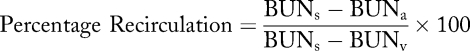ABSTRACT
A mature, functional arteriovenous (AV) access is the lifeline for a hemodialysis (HD) patient as it provides sufficient enough blood flow for adequate dialysis. As the chronic kidney disease (CKD) and end-stage renal disease (ESRD) population is expanding, and because of the well-recognized hazardous complications of dialysis catheters, the projected placement and use of AV accesses for HD is on the rise. Although a superior access than catheters, AV accesses are not without complications. The primary complication that causes AV accesses to fail is stenosis with subsequent thrombosis. Surveying for stenosis can be performed in a variety of ways. Clinical monitoring, measuring flow, determining pressure, and measuring recirculation are all methods that show promise. In addition, stenosis can be directly visualized, through noninvasive techniques such as color duplex imaging, or through minimally invasive venography. Each method of screening has its advantages and disadvantages, and several studies exist which attempt to answer the question of which test is the most useful. Ultimately, to maintain the functionality of the access for the HD patient, a team approach becomes imperative. The collaboration and cooperation of the patient, nephrologist, dialysis nurse and technician, vascular access coordinator, interventionalist, and vascular surgeon is necessary to preserve this lifeline.
Keywords: Hemodialysis, vascular access, surveillance, arteriovenous fistula, arteriovenous graft
The incidence and prevalence of chronic kidney disease (CKD) and end-stage renal disease (ESRD) in the United States (US) continues to increase.1 The incidence of peritoneal dialysis patients has decreased since 2004, while the hemodialysis (HD) population continues to grow.1 In addition, different manners of executing hemodialysis continue to be explored, from in-center methods to those performed at home, such as short daily HD or home nocturnal HD. To achieve effective and adequate HD, blood flow needs to be accessed via either a dialysis central venous catheter or preferably via a permanent hemodialysis arteriovenous (AV) access, such as an AV graft (AVG) or an AV fistula (AVF). An AVF is an artery surgically connected to a vein, while an AVG is a surgically placed conduit of synthetic material connecting an artery to a vein. A mature AV access should be able to deliver a high enough dialysis blood flow (≥300 mL/min) to provide for an adequate dialysis treatment. Primarily because of the infectious complications and increase in mortality associated with catheters,2,3 AVG or AVF are the preferable choice of access. AVF, once mature, are more desirable than AVG, mainly because grafts promote neointimal hyperplasia which can precipitate eventual stenosis.4 Because AVF provide adequate blood flow and have the fewest complications, the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (KDOQI)5 and the Fistula First initiative6 have developed guidelines in attempts to increase the placement and use of AVF for prevalent and incident HD patients to 50 and 60%, respectively, in the United States.5
Despite the fact that AVFs have the least complications and are the preferred method of dialysis access, all permanent accesses are subject to complications associated with low flow, such as failure of maturation, venous stenosis, and thrombosis. On the other hand, states of high flow can result in high output heart failure.7 The complications of flow, plus the infections associated with the access, limb ischemia, and aneurysms, make access-related issues the most common cause of hospitalization for the dialysis patient.5
Because of the measures and guidelines created with the goal of increasing fistula use, and because of the greater prevalence of CKD and ESRD, appropriate surveillance for abnormalities of the dialysis patient's vascular access becomes essential. Currently, several methods of surveillance exist. Some are performed while the patient is not on dialysis, such as changes in physical exam findings or ultrasonography. Others require the patient to be on dialysis, and require specialized equipment or a trained staff, such as measuring access recirculation, flow, or venous pressures.
Ultimately, for a surveillance technique to be successful, it must demonstrate detection of a lesion that would promote failure of the access. Somewhat arbitrarily, stenotic lesions of >50% of the access diameter trigger the need for intervention. Failure of the access is due to thrombosis in up to 80% of the cases,8,9 which is typically a result of neointimal hyperplasia at the venous outflow site causing a stenosis,10 and is found more often in AVG4,11. Thrombosis can also occur without any apparent stenosis, and is thought to be from hypercoagulability, congestive heart failure, hypotension, external compression, or improper cannulation. Available surveillance techniques have therefore been focused on finding stenosis, as this is the most common cause of thrombosis and has an available treatment (angioplasty). The success of this treatment for maintaining long-term access patency and access longevity, however, continues to be questioned and studied.
SURVEILLANCE TECHNIQUES
Clinical Monitoring
The physical examination, if successful and validated for objectively determining stenosis, would be the most valuable surveillance tool, as it is not expensive or time consuming, does not require a specialized staff, does not necessitate sensors or machinery, can be performed at any time, and is noninvasive. Indeed, because of these advantages, it is becoming a valuable component to detect stenosis.
Evaluation of the access requires a systematic approach, as reviewed by Beathard.11 This approach is necessary so that others besides experienced physical diagnosticians can evaluate abnormalities in the access. Inspection, palpation, and auscultation all should be used in conjunction and in a standard fashion. Inspection of the arm, shoulder, breast, neck, and face for edema and the presence of any collateral circulation should be performed first. Palpation of the access directly as well as the anastomosis up to the chest wall is then performed. The pulse and thrill should be assessed for characteristics of intensity and consistency. Two tests should then be performed. The pulse augmentation test evaluates the inflow segment, and is done by occluding the access completely by palpation several centimeters beyond the arterial anastomosis. The test is normal when the portion of the access upstream from the palpation (occlusion) demonstrates augmentation of the pulse. The arm elevation test evaluates the outflow tract, and is done by elevating the arm with the access above the level of the heart and examining the normal collapse of the access. If it fails to collapse or remain plump after elevation, this may signify a stenosis. Finally, auscultation of the access and all areas palpated is necessary, as the character of the bruit or a change in the bruit over time may indicate stenosis.
In addition to physical examination findings, physical characteristics of the access while the patient is on dialysis can be suggestive of access stenosis.4 Prolonged bleeding (>20 minutes) from the needle sites after hemodialysis is finished may represent an underlying outflow stenosis, as the flow will be directed to the area of least resistance. However, prolonged bleeding is not specific for a stenosis as an underlying coagulopathy or therapeutic anticoagulants may contribute as well. Difficulty cannulating the access, especially in one that has been repeatedly cannulated successfully, may indicate stenosis or thrombosis. Recurrent clotting (at least twice per month) may be another sign. Lastly, an unexplained change in the dialysis adequacy measured by urea reduction (URR) or Kt/V in patients on a stable prescription may be another signal of stenosis as the blood flow necessary to provide adequate clearance is diminished. Although these clinical indicators are helpful, they typically are late manifestations of access stenosis. However, the combination of a systematic physical examination and evaluating the characteristics of the access before, during, and after dialysis remain a successful and inexpensive method to determine access dysfunction.
Recirculation
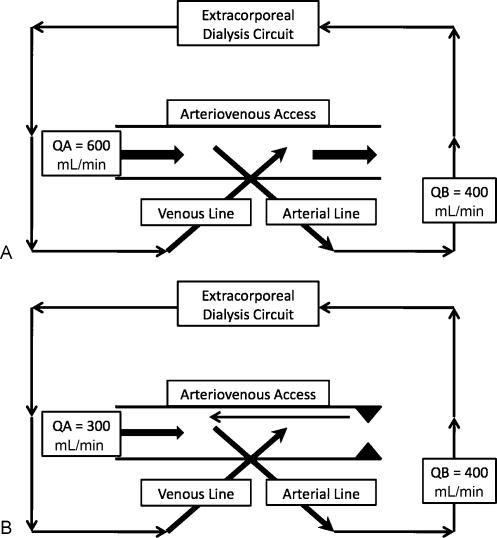
Access recirculation, in the simplest sense, refers to dialyzing blood that has already been dialyzed, by way of blood from the extracorporeal circuit reentering the same circuit (Fig. 1). This occurs if there is intraaccess flow that is less than that of the extracorporeal circuit. Low flow states of the access precipitate recirculation, as the flow rate in the venous needle becomes higher than the fixed flow rate in the access, and the blood which has just been through the dialysis circuit will be redirected toward the arterial needle. The blood will then be directed to the arterial line and be dialyzed again.12 Measuring access recirculation is therefore a screening tool that can be used to detect stenosis, which is the leading cause of intraaccess flow reduction. It is imperative to ensure the needles are in the appropriate position and the lines are not reversed. In addition, stenosis which develops between the venous and arterial needles in the access will not cause recirculation as blood will flow to the area of least resistance; however, it would still be a cause of reduced access flow. As the typical goal blood flow of the hemodialysis treatment is 300 to 450 mL/min, flows lower than this in the access (which would cause recirculation) are already at high risk of thrombosis, and therefore, if recirculation is accurately measured and exists, it is an urgent indication for angioplasty.
Figure 1.
Graphic representation of the hemodialysis circuit and dialysis arteriovenous access. (A) Normal goal treatment flow. Blood is “pulled” from the arterial line and after passing through dialyzer is returned via the venous line. Blood flow in the access (QA) is higher than that of the extracorporeal circuit (QB) and no recirculation is present. (B) Arteriovenous access recirculation caused by significant outlet stenosis. Because the outlet stenosis (triangles) causes low intraaccess flow (QA), returned blood via the venous line has higher flow than acceptable by the access. Blood flow is then directed to the area of the arterial line. Recirculation is calculated in this example to be 25%. (QA = access flow; QB = blood flow in the extracorporeal dialysis circuit; arrows depict the direction of blood flow; triangles depict stenosis.)
Recirculation has classically been measured by urea-based techniques, but non-urea-based methods have been described, such as ultrasound dilution,13,14,15 potassium dilution,16 ionic dialysance,17 glucose infusion,18,19 and thermal dilution. Recirculation of urea, for example, will be present if the blood urea nitrogen (BUN) concentration in the arterial line (BUNa) is less than that of the systemic concentration (BUNs) due to blood reentering the arterial line from the direction of the venous return needle (BUNv). Thus, the percentage recirculation can be calculated according to the following equation:
Determining the percentage of access recirculation is useful to evaluate adequacy of dialysis clearance, but is less helpful to determine if access stenosis is present. Consistency of the urea-based method is poor for surveillance for access stenosis, in part because of arteriovenous (cardiopulmonary recirculation) and venovenous disequilibrium,20 but if the percentage recirculation is >10% stenosis, then should be suspected. Other methods which eliminate the effect of disequilibrium have different thresholds, such as >5% for ultrasound dilution (KDOQI).
Flow
Measuring access blood flow (Q) in an AVF or AVG, either directly or indirectly, theoretically would be helpful in determining stenosis. In addition, it would provide valuable information for clinical diseases associated with high flow states, such as high output congestive heart failure. Flow through a mature functional AVF averages 500 to 800 mL/minute, and in an AVG is typically 600 to 1000 mL/min. Flow is measured either directly, via ultrasound Doppler ultrasonography or magnetic resonance angiography (MRA), or indirectly, using dilution techniques or measuring pressure and determining flow. Indicator dilution techniques have an advantage of being performed during dialysis, as opposed to imaging flow techniques such as Doppler ultrasonography or MRA. Initially established by Krivitski21 in 1995, flow was estimated by using ultrasound velocity to detect dilution in the blood after a bolus of saline was injected into the access. Flow dilution requires special machinery and software, and should be performed earlier in the dialysis treatment to minimize any effects of variable blood pressure. The technique requires the use of two clip sensors, one on the arterial blood line and one on the venous blood line, which are connected to a computer with a data analysis software package. To estimate flow in the access, the hemodialysis machine blood flow is first reduced to 300 mL/min, a temporary recirculation is created by reversing the dialysis bloodlines, and saline (10 cc) is injected into the venous line. This injection must be made to not affect flow in the access. The saline dilutes the red cell mass and changes the Doppler-derived velocity waveform measured by the arterial clip sensor. The flow is then derived by the computer software by using the following relationship to determine the area under the curves as a measure of recirculation:
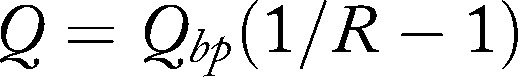
Qbp refers to the blood pump flow and R is degree of recirculation. The measurements should be repeated two or three times for confirmation as there is an expected 5% variability between consecutive measurements.15,21 A high correlation between flow dilution and measured flow using Doppler ultrasound exists over a wide variety of access flows.22,23,24,25 Many methods of flow measurement techniques exist, such as ultrasound, sodium, urea, glucose infusion, differential conductivity, in-line dialysance, or thermal methods. Many modern HD machines have a flow measurement system built in so that flow may be measured without necessitating cumbersome clip sensors. Methods of flow measurements, however, are not universal, and correlation between methods and HD sessions should be established before extrapolating information to the care of patients.26,27,28,29,30,31
Measurements made during Doppler ultrasonography can be used to detect stenotic lesions directly through color duplex imaging (CDI), but can also be used to measure flow. The advantage of this is that both visualization (CDI) and flow can be measured at the same time, but is typically not performed while the patient is on hemodialysis. To maintain precision for this method, it should be performed by trained vascular technicians. Measuring the flow by Doppler depends on accurate readings of velocity and vessel diameter, and with this information flow calculations are possible, typically with specialized software packages. The turbulent nature of an access, especially one with stenosis, and the varying degree of vessel diameter makes a single reading unreliable. To improve accuracy, measurements should be made at two to four places along the graft, and in places where there is not luminal narrowing or turbulent blood flow by color Doppler analysis. Flow can also be measured at the brachial artery, which is upstream from the access, as it provides the access with almost all of the blood flow and has a consistent diameter. This has a direct relationship to access blood flow.32,33,34
MRA can also be reliably used to measure access flow, and provide superior image quality for stenosis, but as there are alternatives, its expense is prohibitive for routine use. More importantly, the clinical risk of nephrogenic systemic fibrosis (NSF) associated with gadolinium exposure in patients with CKD and ESRD35 makes this method obsolete.
Pressure
Measuring pressure in an AV access will provide direct information about access flow, as flow, pressure, and resistance are related:
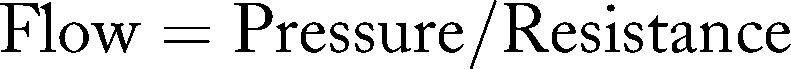
Determining the access pressure or change in pressure could theoretically be predictive of change in flow and therefore a stenosis, and this method is routinely available in HD units. During a hemodialysis treatment, blood flows from the patient's access to the dialyzer via the arterial needle, passes through the venous drip chamber and back to the access via the venous needle. The pressure needed to infuse blood through the venous needle is referred to as the venous pressure. Theoretically, a stenosis in the access would increase the venous pressure. Two methods of estimating this intraaccess pressure are measuring dynamic venous pressure and static venous pressure.
Dynamic venous pressure is measured in the venous drip chamber routinely during hemodialysis while extracorporeal blood is flowing. This should be performed at low blood flow rates (200 to 250 mL/min), which is typically at the beginning of dialysis. At this rate, the resistance to blood flow is from the access itself, and is therefore more reliable to determine pressure. At higher blood flows, such as those from a typical HD treatment (goal blood flow = 300 to 450 mL/min) the resistance is dependent on the blood flow and diameter of the needle. A baseline dynamic venous pressure value should be determined for all patients when the access is initially cannulated successfully, and persistent changes (three times in succession) from that baseline have been shown to be significantly predictive of stenosis.36 Change in dynamic pressure in any individual access is more predictive than an absolute threshold pressure for any access, and needle size and hematocrit need to be regarded for their effect on resistance.
Static venous pressure is the pressure in the access in the absence of extracorporeal blood flow, or prior to the initiation of hemodialysis. This is more reliable than dynamic pressure as there are fewer variables (needle size, blood flow, machine type). It is corrected for the distance between the access and the drip chambers as the height will add additional pressure (0.76 × Δ height) and is also normalized for mean arterial pressure. An increasing ratio of intra-access pressure to blood pressure has been shown to be predictive of graft stenosis.37 Although thresholds of this ratio have been suggested as indicators of stenosis, analysis of trends in individual patients, just as in dynamic venous pressure readings, holds more merit.5
Venous pressure readings in AVGs are more predictive of outlet stenosis close to the anastomotic site, which is the most common place for stenosis to develop, but less accurate for distal stenosis. Pressure readings, if used for surveillance, need to be performed frequently (every 1 to 2 weeks) to improve accuracy.5,38 Dynamic pressures are simpler to perform and record, but are less sensitive and less specific for outlet stenosis than static pressure measurements; however, if averaged for mean arterial pressure and trended together with each treatment, they have been found to be predictive of access thrombosis.39 Lastly, venous pressure readings are, on average, less reliable in an AVF compared with an AVG, as there are typically more collateral veins with a fistula, which dampens the effect of pressure in the access if it is affected by outlet stenosis.
Visualization
Direct imaging of the vascular access can visualize a stenosis, and, if performed by color duplex imaging (ultrasound; CDI) or MRA, has the advantages of being noninvasive as well as being able to determine the flow pattern. In addition, imaging can provide valuable information about nonthrombotic complications of the access. It can evaluate abscess, hematoma, or seroma by imaging surrounding fluid collections. Central stenosis, aneurysms, pseudoaneurysms, or intraluminal thrombi can all be diagnosed. Imaging the access and the surrounding circulation can also determine steal syndrome in patients with symptoms of distal ischemia.
CDI requires trained technicians to maintain accuracy, is not performed while the patient is on dialysis, and has a lack of availability in most dialysis units. Therefore, most dialysis centers refer HD patients with suspected stenosis directly to venography, as this will determine the extent of the stenosis and allow for a treatment at the same time if stenosis is present.
As stated above, MRA can accurately screen for stenosis and access dysfunction40; however, the utility of MRA is limited by the clinical risk of NSF with gadolinium exposure and is no longer recommended for patients with CKD or ESRD.
Ultimately, venography or fistulography is the gold standard for determining AV access stenosis.41,42 Through direct injection of iodinated contrast, it defines the anatomy of the access and the surrounding vasculature, and can be used in conjunction with angioplasty and/or stent placement if a significant stenosis is present. In patients with suspected central stenosis, it is the diagnostic test of choice as it can also provide simultaneous intervention as therapy. When studying AV accesses, stenosis of >50% of the diameter dictates the decision for intervention.43 Although fistulography is an integral part of treatment, its application for routine screening is questionable, as it is invasive, expensive, and time consuming. In addition, in patients with CKD not yet on renal replacement therapy, iodinated contrast should be avoided as it can potentiate renal failure and the need for dialysis.
OUTCOMES OF AV SURVEILLANCE TECHNIQUES
Successful surveillance techniques for AV access failure should be able to detect stenosis that can be treated to prevent thrombosis and prolong the longevity of the access.
Several observational studies have been quite positive for using clinical monitoring and/or recirculation,44,45,46,47,48 flow surveillance via a variety of methods,23,31,49,50,51,52,53,54,55,56 and dynamic and static pressure monitoring36,37 to detect for AV stenosis and thrombosis. As the results of these studies were so robust, several prospective randomized controlled trials have been undertaken to evaluate if surveillance is predictive of thrombosis, and if it provides superior access longevity.
Two prospective blinded studies have evaluated the validity of utilizing a systematic physical examination in detecting access dysfunction compared with angiography36,57,58. The first study36,57 evaluated hemodialysis patients with AVFs. The investigators performed a preangiography systematic physical examination and compared it to angiography in a blinded fashion. There was a strong agreement between the physical examination and angiography for outflow (89% agreement) and inflow (80% agreement) stenosis. The same investigators then duplicated the study for hemodialysis patients with AVGs, and again found a high level of agreement, especially for stenosis at the vein–graft anastomotic site,58 although not as strong of an agreement as found with AVFs. An experienced physical diagnostician is not a prerequisite to determine stenosis, as it has been performed by a trained nephrology fellow with success.59 These studies confirm that a systematic physical examination can be useful to determine stenosis, especially for AVFs, but evidence for using it to determine outcomes of thrombosis and extending access longevity is lacking.
Access recirculation, if present, necessitates a lower blood flow than that of the dialysis circuit. This makes it a relatively late finding12 and, via the urea methods, has not been found to be predictive of access failure or the need for revision.24,49,60 Non-urea-based methods show more promise,16,18,19 but still have not been proven to impact access longevity.
Measuring dynamic pressure in the AV access was one of the first methods of surveillance determined to be successful in observational studies. Recently, Dember et al61 randomized HD patients with AVGs to either static venous pressure monitoring with a trigger for angioplasty of an intragraft to systolic blood pressure ratio of ≥0.4 or clinical observation for graft stenosis to prompt angioplasty. The adjusted results revealed no difference in graft failure from thrombosis and cumulative graft survival was lower in the intervention group compared with the observation group.
Flow monitoring, either through dilutional techniques or Doppler derived, has the potential to be the most accurate determinant of stenosis and thrombosis in AV accesses. Initial studies were performed in HD patients with AVGs. One of the first randomized controlled trials (RCTs) of flow surveillance62 used duplex ultrasound to detect hemodynamically significant (>50%) stenosis in HD patients with AVGs. Once detected and confirmed with venography, the patients were randomized to observation or intervention and followed by duplex ultrasonography. The primary endpoint of graft thrombosis was no different at one-year follow-up. Another RCT63 utilized two surveillance groups (flow monitoring via flow dilution and duplex Doppler ultrasound) compared with a control group of clinical monitoring over a follow-up of 28 months. The results revealed that no group was superior in terms of predicting thrombosis or improving graft longevity at 3 years. The same investigators continued to collect more data prospectively and followed the patients for up to 6 years.64 Their conclusions were that using flow or changes in flow over a long period of time were not predictive of thrombosis or access failure. Moist et al65 investigated whether access flow made a difference when added to dynamic venous pressure and clinical monitoring. In this RCT, the investigators confirmed that the addition of access flow predicted stenosis, but did not increase graft longevity. Similarly, another RCT evaluating the role of routine ultrasound added to clinical monitoring revealed greater detection of stenosis with more angioplasty in the ultrasound group, but no difference in graft thrombosis or survival compared with the control population.66 Finally, one study randomized patients with AVGs to receive surveillance by clinical monitoring with monthly dynamic venous pressures plus access flow (control), compared with another group which had the same monitoring with the addition of Doppler ultrasonography every 3 months. With the addition of ultrasonography performed on a frequent basis, patients had more interventions and longer graft patency.67
In HD patients with AVFs, access flow less than 500 mL/min has been shown to be associated with stenosis.68 RCTs evaluating flow monitoring as surveillance in HD patients with AVFs have been performed in attempts to substantiate this data. The first was in mature virgin AVF, defined as functioning fistulas delivering adequate dialysis by Kt/V, without previous angioplasty or repair. The patients' AVFs were evaluated by clinical monitoring or by flow monitoring. Stenosis detected by flow monitoring of less than 350 mL/min was treated either by angioplasty (72%) or surgical repair (28%) if not considered to be amenable to angioplasty. The preemptive repair group experienced a higher thrombosis-free survival and cumulative survival compared with the clinical monitoring group.69 Another RCT using monthly flow dilution compared with clinical monitoring established a trend (p = 0.09) for flow monitoring to detect stenosis of >50% by angiogram compared with the clinical monitoring in 137 HD patients with AVFs.70 However, the investigators did not evaluate access longevity.
Direct comparisons of surveillance methods have also been studied. The first was a trial by Sands et al71 who randomized patients with AVFs or AVGs to one of three groups: monthly access flow measurements, monthly static venous pressure readings, or a control group. The investigators found that monthly monitoring via flow or static pressure improved thrombosis rates compared with controls, and that flow monitoring also proved to be superior to static venous pressure for access thrombosis. Another RCT from the Netherlands evaluated the utility of venous pressure (dynamic and/or static) or flow dilution or the combination for 125 HD patients with AVGs.72 None of the methods were superior to each other, but all three were superior to historic rates of thrombosis.
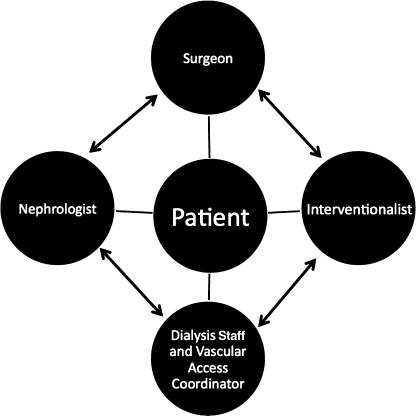
Despite all of the data that exists regarding surveillance of AV access for dialysis patients,73 significant debate still exists in the literature regarding whether access surveillance is useful.5,64,74,75,76,77,78 Clearly, surveillance can successfully detect stenosis, but the outcome of improving graft longevity, especially with surveillance in AVGs, remains speculative. Although also not proven to prolong access survival, establishing a team,44,79 consisting of the patient, dialysis staff, vascular access coordinator, nephrologist, interventionalist, and vascular surgeon (Fig. 2) to meet regularly to care for the access and provide quality improvement is crucial to determine a plan for the patient's future.
Figure 2.
The team approach for managing a patient's vascular access. Cooperation and collaboration of the patient, nephrologist, dialysis nurse and technician, vascular access coordinator, interventionalist, and vascular surgeon are important to preserve the access.
CONCLUSION
Because of the projected increase in placement and use of permanent AV accesses, a successful surveillance system for common complications of the access is important. Using visualization techniques, clinical monitoring, measuring recirculation, determining pressure and measuring flow have all been developed to help determine when a stenosis may occur. Although the observational data was promising, more solid evidence to determine if screening is useful to detect and to prevent thrombosis, especially in AVGs, has been negative. Some data are still promising for using flow monitoring as a surveillance method in AVFs. Future studies should continue to be randomized clinical trials, but should be large and conducted with multiple facilities. Ultimately, integration of a team approach of the patient and the care providers becomes a necessary solution to care for the patient.
REFERENCES
- United States Renal Data System 2007 Annual Data Report. Bethesda, MD: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; 2007.
- Astor B C, Eustace J A, Powe N R, Klag M J, Fink N E, Coresh J, CHOICE Study Type of vascular access and survival among incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J Am Soc Nephrol. 2005;16(5):1449–1455. doi: 10.1681/ASN.2004090748. [DOI] [PubMed] [Google Scholar]
- Lee T, Barker J, Allon M. Tunneled catheters in hemodialysis patients: reasons and subsequent outcomes. Am J Kidney Dis. 2005;46(3):501–508. doi: 10.1053/j.ajkd.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Maya I D, Oser R, Saddekni S, Barker J, Allon M. Vascular access stenosis: comparison of arteriovenous grafts and fistulas. Am J Kidney Dis. 2004;44(5):859–865. [PubMed] [Google Scholar]
- Vascular Access 2006 Work Group Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Tonnessen B H, Money S R. Embracing the fistula first national vascular access improvement initiative. J Vasc Surg. 2005;42(3):585–586. doi: 10.1016/j.jvs.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Basile C, Lomonte C, Vernaglione L, Casucci F, Antonelli M, Losurdo N. The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrol Dial Transplant. 2008;23(1):282–287. doi: 10.1093/ndt/gfm549. [DOI] [PubMed] [Google Scholar]
- Miller C D, Robbin M L, Barker J, Allon M. Comparison of arteriovenous grafts in the thigh and upper extremities in hemodialysis patients. J Am Soc Nephrol. 2003;14(11):2942–2947. doi: 10.1097/01.asn.0000090746.88608.94. [DOI] [PubMed] [Google Scholar]
- Miller P E, Carlton D, Deierhoi M H, Redden D T, Allon M. Natural history of arteriovenous grafts in hemodialysis patients. Am J Kidney Dis. 2000;36(1):68–74. doi: 10.1053/ajkd.2000.8269. [DOI] [PubMed] [Google Scholar]
- Lilly R Z, Carlton D, Barker J, et al. Predictors of arteriovenous graft patency after radiologic intervention in hemodialysis patients. Am J Kidney Dis. 2001;37(5):945–953. doi: 10.1016/s0272-6386(05)80010-1. [DOI] [PubMed] [Google Scholar]
- Beathard G A. An algorithm for the physical examination of early fistula failure. Semin Dial. 2005;18(4):331–335. doi: 10.1111/j.1525-139X.2005.18314.x. [DOI] [PubMed] [Google Scholar]
- Besarab A, Sherman R. The relationship of recirculation to access blood flow. Am J Kidney Dis. 1997;29(2):223–229. doi: 10.1016/s0272-6386(97)90033-0. [DOI] [PubMed] [Google Scholar]
- Alloatti S, Molino A, Bonfant G, Ratibondi S, Bosticardo G M. Measurement of vascular access recirculation unaffected by cardiopulmonary recirculation: evaluation of an ultrasound method. Nephron. 1999;81(1):25–30. doi: 10.1159/000045241. [DOI] [PubMed] [Google Scholar]
- Depner T A, Krivitski N M, MacGibbon D. Hemodialysis access recirculation measured by ultrasound dilution. ASAIO J. 1995;41(3):M749–M753. doi: 10.1097/00002480-199507000-00113. [DOI] [PubMed] [Google Scholar]
- Depner T A, Krivitski N M. Clinical measurement of blood flow in hemodialysis access fistulae and grafts by ultrasound dilution. ASAIO J. 1995;41(3):M745–M749. doi: 10.1097/00002480-199507000-00112. [DOI] [PubMed] [Google Scholar]
- Brancaccio D, Tessitore N, Carpani P, et al. Potassium-based dilutional method to measure hemodialysis access recirculation. Int J Artif Organs. 2001;24(9):606–613. [PubMed] [Google Scholar]
- Mercadal L, Coevoet B, Albadawy M, et al. Analysis of the optical concentration curve to detect access recirculation. Kidney Int. 2006;69(4):769–771. doi: 10.1038/sj.ki.5000154. [DOI] [PubMed] [Google Scholar]
- Alloatti S, Magnasco A, Bonfant G, Pellu V, Paroli V. Comparison of glucose pump test and urea test in measuring blood access flow. J Nephrol. 2004;17(4):559–564. [PubMed] [Google Scholar]
- Magnasco A, Alloatti S, Bonfant G, Copello F, Solari P. Glucose infusion test: a new screening test for vascular access recirculation. Kidney Int. 2000;57(5):2123–2128. doi: 10.1046/j.1523-1755.2000.00063.x. [DOI] [PubMed] [Google Scholar]
- Sherman R A. The measurement of dialysis access recirculation. Am J Kidney Dis. 1993;22(4):616–621. doi: 10.1016/s0272-6386(12)80940-1. [DOI] [PubMed] [Google Scholar]
- Krivitski N M. Theory and validation of access flow measurement by dilution technique during hemodialysis. Kidney Int. 1995;48(1):244–250. doi: 10.1038/ki.1995.290. [DOI] [PubMed] [Google Scholar]
- Besarab A, Lubkowski T, Frinak S, Ramanathan S, Escobar F. Detection of access strictures and outlet stenoses in vascular accesses. Which test is best? ASAIO J. 1997;43(5):M543–M547. [PubMed] [Google Scholar]
- Lindsay R M, Sternby J, Olde B, Persson R, Thatcher M E, Sargent K. Hemodialysis blood access flow rates can be estimated accurately from on-line dialysate urea measurements and the knowledge of effective dialyzer urea clearance. Clin J Am Soc Nephrol. 2006;1(5):960–964. doi: 10.2215/CJN.00810306. [DOI] [PubMed] [Google Scholar]
- May R E, Himmelfarb J, Yenicesu M, et al. Predictive measures of vascular access thrombosis: a prospective study. Kidney Int. 1997;52(6):1656–1662. doi: 10.1038/ki.1997.499. [DOI] [PubMed] [Google Scholar]
- Sands J, Glidden D, Miranda C. Hemodialysis access flow measurement. Comparison of ultrasound dilution and duplex ultrasonography. ASAIO J. 1996;42(5):M899–M901. [PubMed] [Google Scholar]
- Huisman R M, Dijk M van, de Bruin C, et al. Within-session and between-session variability of haemodialysis shunt flow measurements. Nephrol Dial Transplant. 2005;20(12):2842–2847. doi: 10.1093/ndt/gfi142. [DOI] [PubMed] [Google Scholar]
- Lacson E, Jr, Lazarus J M, Panlilio R, Gotch F. Comparison of hemodialysis blood access flow rates using online measurement of conductivity dialysance and ultrasound dilution. Am J Kidney Dis. 2008;51(1):99–106. doi: 10.1053/j.ajkd.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Lopot F, Nejedlý B, Sulková S, Bláha J. Comparison of different techniques of hemodialysis vascular access flow evaluation. J Vasc Access. 2004;5(1):25–32. doi: 10.1177/112972980400500106. [DOI] [PubMed] [Google Scholar]
- Schneditz D, Bachler I, der Sande F M van. Timing and reproducibility of access flow measurements using extracorporeal temperature gradients. ASAIO J. 2007;53(4):469–473. doi: 10.1097/MAT.0b013e31805c1446. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Klarenbach S, Jindal K, et al. Access flow in arteriovenous accesses by optodilutional and ultrasound dilution methods. Am J Kidney Dis. 2005;46(5):933–937. doi: 10.1053/j.ajkd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Wijnen E, Essers S, Meijel G van, et al. Comparison between two on-line reversed line position hemodialysis vascular access flow measurement techniques: saline dilution and thermodilution. ASAIO J. 2006;52(4):410–415. doi: 10.1097/01.mat.0000227680.67901.01. [DOI] [PubMed] [Google Scholar]
- Depairon M, Ferrier C P, Tutta P, Descombes E, Melle G van, Wauters J P. Is there a place for duplex screening of brachial artery in haemodialysis patients with vascular access? Vasa. 2001;30(1):53–58. doi: 10.1024/0301-1526.30.1.53. [DOI] [PubMed] [Google Scholar]
- Lomonte C, Casucci F, Antonelli M, et al. Is there a place for duplex screening of the brachial artery in the maturation of arteriovenous fistulas? Semin Dial. 2005;18(3):243–246. doi: 10.1111/j.1525-139X.2005.18320.x. [DOI] [PubMed] [Google Scholar]
- Wiese P, Blume J, Mueller H J, Renner H, Nonnast-Daniel A B. Clinical and Doppler ultrasonography data of a polyurethane vascular access graft for haemodialysis: a prospective study. Nephrol Dial Transplant. 2003;18(7):1397–1400. doi: 10.1093/ndt/gfg168. [DOI] [PubMed] [Google Scholar]
- Perazella M A, Rodby R A. Gadolinium-induced nephrogenic systemic fibrosis in patients with kidney disease. Am J Med. 2007;120(7):561–562. doi: 10.1016/j.amjmed.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Schwab S J, Raymond J R, Saeed M, Newman G E, Dennis P A, Bollinger R R. Prevention of hemodialysis fistula thrombosis. Early detection of venous stenoses. Kidney Int. 1989;36(4):707–711. doi: 10.1038/ki.1989.250. [DOI] [PubMed] [Google Scholar]
- Besarab A, Sullivan K L, Ross R P, Moritz M J. Utility of intra-access pressure monitoring in detecting and correcting venous outlet stenoses prior to thrombosis. Kidney Int. 1995;47(5):1364–1373. doi: 10.1038/ki.1995.192. [DOI] [PubMed] [Google Scholar]
- White J J, Jones S A, Ram S J, Schwab S J, Paulson W D. Mathematical model demonstrates influence of luminal diameters on venous pressure surveillance. Clin J Am Soc Nephrol. 2007;2(4):681–687. doi: 10.2215/CJN.01070307. [DOI] [PubMed] [Google Scholar]
- Frinak S, Zasuwa G, Dunfee T, Besarab A, Yee J. Dynamic venous access pressure ratio test for hemodialysis access monitoring. Am J Kidney Dis. 2002;40(4):760–768. doi: 10.1053/ajkd.2002.35687. [DOI] [PubMed] [Google Scholar]
- Duijm L E, Liem Y S, der Rijt R H van, et al. Inflow stenoses in dysfunctional hemodialysis access fistulae and grafts. Am J Kidney Dis. 2006;48(1):98–105. doi: 10.1053/j.ajkd.2006.03.076. [DOI] [PubMed] [Google Scholar]
- Beathard G A. Percutaneous transvenous angioplasty in the treatment of vascular access stenosis. Kidney Int. 1992;42(6):1390–1397. doi: 10.1038/ki.1992.431. [DOI] [PubMed] [Google Scholar]
- Schwab S J, Saeed M, Sussman S K, McCann R L, Stickel D L. Transluminal angioplasty of venous stenoses in polytetrafluoroethylene vascular access grafts. Kidney Int. 1987;32(3):395–398. doi: 10.1038/ki.1987.223. [DOI] [PubMed] [Google Scholar]
- Aruny J E, Lewis C A, Cardella J F, et al. Society of Interventional Radiology Standards of Practice Committee Quality improvement guidelines for percutaneous management of the thrombosed or dysfunctional dialysis access. J Vasc Interv Radiol. 2003;14(9 Pt 2):S247–S253. [PubMed] [Google Scholar]
- Allon M, Bailey R, Ballard R, et al. A multidisciplinary approach to hemodialysis access: prospective evaluation. Kidney Int. 1998;53(2):473–479. doi: 10.1046/j.1523-1755.1998.00761.x. [DOI] [PubMed] [Google Scholar]
- Cayco A V, Abu-Alfa A K, Mahnensmith R L, Perazella M A. Reduction in arteriovenous graft impairment: results of a vascular access surveillance protocol. Am J Kidney Dis. 1998;32(2):302–308. doi: 10.1053/ajkd.1998.v32.pm9708617. [DOI] [PubMed] [Google Scholar]
- Daniels I D, Berlyne G M, Barth R H. Blood flow rate and access recirculation in hemodialysis. Int J Artif Organs. 1992;15(8):470–474. [PubMed] [Google Scholar]
- Safa A A, Valji K, Roberts A C, Ziegler T W, Hye R J, Oglevie S B. Detection and treatment of dysfunctional hemodialysis access grafts: effect of a surveillance program on graft patency and the incidence of thrombosis. Radiology. 1996;199(3):653–657. doi: 10.1148/radiology.199.3.8637982. [DOI] [PubMed] [Google Scholar]
- Windus D W, Audrain J, Vanderson R, Jendrisak M D, Picus D, Delmez J A. Optimization of high-efficiency hemodialysis by detection and correction of fistula dysfunction. Kidney Int. 1990;38(2):337–341. doi: 10.1038/ki.1990.206. [DOI] [PubMed] [Google Scholar]
- Bay W H, Henry M L, Lazarus J M, Lew N L, Ling J, Lowrie E G. Predicting hemodialysis access failure with color flow Doppler ultrasound. Am J Nephrol. 1998;18(4):296–304. doi: 10.1159/000013354. [DOI] [PubMed] [Google Scholar]
- Bosman P J, Boereboom F T, Eikelboom B C, Koomans H A, Blankestijn P J. Graft flow as a predictor of thrombosis in hemodialysis grafts. Kidney Int. 1998;54(5):1726–1730. doi: 10.1046/j.1523-1755.1998.00158.x. [DOI] [PubMed] [Google Scholar]
- Lindsay R M, Blake P G, Malek P, Posen G, Martin B, Bradfield E. Hemodialysis access blood flow rates can be measured by a differential conductivity technique and are predictive of access clotting. Am J Kidney Dis. 1997;30(4):475–482. doi: 10.1016/s0272-6386(97)90304-8. [DOI] [PubMed] [Google Scholar]
- McCarley P, Wingard R L, Shyr Y, Pettus W, Hakim R M, Ikizler T A. Vascular access blood flow monitoring reduces access morbidity and costs. Kidney Int. 2001;60(3):1164–1172. doi: 10.1046/j.1523-1755.2001.0600031164.x. [DOI] [PubMed] [Google Scholar]
- Neyra N R, Ikizler T A, May R E, et al. Change in access blood flow over time predicts vascular access thrombosis. Kidney Int. 1998;54(5):1714–1719. doi: 10.1046/j.1523-1755.1998.00145.x. [DOI] [PubMed] [Google Scholar]
- Schwab S J, Oliver M J, Suhocki P, McCann R. Hemodialysis arteriovenous access: detection of stenosis and response to treatment by vascular access blood flow. Kidney Int. 2001;59(1):358–362. doi: 10.1046/j.1523-1755.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- Singh N, Ahmad S, Wienckowski J R, Murray B M. Comparison of access blood flow and venous pressure measurements as predictors of arteriovenous graft thrombosis. J Vasc Access. 2006;7(2):66–73. doi: 10.1177/112972980600700205. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Klarenbach S, Jindal K, et al. Access flow in arteriovenous accesses by optodilutional and ultrasound dilution methods. Am J Kidney Dis. 2005;46(5):933–937. doi: 10.1053/j.ajkd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Asif A, Leon C, Orozco-Vargas L C, et al. Accuracy of physical examination in the detection of arteriovenous fistula stenosis. Clin J Am Soc Nephrol. 2007;2(6):1191–1194. doi: 10.2215/CJN.02400607. [DOI] [PubMed] [Google Scholar]
- Leon C, Orozco-Vargas L C, Krishnamurthy G, et al. Accuracy of physical examination in the detection of arteriovenous graft stenosis. Semin Dial. 2008;21(1):85–88. doi: 10.1111/j.1525-139X.2007.00382.x. [DOI] [PubMed] [Google Scholar]
- Leon C, Asif A. Physical examination of arteriovenous fistulae by a renal fellow: does it compare favorably to an experienced interventionalist? Semin Dial. 2008;21(6):557–560. doi: 10.1111/j.1525-139X.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- Lindsay R M, Blake P G, Bradfield E. Estimation of hemodialysis access blood flow rates by a urea method is a poor predictor of access outcome. ASAIO J. 1998;44(6):818–822. doi: 10.1097/00002480-199811000-00010. [DOI] [PubMed] [Google Scholar]
- Dember L M, Holmberg E F, Kaufman J S. Randomized controlled trial of prophylactic repair of hemodialysis arteriovenous graft stenosis. Kidney Int. 2004;66(1):390–398. doi: 10.1111/j.1523-1755.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- Lumsden A, MacDonald M J, Kikeri D. Prophylactic balloon angioplasty fails to prolong the patency of expanded polytetrafluoroethylene arteriovenous grafts. J Vasc Surg. 1997;26:382–392. doi: 10.1016/s0741-5214(97)70031-4. [DOI] [PubMed] [Google Scholar]
- Ram S J, Work J, Caldito G C, Eason J M, Pervez A, Paulson W D. A randomized controlled trial of blood flow and stenosis surveillance of hemodialysis grafts. Kidney Int. 2003;64(1):272–280. doi: 10.1046/j.1523-1755.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- Ram S J, Nassar R, Work J, Abreo K, Dossabhoy N R, Paulson W D. Risk of hemodialysis graft thrombosis: analysis of monthly flow surveillance. Am J Kidney Dis. 2008;52(5):930–938. doi: 10.1053/j.ajkd.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Moist L M, Churchill D N, House A A, et al. Regular monitoring of access flow compared with monitoring of venous pressure fails to improve graft survival. J Am Soc Nephrol. 2003;14(10):2645–2653. doi: 10.1097/01.asn.0000089562.98338.60. [DOI] [PubMed] [Google Scholar]
- Robbin M L, Oser R F, Lee J Y, Heudebert G R, Mennemeyer S T, Allon M. Randomized comparison of ultrasound surveillance and clinical monitoring on arteriovenous graft outcomes. Kidney Int. 2006;69(4):730–735. doi: 10.1038/sj.ki.5000129. [DOI] [PubMed] [Google Scholar]
- Malik J, Slavikova M, Svobodova J, Tuka V. Regular ultrasonographic screening significantly prolongs patency of PTFE grafts. Kidney Int. 2005;67(4):1554–1558. doi: 10.1111/j.1523-1755.2005.00236.x. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Hirsch D, Clark T W, et al. Access flow monitoring of patients with native vessel arteriovenous fistulae and previous angioplasty. J Am Soc Nephrol. 2002;13(12):2969–2973. doi: 10.1097/01.asn.0000040597.94103.48. [DOI] [PubMed] [Google Scholar]
- Tessitore N, Lipari G, Poli A, et al. Can blood flow surveillance and pre-emptive repair of subclinical stenosis prolong the useful life of arteriovenous fistulae? A randomized controlled study. Nephrol Dial Transplant. 2004;19(9):2325–2333. doi: 10.1093/ndt/gfh316. [DOI] [PubMed] [Google Scholar]
- Polkinghorne K R, Lau K K, Saunder A, Atkins R C, Kerr P G. Does monthly native arteriovenous fistula blood-flow surveillance detect significant stenosis—a randomized controlled trial. Nephrol Dial Transplant. 2006;21(9):2498–2506. doi: 10.1093/ndt/gfl242. [DOI] [PubMed] [Google Scholar]
- Sands J J, Jabyac P A, Miranda C L, Kapsick B J. Intervention based on monthly monitoring decreases hemodialysis access thrombosis. ASAIO J. 1999;45(3):147–150. doi: 10.1097/00002480-199905000-00008. [DOI] [PubMed] [Google Scholar]
- Smits J H, der Linden J van, Hagen E C, et al. Graft surveillance: venous pressure, access flow, or the combination? Kidney Int. 2001;59(4):1551–1558. doi: 10.1046/j.1523-1755.2001.0590041551.x. [DOI] [PubMed] [Google Scholar]
- Tonelli M, James M, Wiebe N, Jindal K, Hemmelgarn B, Alberta Kidney Disease Network Ultrasound monitoring to detect access stenosis in hemodialysis patients: a systematic review. Am J Kidney Dis. 2008;51(4):630–640. doi: 10.1053/j.ajkd.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Allon M. Do we really need periodic monitoring of vascular access for hemodialysis? NephSAP. 2007;6:111–116. [Google Scholar]
- Besarab A. Access monitoring is worthwhile and valuable. Blood Purif. 2006;24(1):77–89. doi: 10.1159/000089442. [DOI] [PubMed] [Google Scholar]
- Paulson W D. Access monitoring does not really improve outcomes. Blood Purif. 2005;23(1):50–56. doi: 10.1159/000082011. [DOI] [PubMed] [Google Scholar]
- Sands J J. Vascular access monitoring improves outcomes. Blood Purif. 2005;23(1):45–49. doi: 10.1159/000082010. [DOI] [PubMed] [Google Scholar]
- White J J, Bander S J, Schwab S J, et al. Is percutaneous transluminal angioplasty an effective intervention for arteriovenous graft stenosis? Semin Dial. 2005;18(3):190–202. doi: 10.1111/j.1525-139X.2005.18307.x. [DOI] [PubMed] [Google Scholar]
- Wijnen E, Planken N, Keuter X, et al. Impact of a quality improvement programme based on vascular access flow monitoring on costs, access occlusion and access failure. Nephrol Dial Transplant. 2006;21(12):3514–3519. doi: 10.1093/ndt/gfl424. [DOI] [PubMed] [Google Scholar]