Abstract
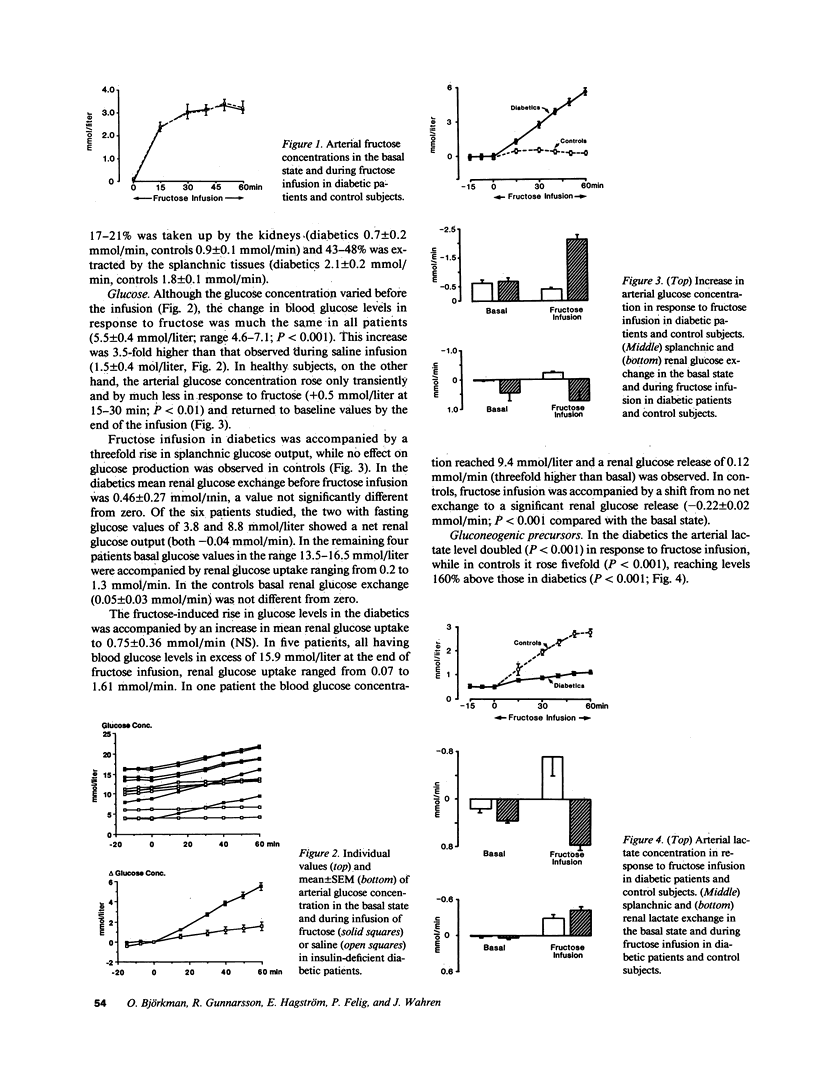
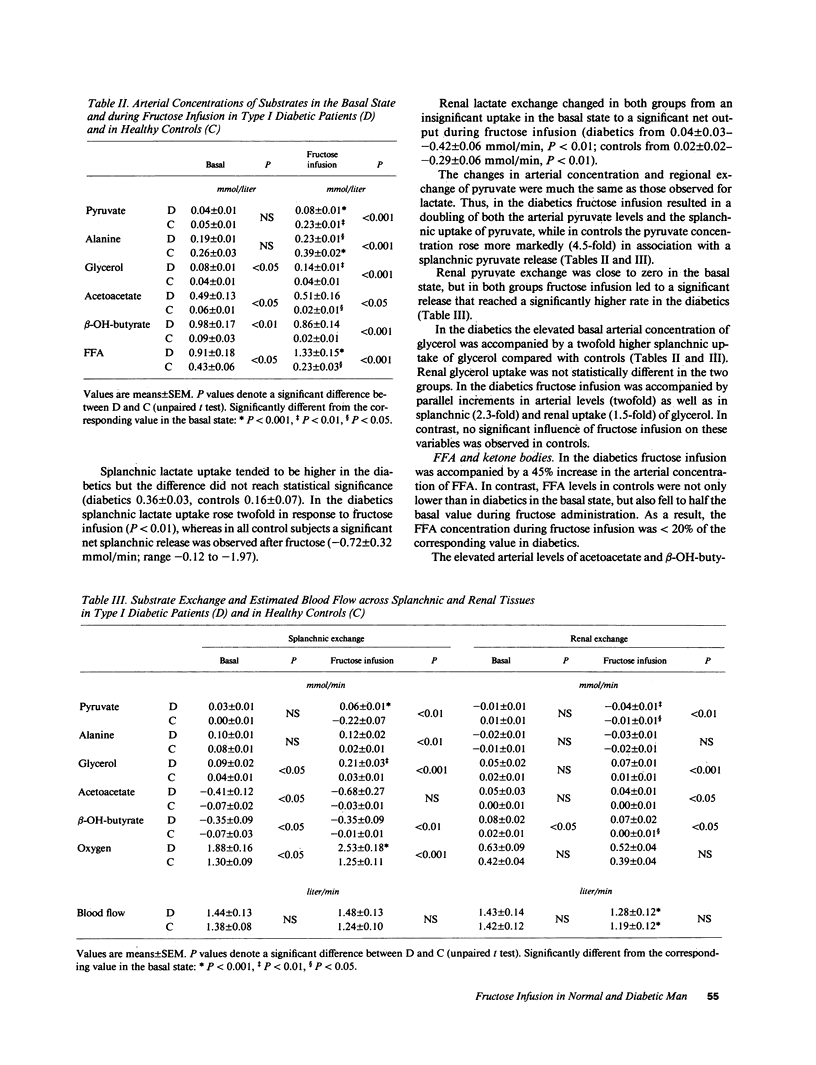
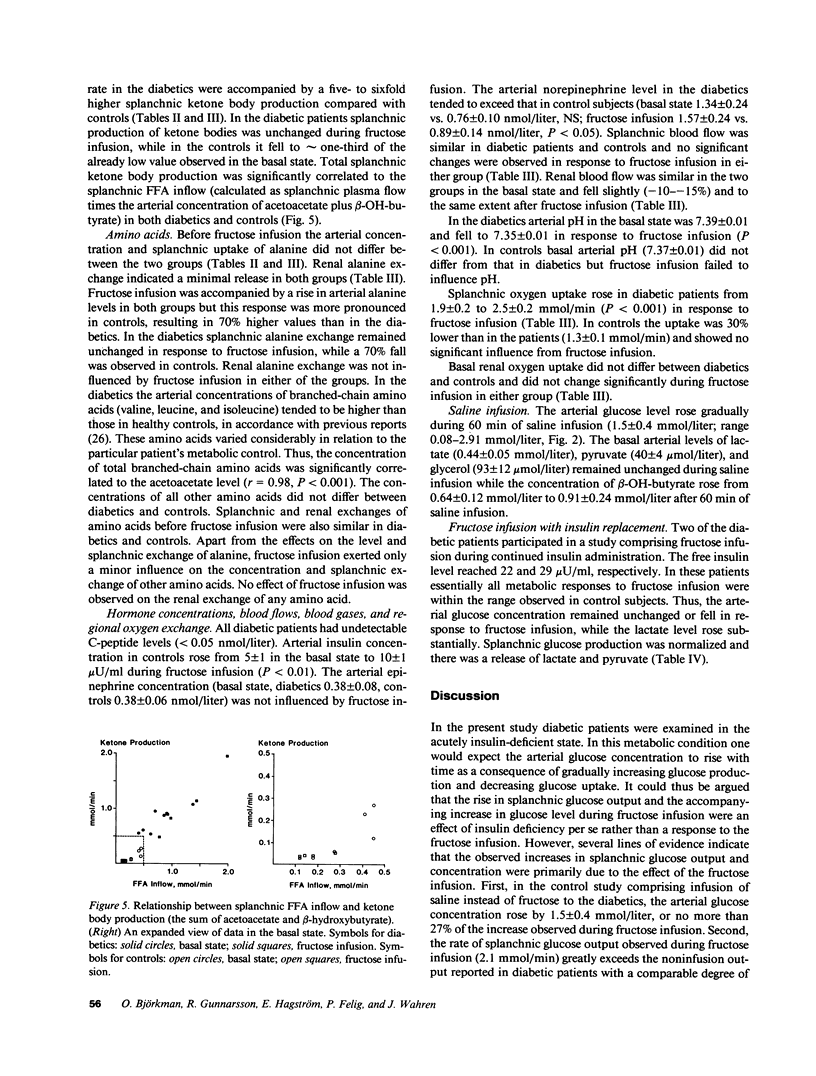
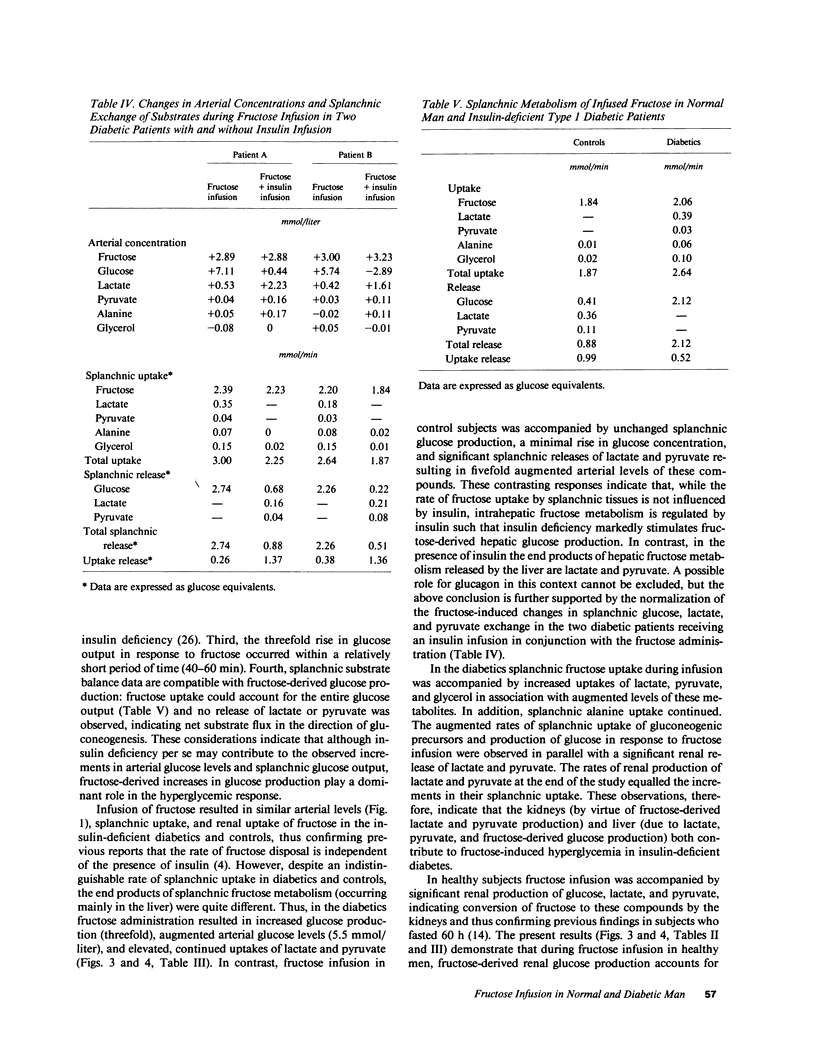
Fructose raises blood glucose and lactate levels in normal as well as diabetic man, but the tissue origin (liver and/or kidney) of these responses and the role of insulin in determining the end products of fructose metabolism have not been fully established. Splanchnic and renal substrate exchange was therefore examined during intravenous infusion of fructose or saline in six insulin-deficient type I diabetics who fasted overnight and in five healthy controls. Fructose infusion resulted in similar arterial concentrations and regional uptake of fructose in the two groups. Splanchnic glucose output increased threefold in the diabetics but remained unchanged in controls in response to fructose infusion, and the arterial glucose concentration rose more in diabetics (+5.5 mmol/liter) than in controls (+0.5 mmol/liter). Splanchnic uptake of both lactate and pyruvate increased twofold in response to fructose infusion in the diabetics. In contrast, a consistent splanchnic release of both lactate and pyruvate was seen during fructose infusion in controls. In diabetics fructose-induced hyperglycemia was associated with no net renal glucose exchange, while there was a significant renal glucose production during fructose infusion in the controls. In both groups fructose infusion resulted in renal output of lactate and pyruvate. In the diabetics this release corresponded to the augmented uptake by splanchnic tissues. In two diabetic patients given insulin infusion, all responses to fructose infusion were normalized. Fructose infusion in diabetics did not influence either splanchnic ketone body production or its relationship to splanchnic FFA inflow. We conclude that in insulin-deficient, mildly ketotic type I diabetes, (a) both the liver, by virtue of lactate, pyruvate, and fructose-derived gluconeogenesis, and the kidneys , by virtue of fructose-derived lactate and pyruvate production, contribute to fructose-induced hyperglycemia; (b) outcome of hepatic fructose metabolism; and (c) fructose does not exert an antiketogenic effect. These data suggest that while total fructose metabolism is not altered in diabetics, intermediary hepatic fructose metabolism is dependent on the presence of insulin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUN C. A rapid method for the determination of para-aminohippuric acid in kidney function tests. J Lab Clin Med. 1951 Jun;37(6):955–958. [PubMed] [Google Scholar]
- Björkman O., Felig P. Role of the kidney in the metabolism of fructose in 60-hour fasted humans. Diabetes. 1982 Jun;31(6 Pt 1):516–520. doi: 10.2337/diab.31.6.516. [DOI] [PubMed] [Google Scholar]
- CRAIG J. W., DRUCKER W. R., MILLER M., OWENS J. E., WOODWARD H., Jr, BROFMAN B., PRITCHARD W. H. Metabolism of fructose by the liver of diabetic and nondiabetic subjects. Proc Soc Exp Biol Med. 1951 Dec;78(3):698–702. doi: 10.3181/00379727-78-19186. [DOI] [PubMed] [Google Scholar]
- DARRAGH J. H., WOMERSLEY R. A., MERONEY W. H. Fructose in the treatment of diabetic ketosis. J Clin Invest. 1953 Dec;32(12):1214–1221. doi: 10.1172/JCI102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze G., Wicklmayr M., Grunst J., Stiegler S., Mehert H. Die Verwertung von Glukose und Fruktose in Leber und Muskel beim Menschen. Int Z Vitam Ernahrungsforsch Beih. 1976;15:31–43. [PubMed] [Google Scholar]
- Dietze G., Wicklmayr M., Mehnert H. Antiketogenic action of fructose in man. Diabetes. 1978 Jul;27(7):709–714. doi: 10.2337/diab.27.7.709. [DOI] [PubMed] [Google Scholar]
- Elliott W. C., Cohen L. S., Klein M. D., Lane F. J., Gorlin R. Effects of rapid fructose infusion in man. J Appl Physiol. 1967 Dec;23(6):865–869. doi: 10.1152/jappl.1967.23.6.865. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L. A gas chromatographic method for the determination of individual free fatty acids in plasma. Clin Chim Acta. 1966 Feb;13(2):266–268. doi: 10.1016/0009-8981(66)90304-4. [DOI] [PubMed] [Google Scholar]
- Heinz F., Schlegel F., Krause P. H. Enzymes of fructose metabolism in human kidney. Enzyme. 1975;19(2):85–92. doi: 10.1159/000458977. [DOI] [PubMed] [Google Scholar]
- MENDELOFF A. I., WEICHSELBAUM T. E. Role of the human liver in the assimilation of intravenously administered fructose. Metabolism. 1953 Sep;2(5):450–458. [PubMed] [Google Scholar]
- MILLER M., CRAIG J. W., DRUCKER W. R., WOODWARD H., Jr The metabolism of fructose in man. Yale J Biol Med. 1956 Dec;29(3):335–360. [PMC free article] [PubMed] [Google Scholar]
- Metz R., Mako M., Stevens T., Franklin J. The metabolism of fructose in diabetes mellitus. J Lab Clin Med. 1967 Mar;69(3):494–503. [PubMed] [Google Scholar]
- Nilsson L. H., Hultman E. Liver and muscle glycogen in man after glucose and fructose infusion. Scand J Clin Lab Invest. 1974 Feb;33(1):5–10. doi: 10.3109/00365517409114190. [DOI] [PubMed] [Google Scholar]
- ROWELL L. B., BLACKMON J. R., BRUCE R. A. INDOCYANINE GREEN CLEARANCE AND ESTIMATED HEPATIC BLOOD FLOW DURING MILD TO MAXIMAL EXERCISE IN UPRIGHT MAN. J Clin Invest. 1964 Aug;43:1677–1690. doi: 10.1172/JCI105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH L. H., Jr, ETTINGER R. H., SELIGSON D. A comparison of the metabolism of fructose and glucose in hepatic disease and diabetes mellitus. J Clin Invest. 1953 Apr;32(4):273–282. doi: 10.1172/JCI102736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYGSTRUP N., WINKLER K., LUNDQUIST F. THE MECHANISM OF THE FRUCTOSE EFFECT ON THE ETHANOL METABOLISM OF THE HUMAN LIVER. J Clin Invest. 1965 May;44:817–830. doi: 10.1172/JCI105194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITALLIE T. B., MORGAN M. C., CATHCART R. T., LEDUC G. G., DOTTI L. B. Peripheral assimilation of fructose in man. Proc Soc Exp Biol Med. 1953 Dec;84(3):713–715. doi: 10.3181/00379727-84-20762. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Felig P., Cerasi E., Luft R. Splanchnic and peripheral glucose and amino acid metabolism in diabetes mellitus. J Clin Invest. 1972 Jul;51(7):1870–1878. doi: 10.1172/JCI106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe B. M., Ahuja S. P., Marliss E. B. Effects of intravenously administered fructose and glucose on splanchnic amino acid and carbohydrate metabolism in hypertriglyceridemic men. J Clin Invest. 1975 Oct;56(4):970–977. doi: 10.1172/JCI108177. [DOI] [PMC free article] [PubMed] [Google Scholar]



