267. doi: 10.1177/152660280100800305. [DOI] [PubMed] [Google Scholar]
- Doty D B, Kongtahworn C, Ehrenhaft J L. Control of proximal subclavian artery injury with internal shunt catheter. Ann Thorac Surg. 1973;15:285–286. doi: 10.1016/s0003-4975(10)65299-x. [DOI] [PubMed] [Google Scholar]
- Scalea T M, Sclafani S J. Angiographically placed balloons for arterial control: a description of a technique. J Trauma. 1991;31:1671–1677. doi: 10.1097/00005373-199112000-00018. [DOI] [PubMed] [Google Scholar]
- Kidney D D. In: Dyett JF, Ettles DF, Nicholson AA, Wilson SE, editor. Textbook of Endovascular Procedures. Philadelphia: Churchill Livingstone; 2000. The endovascular approach to trauma. pp. 313–327.
- Marin M L, Veith F J, Cynamon J, et al. Transfemoral endoluminal repair of a penetrating vascular injury. J Vasc Interv Radiol. 1994;5:592–594. doi: 10.1016/s1051-0443(94)71559-1. [DOI] [PubMed] [Google Scholar]
- Maras D, Lioupis C, Magoufis G, Tsamopoulos N, Moulakais K, Andrikopoulos V. Covered stent-graft treatments of traumatic internal carotid artery pseudoaneurysms: a review. Cardiovasc Intervent Radiol. 2006;29:958–968. doi: 10.1007/s00270-005-0367-7. [DOI] [PubMed] [Google Scholar]
- Smith T P. Embolization in the external carotid artery. J Vasc Interv Radiol. 2006;17:1897–1913. doi: 10.1097/01.RVI.0000247301.64269.27. [DOI] [PubMed] [Google Scholar]
- Yee L F, Olcott E W, Knudson M M, et al. Extraluminal, transluminal and observational treatment for vertebral artery injuries. J Trauma. 1995;39:480–486. doi: 10.1097/00005373-199509000-00014. [DOI] [PubMed] [Google Scholar]
- White R, Krajcer Z, Johnson M, Williams D, Bacharach M, O'Malley E. Results of a multicenter trial for the treatment of traumatic vascular injury with a covered stent. J Trauma. 2006;60:1189–1196. doi: 10.1097/01.ta.0000220372.85575.e2. [DOI] [PubMed] [Google Scholar]
- Demetriades D, Chahwan S, Gomez H, et al. Penetrating injuries of the subclavian and axillary arteries. J Am Coll Surg. 1999;188:290–295. doi: 10.1016/s1072-7515(98)00289-0. [DOI] [PubMed] [Google Scholar]
- Cronin P, McPherson S J, Meaney J F, Mavor A. Venous covered stent: successful occlusion of a symptomatic internal iliac arteriovenous fistula. Cardiovasc Intervent Radiol. 2002;25:323–325. doi: 10.1007/s00270-001-0116-5. [DOI] [PubMed] [Google Scholar]