ABSTRACT
Hemodialysis access grafts are an important component of the treatment of patients with renal failure. Because access sites are limited, maximizing graft lifespan is of major importance to dialysis patients. Pseudoaneurysm formation is a rare, but important complication potentially limiting the longevity of dialysis grafts. With rapidly advancing technology, placement of stent grafts in patients with end-stage renal disease is an important step in prolonging the life of the graft. We conducted a review of the literature regarding stent-graft use for hemodialysis access. In addition, we looked at our experience utilizing the Viabahn® (W. L. Gore & Associates, Newark, DE) stent graft in pseudoaneurysm repair. Our patients achieved primary patency of their grafts for 1, 5, and 9 months, respectively. No complications related to stent-graft implementation have been encountered in six stent-graft implants over the course of 29 months.
Keywords: Arteriovenous grafts, hemodialysis pseudoaneurysm, stent graft repair
With the advancement of endovascular stent technology in the past two decades, much progress has been made in the prolongation of graft life for hemodialysis patients. With end-stage renal disease on the rise, extending the life of an arteriovenous (AV) fistula or graft is critical in the survival of these patients. According to the Quality Improvement Guidelines of the Society of Interventional Radiology, indications for stent placement are well defined.1 Although practice guidelines for stent-graft use for dialysis patients have not been formally defined, published literature suggests that stent grafts can effectively treat pseudoaneurysms and prolong graft life.
Stenosis and thrombosis are the most common causes of failing grafts2; however, pseudoaneurysm formation can contribute considerably toward graft failure. Pseudoaneurysms usually arise from repeated puncture by dialysis needles in a limited area of the graft. This repetitive action contributes to the destruction of existing graft material. In addition, recent literature states that venous outflow stenosis with resultant increase in intragraft pressure also promotes pseudoaneurysm formation.3 Pseudoaneurysms pose a danger to the dialysis patient because they predispose to graft thrombosis, jeopardize the integrity of the graft, and lead to possible rupture. The National Kidney Foundation Disease Outcomes Quality Initiative (KDOQI) established guidelines for treatment of pseudoaneurysms consisting of the following:
The number of cannulation sites are limited by the presence of a large pseudoaneurysm.
The pseudoaneurysm threatens the integrity of the overlying skin.
There is symptomatic pain or throbbing.
There is a developing infection.
The KDOQI guidelines also advise avoiding cannulation if there is evidence of the pseudoaneurysm increasing in size.4
Traditional treatment of pseudoaneurysms involved surgical excision or ligation and subsequent insertion of new graft material. By treating the pseudoaneurysm with an endovascular stent graft, patients can avoid invasive surgery and prolong the life of their existing graft. In this article, we will review the existing literature and present our experience with stent-graft placement to explore treatment options and outcomes for patients with pseudoaneurysm formation in dialysis AV grafts.
CASE STUDIES
At our institution, we have placed stent grafts for the treatment of pseudoaneurysms in hemodialysis AV grafts in four patients from July of 2006 to October of 2008. Six stent grafts were deployed for indications including thrombus formation and enlarging pseudoaneurysms. We exclusively used Viabahn® (W. L. Gore & Associates, Newark, DE) stent grafts (Fig. 1). In all our patients, we attempted to aspirate blood from the pseudoaneurysm sac immediately after insertion of the stent graft. This technique proved successful in all but one case, likely due to the presence of recalcitrant, chronic thrombus within the pseudoaneurysm sac. This patient was also heparin allergic; therefore, the procedure was performed without anticoagulation.
Figure 1.
Digital photograph of an externally deployed Viabahn® stent-graft (W. L. Gore & Associates, Newark, DE) demonstrating flexible expanded polytetrafluoroethylene (ePTFE) material with surrounding external metal stent.
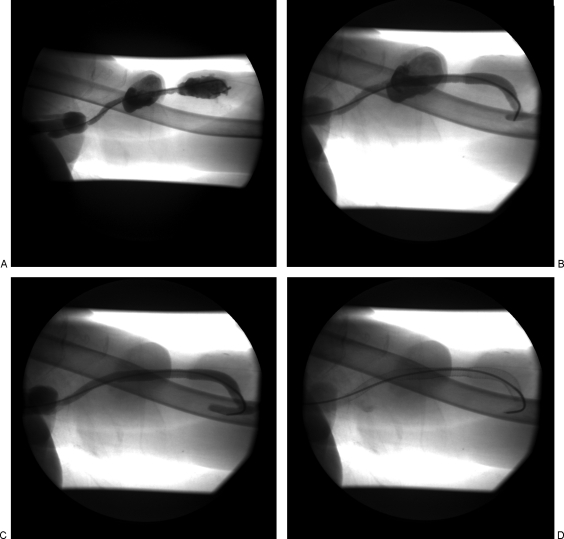
Our first case is a 61-year-old woman with a left upper arm brachial artery to axillary vein graft placed in April 2004, with two pseudoaneurysms in tandem at both the arterial and venous limbs of the graft discovered in May 2008. This patient had a long history of central venous stenoses requiring three separate percutaneous transluminal angioplasty (PTA) treatments in the past year including a pharmacomechanical graft thrombectomy 2 months prior to stent-graft placement. As seen in Fig. 2, the large pseudoaneurysms in tandem near the arterial and venous anastomoses precluded direct access to the dialysis AV graft. In addition, we believed a sufficient landing zone for the stent graft to exclude the more central pseudoaneurysm was unattainable with the high axillary vein anastomosis and technically difficult left axilla approach. Therefore, a right common femoral vein approach was performed. An 18-gauge needle was used to gain access into the right common femoral vein. A 5-F Berenstein catheter (Boston Scientific, Natick, MA) was advanced into the arterial limb of the graft and positioned in the native artery adjacent to the arterial anastomosis. A fistulogram was performed to evaluate the AV graft inflow and venous outflow. Over a 0.035-inch Bentson guide wire (Cook Medical, Bloomington, IN), a long 9-F guiding catheter was positioned within the left subclavian–axillary vein junction. Under direct fluoroscopic guidance, successful exclusion of the pseudoaneurysm near the arterial anastomosis was performed with precise deployment of an 8 × 100 mm Viabahn® stent graft just beyond the anastomosis and at least 10 mm beyond the pseudoaneurysm segment. In similar fashion, a second Viabahn® stent graft of the same length was deployed to ensure sufficient overlap and coverage of the more central pseudoaneurysm. An 8-mm diameter angioplasty balloon was used to ensure adequate apposition of the walls of the stent graft to the hemodialysis graft. Follow-up fistulogram confirmed adequate stent-graft placement without endoleak. The patient's AV graft remained patent and functional without intervention for 5 months until her death from an unrelated traumatic event.
Figure 2.
(A) Left upper arm brachial artery to axillary vein graft with two intragraft pseudoaneurysms in tandem. (B) 8 × 100 mm Viabahn® (W. L. Gore & Associates, Newark, DE) covered stent deployed across the more peripheral pseudoaneurysm. (C) A second 8 × 100 mm Viabahn® covered stent was deployed in an overlapping fashion to exclude the more central pseudoaneurysm. (D) Residual contrast outlines the pseudoaneurysm sacs and the overlying skin deformity.
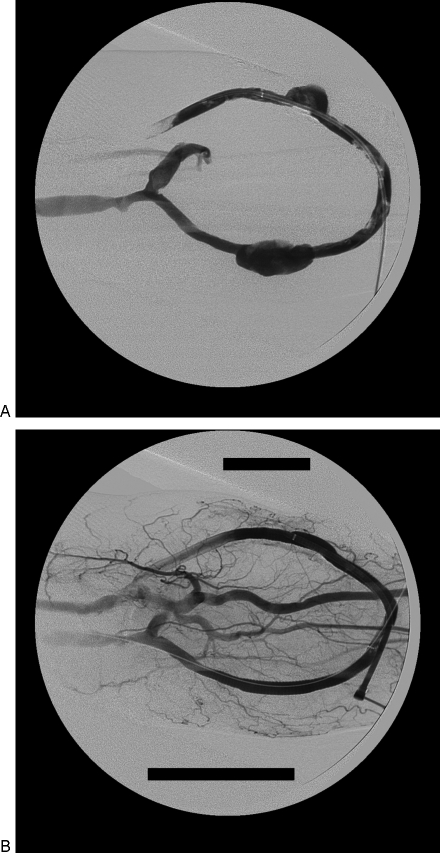
Our next case is a 41-year-old man with a left brachial artery to brachial vein forearm loop graft who was emergently transferred to our institution for treatment of a ruptured (external) iliac-artery aneurysm (Fig. 3). His hemodialysis graft thrombosed likely due to hypotension. Two days later he underwent pharmacomechanical thrombectomy of his left forearm AV graft with subsequent angiography revealing pseudoaneurysm formation at both the arterial and venous limbs of the grafts corresponding to the hemodialysis access puncture sites. The two pseudoaneurysms were treated separately with Viabahn® 8 mm × 100 mm stent grafts with successful exclusion of the pseudoaneurysms. The graft remains patent and functional over 3 months without subsequent intervention.
Figure 3.
(A) A 41-year-old woman with a left brachial artery to brachial vein forearm loop graft, who underwent a pharmacomechanical thrombectomy revealing pseudoaneurysm formation at both the arterial and venous limbs of the grafts at the expected locations of the hemodialysis access puncture sites. Residual intragraft thrombus is visualized. (B) The two pseudoaneurysms were separately treated and successfully excluded with two Viabahn® (W. L. Gore & Associates, Newark, DE) 8 mm × 100 mm stent grafts.
Our third case is a 64-year-old woman with a left upper arm brachial artery to basilic vein graft placed in August 2003. A subcentimeter intragraft pseudoaneurysm was initially present 2 years prior to stent-graft placement. Since that time, the patient underwent pharmacomechanical thrombectomy and two separate PTA treatments for venous anastomotic strictures. A slowly expanding 2-cm-sized pseudoaneurysm at the arterial limb of the graft was treated with a 7 mm × 50 mm Viabahn® stent graft in June 2006. Nine months later the patient underwent dialysis graft angiography and dilation for treatment of stenosis at the venous anastomosis, revealing a patent graft without recurrence of the pseudoaneurysm.
The last case is a 72-year-old man with a left brachial artery to basilic vein graft placed in May 2003 who had undergone three pharmacomechanical graft thrombectomy treatments in a 2-year period. He had several 2 to 3 cm pseudoaneurysms in tandem with associated focal intragraft stenoses within the proximal half of the graft. A single Viabahn® stent graft measuring 7 mm × 15 cm was used to reconstruct the long segment of damaged synthetic graft. One month later the dialysis graft thrombosed due to recurrent high-grade venous outflow lesions. The patency of the AV graft was restored and subsequent fistulogram revealed no evidence of recurrent pseudoaneurysm. Two months later the graft was surgically revised due to recurrent AV graft thrombosis, presumably due to the high-grade venous stricture.
DISCUSSION
Pseudoaneurysm formation is a dangerous outcome for the dialysis patient. By developing ways of excluding pseudoaneurysms in a noninvasive manner, physicians may have a better chance of preserving hemodialysis access. Early studies of expanded polytetrafluoroethylene (ePTFE) grafts such as that of Cinat et al5 emphasize the importance of the development of synthetic grafts in extending the life expectancy of patients with end-stage renal disease. When introduced, ePTFE provided additional options for patients who were in need of vascular access beyond the traditional autogenous fistula due to anatomic constraints.6
There have been various manufacturers of endoluminal stent grafts since ePTFE was introduced in 1976. Technical advances have made it possible for manufacturers to construct stents of various strength and flexibility. Stent grafts used for dialysis AV graft pseudoaneurysm repair include Viabahn®, Wallgraft® (Boston Scientific, Natick, MA), and Fluency® (Bard Peripheral Vascular, Tempe, AZ), with most documented experience using Viabahn® and Wallgraft® stent grafts.3,6,7,8,9,10,11,12,13 The main difference in the construction of these two grafts relates to the position of the stent—the Viabahn is constructed with ePTFE material lining an external metal stent. The Wallgraft® is constructed with woven polyester material external to the metal stent. It has been hypothesized that having the synthetic material external to the stent allows for better incorporation into the surrounding tissue and less chance for development of pseudoaneurysms after repeated needle puncture.11,13 In either form, both are self-expanding covered stents that are flexible and provide enough durability to withstand repeated dialysis punctures.
However, the composition of the metal skeleton may have importance as well. Clark14 described the use of Nitinol stents, which are composed of an alloy of nickel and titanium with “memory” enabling transformation from catheter to its expanded form once in place. Viabahn® is made up of a Nitinol exterior with PTFE liner. The Wallgraft® stent is a shape-memory alloy- (Mediloy) based stent graft with a polyester, externally mounted graft material. Reports by Vogel and Parise15,16 support Clark's belief that Nitinol and other SMART (shape-memory alloy recoverable technology) stents provide longer primary graft patency and go even further to suggest that these stents have an advantage over Wallgraft® stents.
Sapoval et al17 followed 14 patients with situations that limited the use of Wallstents® such as restenosis or residual stenosis. Four of the patients had rupture or pseudoaneurysms prior to stent placement. The patients were treated with implantation of the covered Cragg Endopro® (MinTec, LaCiotat, France) stent. They concluded that the stent was suitable for achieving patency, but did not prevent restenosis. For this reason they no longer use this particular stent in their practice. Hausegger et al7 had similar results in that all three of their pseudoaneurysm case studies needed reintervention within 8 months when using the Cragg Endopro® stent. Despite the fact that all three cases experienced exclusion of their pseudoaneurysms upon stent placement, the stent did not withstand long-term puncture for dialysis. The authors cite their lack of control over where dialysis was administered as the reason for this. Although the Cragg EndoPro® stent was suitable in excluding the pseudoaneurysm, it was deemed inappropriate for long-term management of pseudoaneurysms.
Najibi et al9 followed 10 patients with pseudoaneurysm repair using Wallgraft® stent grafts. Upon follow-up at 6 months, seven patients showed evidence of pseudoaneurysm exclusion. Two patients experienced graft thrombosis at 3 weeks and 3 months, respectively. One patient had early thrombosis immediately following the stent-graft placement.
A recent report by Barshes et al3 specifically supports the durability of the Wallgraft® and Viabahn® stent grafts for pseudoaneurysm repair. They followed 26 patients with AV graft pseudoaneurysms over 5 years: 32 stent grafts were placed. All patients received hemodialysis within 48 hours. Over the course of the study, only four patients underwent revision of the graft. During the follow-up period (mean 9 months), Kaplan-Meier analysis demonstrated a primary patency rate of 82% at one month and 28% at 6 months. 21 patients needed only one endograft to completely exclude the pseudoaneurysm. Four patients needed graft revisions due to return of pseudoaneurysm pulsatility (two patients) or persistently large pseudoaneurysm size (two patients). The mean diameter of the large pseudoaneurysms was 4.3 ± 1.5 cm. Four other patients incurred early thrombosis of their graft at 17, 29, 41, and 63 days, respectively. There was no significant difference between the Viabahn® or Wallgraft® stent grafts.
It should be noted that the Fluency® graft is another covered stent that shares some properties with the Wallgraft® and Viabahn® stents. Moszkowicz et al reported the use of Fluency Plus® stent grafts for pseudoaneurysm repair. They found that of the 28 stent grafts placed in 16 patients, four experienced complications (three with endoleak and one with stent migration).18 Fluency® is a nitinol stent encapsulated with ePTFE along the entire length, minus the flared ends. Although the Fluency® shares some similarities with the other two stents, the evidence is limited and should be studied further.
Criado et al19 found that exclusion of pseudoaneurysms can be achieved with a high degree of technical success, low morbidity rate, and short hospital stay. Today, pseudoaneurysm repair is usually performed as an outpatient procedure. As with Ryan,11 this study encourages early dialysis venipuncture to return the patient to an uninterrupted dialysis regimen. The durability of the graft as described by Barshes also supports the success of early hemodialysis after pseudoaneurysm stent-graft repair. Rhodes and Silas demonstrated that the Wallgraft® can withstand repeated dialysis puncture after pseudoaneurysm repair.10 In their study, the stent-graft location was marked and nurses were instructed to avoid the stent for 2 to 3 weeks following insertion to encourage integration of the graft with the surrounding tissue—a similar technique employed in the Ryan study. Two of their four patients underwent dialysis the following day and the other two waited for 3 weeks. There was no significant difference in outcome between the two groups.
We also believe that early postprocedural dialysis access is essential to consider endovascular repair a viable and effective option to treat dialysis graft pseudoaneurysms. The early paradigm of waiting weeks after stent graft insertion to access the dialysis graft is no longer used.10,12,13 This conservative approach essentially forfeits the advantages of endovascular versus surgical repair. Endovascular repair obviates the need to interrupt use of the dialysis graft. Furthermore, no maturation period of the graft is needed, and undergoing another procedure to gain additional venous access for percutaneous catheter hemodialysis is avoided.
Successful stent graft exclusion of dialysis graft pseudoaneurysms and their long-term results may be a direct result of the initial pseudoaneurysm size. Primary patency results following pseudoaneurysm repair ranged from 20 to 43%5,13,20 over the course of one year. Those with larger-diameter pseudoaneurysms faired poorly when compared with smaller pseudoaneurysms. The four patients who underwent revision in the Barshes study had a mean pseudoaneurysm size of 4.3 ± 1.5 cm.3 Vesley et al13 had two patients requiring surgical repair because the larger pseudoaneurysms did not decrease in size. The precise diameter of these large pseudoaneurysms was not stated, but larger pseudoaneurysms are more prone to leakage, recurrence, or migration into the pseudoaneurysm sac despite stent placement. Further studies on exact diameter measurements of pseudoaneurysms impacting success rate of stent placement would be valuable. Undergoing stent placement may not be a viable or cost-effective treatment option for those with larger pseudoaneurysms.
Multiple reports describe repeated needle cannulation of dialysis grafts—particularly at the same location—leading to damage and breakdown of the graft material and subsequent pseudoaneurysm formation.8,10,13,18,21 This was readily evident with the case of our 41-year-old man (Fig. 2). Two pseudoaneurysms arose from the sites of repetitive dialysis puncture. As previously proposed, a systematic rotation of needle punctures to alternate sites should be strictly followed.11
The multifactorial nature of developing dialysis graft pseudoaneurysms invariably includes intragraft flow dynamics and increased pressures11,12,22 as most dialysis AV graft stenoses recur at the venous anastomosis or peripheral outflow veins. In fact, many of the reported fistulograms after pseudoaneurysm stent-graft repair revealed persistent exclusion of the pseudoaneurysm with venous anastomotic stenosis recurrence.11,12 Silas and Bettmann followed three patients who presented with pseudoaneurysms and recurrent thrombosis. All three patients had Wallgraft® stent grafts used for revisions. The first patient acquired a skin ulcer one month following the procedure, which became infected and the graft needed to be surgically revised. The second patient underwent angioplasty at 5 months following stent placement due to recurrent stenosis at the venous anastomosis. The third patient underwent revision angioplasty at 17 months following stent placement due to venous stenosis.13 There is a correlation between pseudoaneurysm formation and venous outflow stenosis as the primary cause. Several articles we reviewed documented venous anastomotic stenosis as the reason for pseudoaneurysm formation.7,11,12,13,18,21 Similarly, several studies demonstrated venous anastomotic stenosis following pseudoaneurysm repair.7,11,12 It is clear that venous stenosis is a major factor in both inciting development of pseudoaneurysms as well as the cause of complications leading to failure of the graft despite pseudoaneurysm repair. Thus, despite efforts in successfully excluding pseudoaneurysms using stent grafts, the limiting factor of dialysis graft patency is defined by venous outflow lesions.
CONCLUSION
In conclusion, multiple studies have demonstrated the repair of dialysis AV graft pseudoaneurysms with stent-graft exclusion. Our four case studies provide further support for the safety and efficacy of Viabahn® stent grafts. The Wallgraft® and Viabahn® stent grafts are safe, flexible and durable treatment options for patients with AV graft pseudoaneurysms. The composition of these devices is such that repeated dialysis puncture can be endured for many years. Stent grafts are an important treatment option to improve graft patency due to pseudoaneurysms. However, the life of dialysis grafts remains limited by venous outflow lesions and complications thereof. By excluding pseudoaneurysms and continuing timely dialysis graft maintenance, the goal of prolonged graft preservation is attainable.
REFERENCES
- Aruny J E, Lewis C A, Cardella J F, et al. Quality improvement guidelines for percutaneous management of the thrombosed or dysfunctional dialysis access. Standards of Practice Committee of the Society of Cardiovascular and Interventional Radiology. J Vasc Interv Radiol. 1999;10(4):491–498. doi: 10.1016/s1051-0443(99)70071-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez H E, Leon L, Schalch P, Labropoulous N, Borge M, Kalman P G. Arteriovenous access: managing common problems. Perspect Vasc Surg Endovasc Ther. 2005;17:155–166. doi: 10.1177/153100350501700221. [DOI] [PubMed] [Google Scholar]
- Barshes N R, Annambhotla S, Bechara C, et al. Endovascular repair of hemodialysis graft-related pseudoaneurysm: an alternative treatment strategy in salvaging failing dialysis access. Vasc Endovascular Surg. 2008;42(3):228–234. doi: 10.1177/1538574408314443. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation K/DOQI clinical practice guidelines for vascular access. Available at: http://www.kidney.org/PROFESSIONALS/kdoqi/guideline_upHD_PD_VA/va_guide6.htm. Accessed November 9, 2008. Available at: http://www.kidney.org/PROFESSIONALS/kdoqi/guideline_upHD_PD_VA/va_guide6.htm
- Cinat M E, Hopkins J, Wilson S E. A prospective evaluation of PTFE graft patency and surveillance techniques in hemodialysis access. Ann Vasc Surg. 1999;13(2):191–198. doi: 10.1007/s100169900241. [DOI] [PubMed] [Google Scholar]
- Roy-Chaudhury P, Kelly B S, Melhem M, et al. Vascular access in hemodialysis: issues, management, and emerging concepts. Cardiol Clin. 2005;23(3):249–273. doi: 10.1016/j.ccl.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Hausegger K A, Tiessenhausen K, Klimpfinger M, Raith J, Hauser H, Tauss J. Aneurysms of hemodialysis access grafts: treatment with covered stents: a report of three cases. Cardiovasc Intervent Radiol. 1998;21(4):334–337. doi: 10.1007/s002709900271. [DOI] [PubMed] [Google Scholar]
- Lin P H, Johnson C K, Pullium J K, et al. Transluminal stent graft repair with Wallgraft endoprosthesis in a porcine arteriovenous graft pseudoaneurysm model. J Vasc Surg. 2003;37(1):175–181. doi: 10.1067/mva.2002.87. [DOI] [PubMed] [Google Scholar]
- Najibi S, Bush R L, Terramani T T, et al. Covered stent exclusion of dialysis access pseudoaneurysms. J Surg Res. 2002;106(1):15–19. doi: 10.1006/jsre.2002.6389. [DOI] [PubMed] [Google Scholar]
- Rhodes E S, Silas A M. Dialysis needle puncture of Wallgrafts placed in polytetrafluoroethylene hemodialysis grafts. J Vasc Interv Radiol. 2005;16(8):1129–1134. doi: 10.1097/01.RVI.0000167852.14245.05. [DOI] [PubMed] [Google Scholar]
- Ryan J M, Dumbleton S A, Doherty J, Smith T P. Technical innovation. Using a covered stent (Wallgraft) to treat pseudoaneurysms of dialysis grafts and fistulas. AJR Am J Roentgenol. 2003;180(4):1067–1071. doi: 10.2214/ajr.180.4.1801067. [DOI] [PubMed] [Google Scholar]
- Silas A M, Bettmann M A. Utility of covered stents for revision of aging failing synthetic hemodialysis grafts: a report of three cases. Cardiovasc Intervent Radiol. 2003;26(6):550–553. doi: 10.1007/s00270-003-0013-1. [DOI] [PubMed] [Google Scholar]
- Vesely T M. Use of stent grafts to repair hemodialysis graft-related pseudoaneurysms. J Vasc Interv Radiol. 2005;16(10):1301–1307. doi: 10.1097/01.RVI.0000175903.38810.13. [DOI] [PubMed] [Google Scholar]
- Clark T WI. Nitinol stents in hemodialysis access. J Vasc Interv Radiol. 2004;15(10):1037–1040. doi: 10.1097/01.RVI.0000136029.88286.48. [DOI] [PubMed] [Google Scholar]
- Vogel P M, Parise C. Comparison of SMART stent placement for arteriovenous graft salvage versus successful graft PTA. J Vasc Interv Radiol. 2005;16(12):1619–1626. doi: 10.1097/01.RVI.0000179792.23867.01. [DOI] [PubMed] [Google Scholar]
- Vogel P M, Parise C. SMART stent for salvage of hemodialysis access grafts. J Vasc Interv Radiol. 2004;15(10):1051–1060. doi: 10.1097/01.RVI.0000129915.48500.DC. [DOI] [PubMed] [Google Scholar]
- Sapoval M R, Turmel-Rodrigues L A, Raynaud A C, Bourquelot P, Rodrigue H, Gaux J C. Cragg covered stents in hemodialysis access: initial and midterm results. J Vasc Interv Radiol. 1996;7(3):335–342. doi: 10.1016/s1051-0443(96)72863-4. [DOI] [PubMed] [Google Scholar]
- Moszkowicz A, Behrens G, Gueyikian S, Patel N, Ferral H. Occlusion of a rapidly expanding hemodialysis graft pseudoaneurysm with placement of a stent graft. Semin Intervent Radiol. 2007;24:34–37. doi: 10.1055/s-2007-971183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado E, Marston W A, Ligush J, Mauro M A, Keagy B A. Endovascular repair of peripheral aneurysms, pseudoaneurysms, and arteriovenous fistulas. Ann Vasc Surg. 1997;11(3):256–263. doi: 10.1007/s100169900043. [DOI] [PubMed] [Google Scholar]
- Farber A, Barbey M M, Grunert J H, Gmelin E. Access-related venous stenoses and occlusions: treatment with percutaneous transluminal angioplasty and Dacron-covered stents. Cardiovasc Intervent Radiol. 1999;22(3):214–218. doi: 10.1007/s002709900369. [DOI] [PubMed] [Google Scholar]
- Hein A N, Vesely T M. Use of the percutaneous thrombolytic device for the treatment of thrombosed pseudoaneurysms during mechanical thrombectomy of hemodialysis grafts. J Vasc Interv Radiol. 2002;13:201–204. doi: 10.1016/s1051-0443(07)61939-3. [DOI] [PubMed] [Google Scholar]
- Sullivan K L, Besarab A, Bonn J, Shapiro M J, Gardiner G A, Jr, Moritz M J. Hemodynamics of failing dialysis grafts. Radiology. 1993;186(3):867–872. doi: 10.1148/radiology.186.3.8430200. [DOI] [PubMed] [Google Scholar]