ABSTRACT
Autogenous arteriovenous fistulas are the preferred vascular access in patients undergoing hemodialysis. Increasing fistula prevalence depends on increasing fistula placement, improving the maturation of fistula that fail to mature and enhancing the long-term patency of mature fistula. Percutaneous methods for optimizing arteriovenous fistula maturation will be reviewed.
Keywords: Arteriovenous fistula maturation, dialysis access, angioplasty, collateral embolization
Autogenous arteriovenous fistulas (AVFs) are considered the most reliable long-term vascular access in patients undergoing hemodialysis. Compared with prosthetic arteriovenous grafts (AVG) and tunneled catheters, AVFs require fewer interventions, are less susceptible to failure due to infection and thrombosis, and have been shown to improve patient survival.1
In an effort to increase the appropriate use of AVFs for hemodialysis, the Centers for Medicare and Medicaid Services (CMS) and other provider representatives recommended adoption of the National Vascular Access Improvement Initiative (now recognized as the AV Fistula First Breakthrough Coalition or FFBI) in 2003. The original goal of the agency's first breakthrough initiative, “Fistula First,” was to achieve at least 40% fistula prevalence. Having achieved the goal 10 months early, in August 2005, CMS has reset the goal to 65% by 2009.2 The most recent data indicate that the AVF rate reached 51.8% in January 2009.3
Increasing fistula prevalence depends on increasing fistula placement, improving the maturation of fistula that fail to mature, and enhancing the long-term patency of mature fistula. This review will focus on the optimization of fistulas that fail to mature using percutaneous techniques.
Fistula maturation depends on several changes involving the vein such as increased blood flow, increased vein diameter, and increased visibility of the vein. Successful fistula creation results in easy cannulation within 90 days of placement and adequate blood flow to support dialysis. Per the K/DOQI guidelines an adequate AV fistula resides ~0.6 cm from the skin surface, has a flow >600 mL/min, and a diameter >0.6 cm.1
Traditionally, one quarter to one third of all autogenous hemodialysis AVF created never mature. Current practice has focused on the prevention of delayed fistula maturation and early fistula failure through the appropriate preoperative selection of arterial and venous vessels, as well as procedures most suitable for the individual patient. In fact, K/DOQI vascular access guidelines recommend universal preoperative venous mapping. However, the impact of preoperative imaging on successful AVF creation is unclear. Patel et al4 showed that preoperative duplex ultrasound (US) scanning and venography increased the creation of AVFs by identifying veins that are not clinically evident. However, the functional maturation rate decreased from 73 to 57% after implementation of preoperative imaging and more aggressive vein use. In contrast, a study evaluating preoperative US mapping showed an early fistula failure rate of only 8% compared with 35% when preoperative imaging was not performed.5 Additionally, there is controversy regarding the ideal preoperative imaging modality. Although US is noninvasive and avoids the use of potentially nephrotoxic contrast agents, it is less sensitive than venography for examining the central veins. A recent study suggests that venography may identify clinically occult veins usable for access and anatomic variants, which may affect choice of access site.6 Further complicating the issue is that despite selection of the best available artery and vein, fistula maturation rates have not changed.1,4,7,8
Though there is a relatively limited understanding of why fistulas fail, several studies have provided insight into the problem of nonmaturation. A review by Roy-Chaudhury et al9 summarizes the pathophysiology of early AVF failure. Genetic predisposition, low shear stress, increases in transmural pressure, turbulence, differences in compliance between arteries and veins, and vascular injury of the mobilized segment all contribute to neointimal hyperplasia and adverse vascular remodeling. Failure due to thrombosis immediately following surgery is usually secondary to technical errors, judgment error regarding vessel adequacy, or a period of extreme hypotension.10 Peripheral location of the first fistula, female sex, diabetes mellitus, and surgical expertise are the main predictive factors of early fistula failure.11
Fistulae that are going to mature do so within the first several weeks. Various studies have documented that an increase in blood flow begins soon after the construction of the fistula in the majority of cases.11,12,13,14 By combining venous diameter (>0.4 cm) and flow volume (>500 mL/min) during duplex Doppler US evaluation within the first 4 months after access construction, one can predict the likelihood of maturating fistula.11 Physical examination also provides a simple means for predicting eventual fistula maturity.15 Per the K/DOQI guidelines, all AVFs should be routinely evaluated at 4 weeks. By 4 weeks, normal postoperative edema will have resolved and it is expected that the majority of fistulae with have reached maximum flow. If there is evidence of dysfunction, early intervention is advocated to identify and treat the underlying problem.
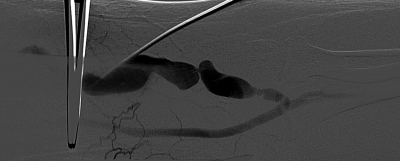
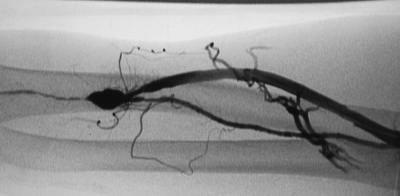
Early fistula failure is frequently due to anatomic lesions that may exist anywhere within the access circuit. Several studies have described the lesions responsible for early fistula failure (Table 1).7,16,17,18,19 Fistulae that fail to mature may have at least one stenotic lesion within the access circuit and often have multiple lesions (Fig. 1). Arterial inflow lesions include anatomically small vessels and stenoses due to atherosclerotic disease (Fig. 2). These are found in an increasing number of elderly, diabetic, and hypertensive patients. Arterial anastomotic and venous swing point stenoses are commonly seen acquired lesions as these are the sites of surgical creation and mobilization of the artery and vein (Figs. 3 and 4). Venous outflow lesions are the most common lesions found in fistulae that fail to mature. Venous outflow lesions, including the central veins, may be due to preexisting anomalies, such as anatomically small veins, fibrotic or stenotic veins, or sites of prior puncture or catheter placement (Figs. 5–7). In addition, venous outflow problems may be due to accessory veins or side branches (Fig. 8).
Table 1.
Location of Lesions in Nonmaturing Fistulae
| Author | Number of Fistulas | Arterial Inflow | Arterial Anastomosis | Swing Point | Outflow Vein | Central Vein | Accessory Vein | Multiple |
|---|---|---|---|---|---|---|---|---|
| Turmel-Rodgrigues et al (2001)16 | 69 | 6% | 55% | 39% | 25% | |||
| Beathard et al (2003)7 | 100 | 4% | 38% | 43% | 36% | 9% | 46% | 40% |
| Falk (2006)17 | 65 | 8% | 6% | 25% | 33% | 2% | 19% | 29% |
| Nassar et al (2006)18 | 118 | 5% | 47% | 64% | 59% | 8% | 29% | 71% |
| Clark et al (2007)19 | 101 | 6% | 4% | 38% | 49% | 3% | 4% | 42% |
Figure 1.
Left upper extremity brachiocephalic fistula with multifocal proximal stenoses.
Figure 2.
Right forearm radiocephalic fistula that failed to mature due to poor inflow. Atherosclerotic disease affects the native radial artery.
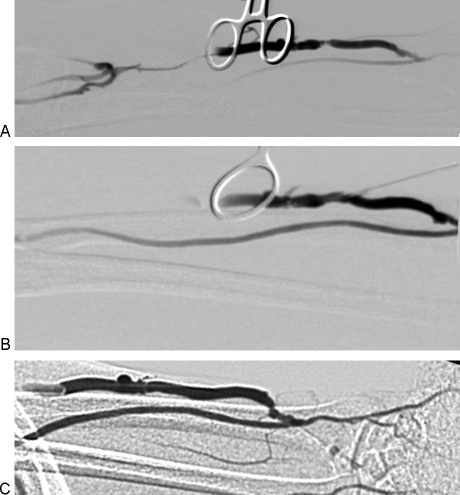
Figure 3.
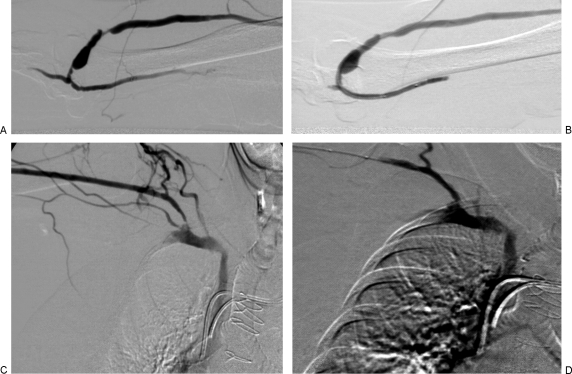
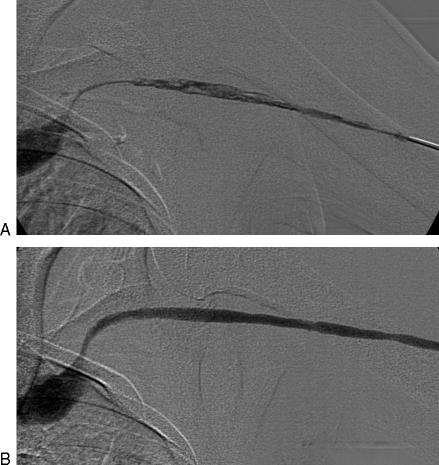
(A) Right upper extremity brachiocephalic fistula with juxta-anastomotic and swing point stenoses. Focal narrowing just proximal to the anastomosis is likely the result of clamp injury. (B) Following 5 mm angioplasty, there is resolution of both stenoses. (C) An additional narrowing involving the cephalic arch also contributed to fistula nonmaturation in this patient. (D) Following 6 mm angioplasty, the cephalic stenosis has improved. Note the diminished flow through collateral veins. Over the course of the following 3 weeks, the fistula became functional.
Figure 4.
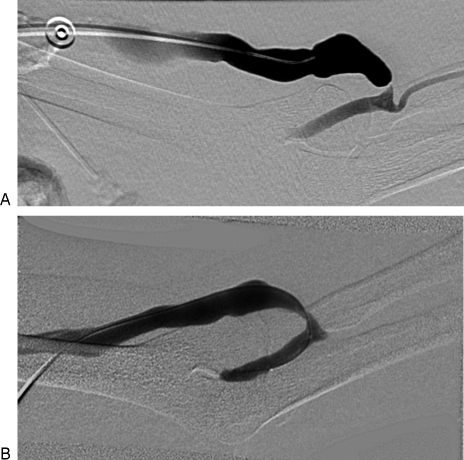
(A) Left arm brachiocephalic fistula with severe juxta-anastomotic narrowing. Poor clearance was reported during hemodialysis. (B) Following 4 mm angioplasty, the caliber of the fistula improved. After 2 weeks, no further difficulties were encountered.
Figure 5.
(A) Left arm radiocephalic fistula that did not mature due to a focal venous outflow narrowing. The focal narrowing in the cephalic vein was likely due to previous venipuncture. (B) Following 5 mm angioplasty, there is no residual stenosis. (C) Two months later, the fistula increased in size and was suitable for dialysis.
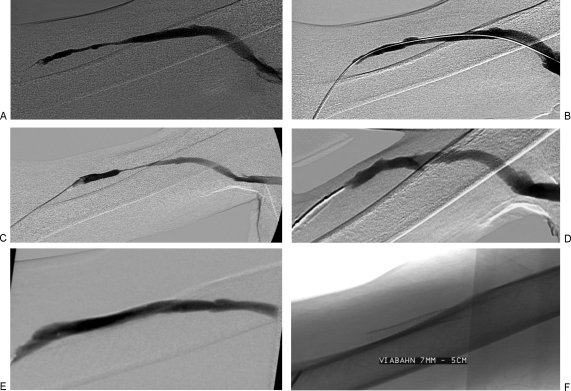
Figure 6.
(A) Right upper arm brachiocephalic fistula with long segment outflow narrowing. (B) Following 6 mm angioplasty, the stenosis improved. (C) Though the fistula matured, after 7 months the patient developed recurrent stenosis. (D) Angioplasty with a 7 mm balloon again yielded a good technical result. (E) At 12 months, the patient returned with recurrent narrowing. It was resistant to angioplasty and a 7 × 50 mm stent graft was deployed. (F) The stent graft consists of an external nitinol stent and expanded polytetrafluoroethylene (ePTFE) lining.
Figure 7.
(A) The left upper extremity brachiobasilic fistula did not mature because of a chronic total occlusion of the left brachiocephalic vein. (B) The narrowing was resistant to angioplasty. A 12 × 60 mm self-expanding stent was placed. Note the resolution of flow through collateral veins.
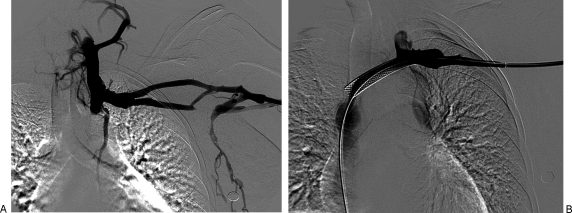
Figure 8.
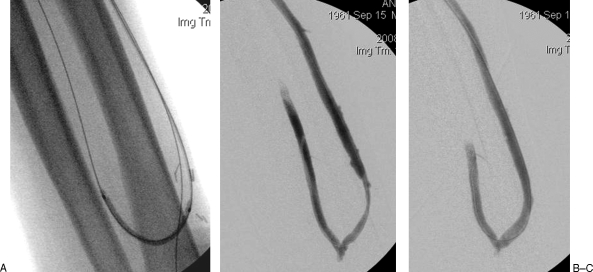
(A) Left radiocephalic fistula that failed to mature due to multiple accessory veins. (B) The accessory veins were successfully embolized and the fistula matured without further intervention.
Though a variety of anatomic lesions may contribute to fistula failure, several studies have emphasized that the majority of these failed fistula can be salvaged using percutaneous techniques (Table 2).7,8,16,17,18,19,20,21,22,23 Using predominantly balloon angioplasty (arterial and/or venous) with sequential dilation if needed and ligation or embolization of collateral side branches, a fistula that has failed to mature can be salvaged. The use of cutting balloon angioplasty for primary treatment of venous stenoses has shown promise.24 Cutting balloon angioplasty has also compared favorably to high pressure balloon angioplasty.25 However, these studies have not focused on the salvage of immature fistulae. In a series of 41 patients treated with primary cutting balloon angioplasty for venous stenoses, primary patency rates were 88, 73, and 34% at 6, 12, and 24 months, respectively.26 In only five of these patients was a nonmaturing fistula the indication for the intervention and the results did not specifically address the success rates based on indication. Occasionally, thrombectomy procedures are required to salvage these fistulas (Figs. 9 and 10). When percutaneous interventions fail, surgical options such as reanastomosis, revision utilizing patch angioplasty or vein interposition, hybrid creation utilizing a prosthetic segment, or superficialization will be necessary.10
Table 2.
Salvage of Nonmaturing Fistulas
| Author | Number of Fistulas | Fistula Age (Months) | Interventions | Salvage Rate (%) | 1-Year Patency |
|---|---|---|---|---|---|
| PTA, percutaneous transluminal angioplasty. | |||||
| Beathard et al (1999)20 | 63 | 4.2 | PTA, ligation, banding | 82.5 | 75% Secondary |
| Turmel-Rodrigues et al (2001)16 | 69 | 2.5 | PTA, aspiration thrombectomy | 97 | 39% Primary 79% Secondary |
| Faiyez et al (2002)21 | 17 | 4.0 | Ligation | 88 | |
| Tordoir et al (2003)8 | 17 | 4.0 | PTA, surgical revision | 47 | |
| Beathard et al (2003)7 | 100 | 4.7 | PTA, ligation or embolization | 92 | 68% Secondary |
| Shin et al (2005)22 | 19 | 1.8 | PTA, ligation | 74 | 61% Primary 82% Secondary |
| Falk (2006)17 | 65 | 2.7 | PTA, ligation, banding, thrombectomy | 74 | |
| Nassar et al (2006)18 | 118 | 4 | PTA, embolization | 83 | 62% Primary 93% Secondary |
| Song et al (2006)23 | 22 | 2.7 | PTA, stenting | 96 | 28% Primary 85% Secondary |
| Clark et al (2007)19 | 101 | 2.5 | PTA, ligation | 88 | 34% Primary 75% Secondary |
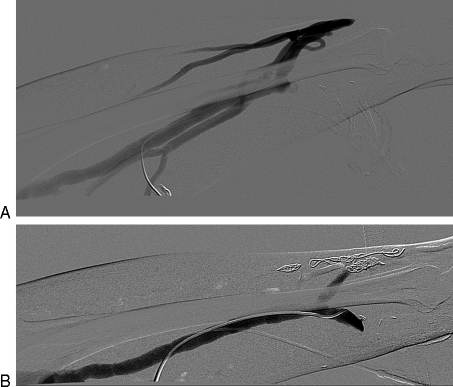
Figure 9.
(A) Immature left radiocephalic fistula that thrombosed prior to initiation of dialysis. Note the high-grade cephalic arch stenosis and extensive clot in the cephalic vein. (B) After administration of 4 mg alteplase and 6 mm balloon angioplasty, flow was restored and the cephalic arch stenosis improved.
Figure 10.
(A) A 46-year-old man presented with a 3-week-old thrombosed immature left radiocephalic fistula. Sequential balloon dilation of the cephalic vein was performed using 2, 3, and 4 mm balloons. The balloon was punctured for retrograde access and the radial artery and anastomosis were dilated with a 2 mm balloon. (B) Flow was reestablished and the fistula became functional. However, 4 months later, there was a decreased thrill on exam and angiography demonstrated a swing point stenosis. (C) Following 6 mm angioplasty, there was resolution of the venous narrowing.
Usually, more than one procedure is required to salvage a fistula; however, salvage rates of 75 to 95% can be achieved with one-year primary patency rates of 30 to 60%. With repeat percutaneous interventions, one-year secondary patency rates can exceed 75%. Careful surveillance and aggressive follow up after placement with early intervention allow these high salvage rates. This approach may help interventionalists conform to the National Kidney Foundation's Dialysis Quality Outcomes Initiative guidelines prescribed for vascular access.
REFERENCES
- Vascular Access 2006 Work Group Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- The Centers for Medicare and Medicaid Services Available at: http://www.cms.hhs.gov/ESRDQualityImproveInit/04_FistulaFirstBreakthrough.asp. Available at: http://www.cms.hhs.gov/ESRDQualityImproveInit/04_FistulaFirstBreakthrough.asp [PubMed]
- Fistula First Dashboard. Available at: http://www.esrdncc.org/index/cms-filesystem-action?file=/fistulafirstdashboard2.pdf. Accessed April 13, 2009. Available at: http://www.esrdncc.org/index/cms-filesystem-action?file=/fistulafirstdashboard2.pdf
- Patel S T, Hughes J, Mills J L., Sr Failure of arteriovenous fistula maturation: an unintended consequence of exceeding dialysis outcome quality Initiative guidelines for hemodialysis access. J Vasc Surg. 2003;38(3):439–445. discussion 445. doi: 10.1016/s0741-5214(03)00732-8. [DOI] [PubMed] [Google Scholar]
- Silva M B, Jr, Hobson R W, II, Pappas P J, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg. 1998;27(2):302–307. discussion 307–308. doi: 10.1016/s0741-5214(98)70360-x. [DOI] [PubMed] [Google Scholar]
- Hyland K, Cohen R M, Kwak A, et al. Preoperative mapping venography in patients who require hemodialysis access: imaging findings and contribution to management. J Vasc Interv Radiol. 2008;19(7):1027–1033. doi: 10.1016/j.jvir.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Beathard G A, Arnold P, Jackson J, Litchfield T, Physician Operators Forum of RMS Lifeline Aggressive treatment of early fistula failure. Kidney Int. 2003;64(4):1487–1494. doi: 10.1046/j.1523-1755.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- Tordoir J H, Rooyens P, Dammers R, der Sande F M van, de Haan M, Yo T I. Prospective evaluation of failure modes in autogenous radiocephalic wrist access for haemodialysis. Nephrol Dial Transplant. 2003;18(2):378–383. doi: 10.1093/ndt/18.2.378. [DOI] [PubMed] [Google Scholar]
- Roy-Chaudhury P, Spergel L M, Besarab A, Asif A, Ravani P. Biology of arteriovenous fistula failure. J Nephrol. 2007;20(2):150–163. [PubMed] [Google Scholar]
- Spergel L M, Ravani P, Roy-Chaudhury P, Asif A, Besarab A. Surgical salvage of the autogenous arteriovenous fistula (AVF) J Nephrol. 2007;20(4):388–398. [PubMed] [Google Scholar]
- Robbin M L, Chamberlain N E, Lockhart M E, et al. Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology. 2002;225(1):59–64. doi: 10.1148/radiol.2251011367. [DOI] [PubMed] [Google Scholar]
- Won T, Jang J W, Lee S, Han J J, Park Y S, Ahn J H. Effects of intraoperative blood flow on the early patency of radiocephalic fistulas. Ann Vasc Surg. 2000;14(5):468–472. doi: 10.1007/s100169910082. [DOI] [PubMed] [Google Scholar]
- Corpataux J M, Haesler E, Silacci P, Ris H B, Hayoz D. Low-pressure environment and remodelling of the forearm vein in Brescia-Cimino haemodialysis access. Nephrol Dial Transplant. 2002;17(6):1057–1062. doi: 10.1093/ndt/17.6.1057. [DOI] [PubMed] [Google Scholar]
- Yerdel M A, Kesenci M, Yazicioglu K M, Döşeyen Z, Türkçapar A G, Anadol E. Effect of haemodynamic variables on surgically created arteriovenous fistula flow. Nephrol Dial Transplant. 1997;12(8):1684–1688. doi: 10.1093/ndt/12.8.1684. [DOI] [PubMed] [Google Scholar]
- Beathard G A. An algorithm of the physical examination of early fistula failure. Semin Dial. 2005;18:331–335. doi: 10.1111/j.1525-139X.2005.18314.x. [DOI] [PubMed] [Google Scholar]
- Turmel-Rodrigues L, Mouton A, Birmelé B, et al. Salvage of immature forearm fistulas for haemodialysis by interventional radiology. Nephrol Dial Transplant. 2001;16(12):2365–2371. doi: 10.1093/ndt/16.12.2365. [DOI] [PubMed] [Google Scholar]
- Falk A. Maintenance and salvage of arteriovenous fistulas. J Vasc Interv Radiol. 2006;17(5):807–813. doi: 10.1097/01.RVI.0000217928.43396.35. [DOI] [PubMed] [Google Scholar]
- Nassar G M, Nguyen B, Rhee E, Achkar K. Endovascular treatment of the “failing to mature” arteriovenous fistula. Clin J Am Soc Nephrol. 2006;1(2):275–280. doi: 10.2215/CJN.00360705. [DOI] [PubMed] [Google Scholar]
- Clark T W, Cohen R A, Kwak A, et al. Salvage of nonmaturing native fistulas by using angioplasty. Radiology. 2007;242(1):286–292. doi: 10.1148/radiol.2421051718. [DOI] [PubMed] [Google Scholar]
- Beathard G A, Settle S M, Shields M W. Salvage of the nonfunctioning arteriovenous fistula. Am J Kidney Dis. 1999;33(5):910–916. doi: 10.1016/s0272-6386(99)70425-7. [DOI] [PubMed] [Google Scholar]
- Faiyaz R, Abreo K, Zaman F, Pervez A, Zibari G, Work J. Salvage of poorly developed arteriovenous fistulae with percutaneous ligation of accessory veins. Am J Kidney Dis. 2002;39(4):824–827. doi: 10.1053/ajkd.2002.32003. [DOI] [PubMed] [Google Scholar]
- Shin S W, Do Y S, Choo S W, Lieu W C, Choo I W. Salvage of immature arteriovenous fistulas with percutaneous transluminal angioplasty. Cardiovasc Intervent Radiol. 2005;28(4):434–438. doi: 10.1007/s00270-003-0211-x. [DOI] [PubMed] [Google Scholar]
- Song H H, Won Y D, Kim Y O, Yoon S A. Salvaging and maintaining non-maturing Brescia-Cimino haemodialysis fistulae by percutaneous intervention. Clin Radiol. 2006;61(5):404–409. doi: 10.1016/j.crad.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Singer-Jordan J, Papura S. Cutting balloon angioplasty for primary treatment of hemodialysis fistula venous stenoses: preliminary results. J Vasc Interv Radiol. 2005;16(1):25–29. doi: 10.1097/01.RVI.0000144868.50440.8A. [DOI] [PubMed] [Google Scholar]
- Wu C C, Lin M C, Pu S Y, et al. Comparison of cutting balloon versus high-pressure balloon angioplasty for resistant venous stenoses of native hemodialysis fistulas. J Vasc Interv Radiol. 2008;19:877–883. doi: 10.1016/j.jvir.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Bhat R, McBride K, Chakraverty S, Vikram R, Severn A. Primary cutting balloon angioplasty for treatment of venous stenoses in native hemodialysis fistulas: long-term results from three centers. Cardiovasc Intervent Radiol. 2007;30(6):1166–1170. discussion 1171–1172. doi: 10.1007/s00270-007-9143-1. [DOI] [PubMed] [Google Scholar]