ABSTRACT
Therapeutic embolization is a common procedure in interventional radiology. A wide variety of agents are available, and each has its own place and use. Additionally, many new agents have appeared on the market in the past several years. The aim of this review article is to give a brief description of available agents, guide appropriate selection, and familiarize the reader regarding appropriate use and limitations.
Keywords: Embolization, Gelfoam, coils, glue, Onyx, polyvinyl alcohol, Embosphere
Therapeutic embolization is the intentional endovascular occlusion of an artery or vein. The embolic agent of choice depends on the desired clinical outcome, as well as the inherent properties and behavior of the agent. Familiarity with the unique characteristics of different agents is essential for clinical success.
Historically, the first agent used for embolotherapy was autologous blood clot. This was easily and quickly obtained and was inherently biocompatible. The drawback of autologous blood clot is that as the body's natural clot lysis dramatically limits the durability of occlusion; recanalization can recur within hours to days. The next agents developed were fascial strips harvested from dura and tensor fascia lata. Silk threads were also historically used as embolic agents, notably for intracranial vascular malformations. Today with the advent of modern liquid and particulates, silk, clot, and fascia are not used.
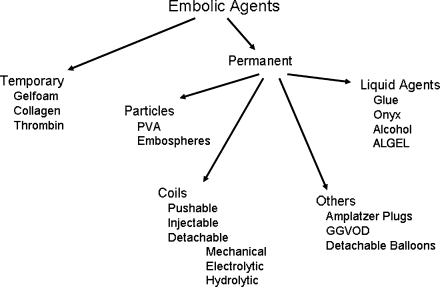
Modern embolic agents are either temporary or permanent. Permanent agents are more common, and there are many applicable subsets including liquid agents, particulates, coils, and detachable plugs and balloons (Fig. 1).
Figure 1.
Divisions of embolic agents. PVA, polyvinyl alcohol; GGVOD, Grifka-Gianturco vascular occlusion device.
GELATIN FOAM
Gelatin foam (Gelfoam, Upjohn, Kalamazoo, MI) has been used as an intravascular embolization agent for more than 30 years, with the first intravascular use in 1964 for cavernous carotid fistulas.1 Since that time, Gelfoam has evolved into a common agent used for a variety of applications. Use of Gelfoam is off-label for embolization, but its use is within the standard of care.2
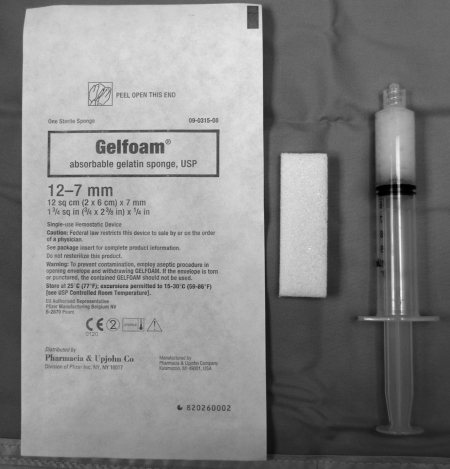
Gelatin foam is a biologic substance made from purified skin gelatin. It is available in sterile sheets and as a powder comprised of 40 to 60 μm particles (Fig. 2). Sheets can be cut into a variety of shapes. Gelfoam cut into small 1 to 2–mm pieces can be mixed with dilute contrast and injected as pledgets, or be prepared as slurry (Fig. 3). Another standard use of gelatin foam is to form a small torpedo that can be injected into the target vessel for a more proximal occlusion. Gelatin foam particles, however, can aggregate or swell on hydration into larger particles.
Figure 2.
Gelfoam package, slab, and slurry (left to right).
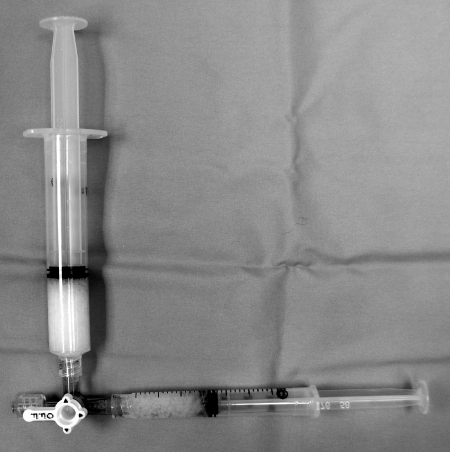
Figure 3.
Setup for particle embolization. Larger syringe is reservoir and smaller is for injection. Open end of the three-way connects to the catheter. This setup is for all particles as well as gelfoam slurry.
Gelatin foam causes mechanical obstruction, slowing blood flow and hastening thrombus formation. In addition, it provides a scaffold for clot formation. Gelfoam embolization provides temporary vessel occlusion, allowing recanalization in a few weeks. The temporary nature of gelatin foam occlusion can be either an advantage or disadvantage depending on the clinical situation (e.g., temporary nature is advantageous in case of hemoptysis or trauma). Other advantages of Gelfoam include low cost, versatility of use, and extensive clinical experience. However, it has been postulated that Gelfoam can be associated with infection due to trapped air bubbles. Further, gelatin foam powder can potentially cause ischemia due to the small size (especially at sizes < 70 μm) of the particles, allowing distal embolization.
POLYVINYL ALCOHOLPARTICLES
First introduced as an intravascular embolization agent in 1974 in the form of a sponge,3,4 current intravascular use of polyvinyl alcohol (PVA) (Boston Scientific/Target Therapeutics, Cork Ltd., Cork, Ireland, Cordis J&J Endovascular, Miami, FL, USA) is primarily in the form of particles. The particles are made from a PVA foam sheet that is vacuum dried and rasped into particles. The particles are filtered with sieves and are available in sizes ranging from 100 μm to 1100 μm. Because of the method of preparation, PVA particles are slightly irregular in size (Figs. 4 and 5). Particles are sorted by their ability to pass through a larger sieve, but not through a smaller sieve. Thus, oblong particles may have a long axis that is much longer than the stated size.5 One study evaluating actual size of PVA particles found that the majority were larger than the stated minimum size, with a few very small particles observed once the PVA particles were suspended in solution.5 Polyvinyl alcohol particles are irregular in shape, which promotes aggregation. They can be oblong, oval, irregular, sharp, and angulated with small fragments after suspension.6
Figure 4.

Polyvinyl alcohol particles magnified, showing irregular nature and variation in size.
Figure 5.

Polyvinyl alcohol vial.
Polyvinyl alcohol particles provide permanent occlusion by adherence to the vessel wall, causing stagnation of flow, in addition to lodging in the smallest vessel into which they will fit.7 The results are an inflammatory reaction and focal angionecrosis, with vessel fibrosis developing over time. Polyvinyl alcohol particles are biocompatible, and there is vast cumulative clinical experience with PVA particle embolization.
The major disadvantage of PVA particles is their tendency to aggregate, occluding vessels more proximally than might be expected based on stated size. Particle clumping can also cause catheter occlusion, which is preventable by dilution of particles, proper suspension, and slow infusion. In addition, PVA particles can accumulate in the catheter hub6 and theoretically cause subsequent nontarget embolization when the catheter is flushed.
TRIS-ACRYL GELATIN MICROSPHERES
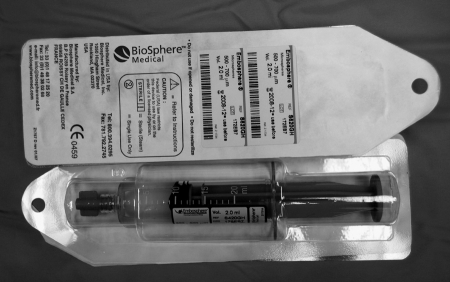
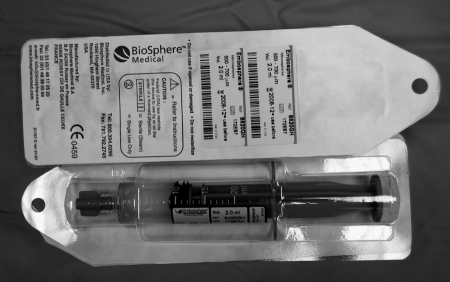
Use of tris-acryl gelatin microspheres (TAGM) (Embospheres, Biosphere Medical, Rockland, MA) was approved by the U.S. Food and Drug Administration (FDA) in 2000 and gained specific approval for uterine fibroid embolization in December 2002.8 Embospheres are available in six size ranges: 40 to 120 μm, 100 to 300 μm, 300 to 500 μm, 500 to 700 μm, 700 to 900 μm, and 900 to 1200 μm. Embospheres are packaged in 20-mL prefilled syringes containing 2 mL of spheres in saline (Fig. 6). Embosphere gold particles are colored for visibility.
Figure 6.
Prefilled Embosphere syringe.
Tris-acryl gelatin microspheres are made from an acrylic polymer matrix impregnated and embedded with porcine gelatin.9 They are nonresorbable hydrophilic particles that are precisely calibrated by size. In addition, TAGM can be temporarily compressed by 20 to 30% of their initial diameter.
Unlike PVA particles, TAGM are smooth and spherical in shape and fragmentation is not observed (Fig. 7).8 Particle accumulation in the catheter hub, particle aggregation fragmentation, and catheter occlusion are uncommon with TAGM. In fact, larger TAGM may deform allowing delivery through a microcatheter with a lumen smaller than the maximum diameter of the sphere.8,9 By avoiding particle aggregation, Embospheres may penetrate into smaller vessels compared with PVA particles of the same size. Embospheres cause vascular occlusion by lodging in vessels and inciting a histological reaction similar to PVA particles when studied at 48 hour and 4 weeks.8 Embospheres are still identifiable in the tissue at 4 weeks, both intra- and extravascularly, and degeneration of the spheres has not been observed.10
Figure 7.

Magnified view of Embospheres showing uniform nature.
Disadvantages of Embospheres include the need for intermittent agitation to prevent sedimentation and maintain suspension. In addition, Embospheres are partly composed of porcine gelatin, which has allergic potential. Careful attention to sizing is necessary. The same size of Embosphere will penetrate more deeply compared with PVA and could cause unintended ischemia in some vascular beds such as the colon. The difference in effective particle size when using PVA particles versus Embospheres can be significant.10
COILS
Gianturco et al designed and described the use of coils for embolotherapy in 1975.11 The original coil was a 5-cm curled segment from a 0.038-inch guidewire. Later, wool strands were attached to the coils to increase thrombogenicity. The original coils were only compatible with a thin-walled, preferably Teflon-coated, 7F system. Treatment of arteriovenous malformations (AVMs) was among the first uses described.11,12,13 The “mini-coil,” introduced in 1978, could be delivered through a nontapered 5F system.14
Coils are permanent embolic agents that come in a variety of shapes and sizes. In general, they are easy to see, control, and deploy. They are typically used for occlusion of larger vessels and cause complete occlusion equivalent to surgical ligation. Coils cause vessel occlusion by inducing thrombosis. Deployment of a coil acts by physically slowing or stopping blood flow, providing a thrombogenic surface for clot formation, and causing vessel wall damage that results in release of thrombogenic factors. Bare coils can only partially embolize vessels by pure mechanical occlusion. Because coil embolization depends on the ability of the patient to form thrombus, coagulopathic states such as thrombocytopenia, platelet dysfunction, and abnormal clotting factors may hinder complete vessel occlusion. Time to occlusion also depends on the type of coil used, as well as the rate of flow of the target vessel embolized. Typically, thrombosis occurs within less than 5 minutes after deployment. Over time, coils induce a chronic inflammatory response with organized thrombus and subsequent neointimal formation and fibrosis.
Potential complications of coil embolization include occlusion of a nontarget vessels and coil migration. Coil migration can result in pulmonary embolism, stroke, or myocardial infarction, particularly when treating high-flow conditions such as AVMs or arteriovenous fistulas (AVFs). In the presence of left-to-right shunts, paradoxical coil embolization is possible. Other complications of coil embolization include vessel dissection, perforation, and vessel rupture. To minimize the risk of vessel rupture, soft detachable coils have been developed. These coils come in a variety of stiffness levels, including ultrasoft, soft, standard, firm, and stretch resistant. Infection and allergic reaction to coils are uncommon. There have been seven published case reports of infection related to coil deployment. Colonization of coils with micro-organisms can occur during coil insertion or develop in the setting of bacteremia.15 The use of preprocedural antibiotics during coil placement is controversial and has not been shown to decrease the chances of infection.
In general, coils should be sized 20 to 30% larger than what the vessel measures on predeployment angiogram to prevent distal embolization or migration. Placement of an undersized coil risks its distal embolization away from the intended location. Attempting to place an oversized coil may result in the coil not forming the intended shape or even straightening in the vessel.
Coils are generally made of steel or platinum. Although more expensive, platinum coils are more malleable and radiopaque and are easier to see under fluoroscopy compared with similarly sized and shaped steel coils. Coils range in size from 0.008 to 0.052 inches. Standard angiographic catheters are used to deliver 0.035/0.038 steel coils. Once advanced into the catheter, however, standard steel coils cannot be retrieved without removing the entire catheter with the coil still inside. If a vessel is too small to allow passage of a standard size catheter, a microcatheter may be used to select the target vessel and a microcoil can be used. Microcoils are typically made of platinum wire from 0.012 to 0.018 inches in diameter.
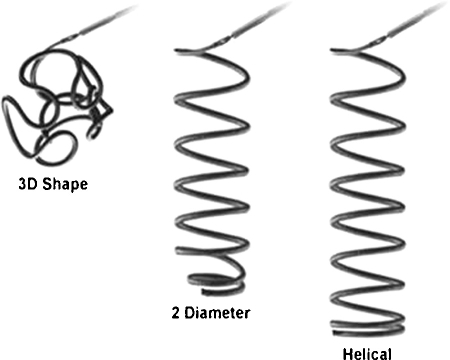
Coils are available in various lengths, diameters, and shapes (Figs. 8 and 9). Coils are available in lengths from 1 to 300 mm and in diameters ranging from 1 to 27 mm. Available coil shapes include J- or C-shaped, helical, conical, tornado, straight, and complex three dimensional (3D) shapes. Coils may be bare or fibered with material such as Dacron, nylon fibers, polyester, wool, silk, or PVA embedded within them to increase thrombogenicity. In summary, there is a vast array of different coils and the choice of type of coil depends on the vessel, aneurysm, and so forth. being treated, as well as the overall clinical situation.
Figure 8.
Various coil shapes.
Figure 9.
Different coil configurations, usually seen with detachable coils.
In general, densely packing the target vessel is recommended to achieve complete embolization. Using a guide catheter for support with a microcatheter will provide more purchase to tightly pack coils and avoid secondary coil compaction. The key to achieving thorough thrombosis is cross-sectional occlusion.16 This can be achieved via different techniques. A scaffold technique involves initially deploying a higher radial force coil, followed by a softer coil. In the anchor technique, a distal coil is anchored in a branch vessel then packed proximally.17 This prevents distal migration and results in a tighter coil pack. An inflated compliant occlusion balloon may be used as the coil delivery catheter to prevent unwanted distal embolization, especially in high-flow AVFs.
The methods of coil delivery are numerous and include simple pushing, injecting, and commercial detachment systems.
Pushable Coils
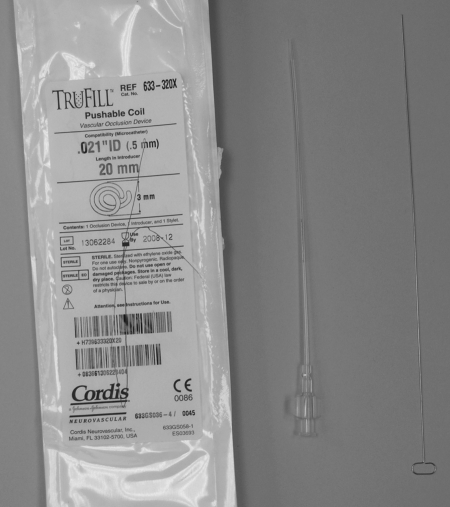
Pushable coils (Fig. 10) are the most commonly used in the interventional radiology arena. A special guidewire with a bulbous tip (Fig. 11) is used to physically push the coil through an end-hole catheter into the desired position. Advantages of pushable coils include ready availability, relative cost, and ease of use. Disadvantages are that pushable coils may not be repositioned once deployed; can become trapped at sharp curves of the catheter; and, if incompatible with the catheter, can become irretrievably jammed in the catheter. Care should be taken not to advance the guidewire used as a coil pusher beyond the catheter tip after the coil is released, as the coil may be inadvertently advanced too far distally. Also, when there is little purchase in the target vessel, and/or when using an oversized coil, deploying a simple pushable coil may result in the catheter backing out of the vessel resulting in a precarious situation that could result in nontarget embolization.
Figure 10.
Pushable coil. Left to right: package, loaded introducer, initial introducing stylet.
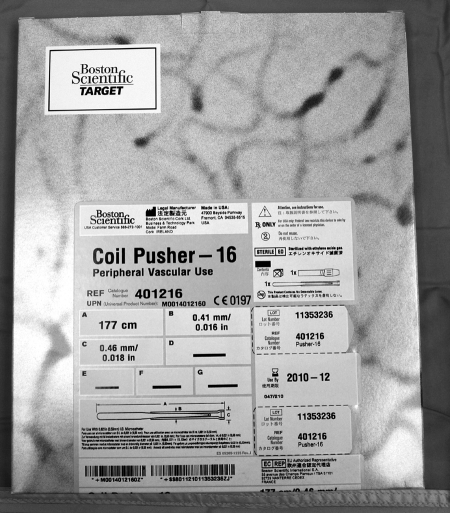
Figure 11.
Coil pusher.
Injectable Coils
Injectable coils are simply standard pushable coils that tolerate injection through a catheter via a small syringe filled with saline, saving time by not having to use a pusher wire. This method relies on secure and adequate vessel purchase, and is generally quicker but less precise than other methods. Vigorous injection can result in pushing the catheter back substantially and risking nontarget embolization. Soft 0.035 and 0.038 coils can be delivered via injection, but this method is more commonly used with microcatheters and soft platinum coils.
Liquid Coils
Liquid coils are also deployed by forceful injection of contrast through the catheter after loading the coil (Fig. 12). These are extremely soft, nonfibered platinum coils of 0.008-to-0.016-inch diameters. Although made of metal, liquid coils can be delivered across tight bends and conform to the space into which they are injected.18 Effectiveness depends on coil compaction, coil mass and physical obstruction. Delivery of a liquid coil is through a preloaded introducer. An adapter is required if using the 0.008 coil. The coil is loaded into the microcatheter using a 1-mL syringe of saline. A 3-mL saline syringe is used to advance and deploy the coil. Advantages of liquid coils include tight coil compaction, ability to accommodate to tortuous anatomy, and ability to flow to a target distal to the catheter if desired. Liquid coils are optimal for treating tortuous moderate size vessels or multiple vessels. Multiple platinum microcoils, delivered over several sessions, may be required to treat AVMs.
Figure 12.
Liquid coil packaging.
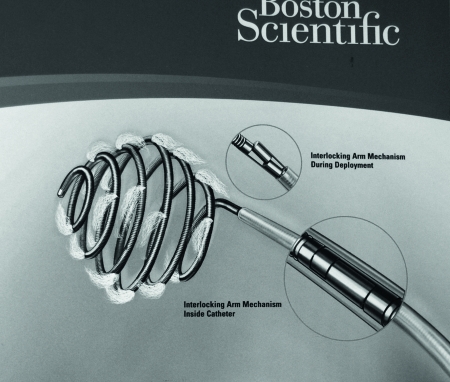
Detachable Coils
The first detachable coil was described in 1977 by Professor Cesare Gianturco and consisted of a coil attached to an introducer wire through a proximal corkscrew on the coil. Gianturco first used these coils to embolize renal tumors. Current detachable coils deploy by a variety of mechanisms, including mechanically, by electrolysis, and via hydrostatic means. They come in a variety of shapes such as 3D basket type and two-dimensional (2D) helical type. Disadvantages of detachable coils include expense and longer setup time. In addition, detachable coils are not generally necessary for many indications in the typical interventional setting.
Mechanical detachment includes interlocking mechanical detachment and screw-release mechanisms. The interlocking mechanism uses small metal beads, or hinge, at the proximal coil tip and end of the wire (Fig. 13). The coil is fastened by overlapping the beads hinge. Within the catheter the coil is attached, but once out of the catheter the beads separate and the coil is released. The disadvantage of this system is that there is often friction between the microcoil and microcatheter. When embolizing through tortuous vessels, this friction can limit delivery. With a screw-release mechanism, spiral threads are on the proximal coiled tip and on the wire. A mandrill is passed through the wire and coil. The coil is attached by clockwise rotation, so to release the coil the wire is rotated counterclockwise. The main disadvantage of this system is that the coil can rotate or flip at detachment by inadvertent detachment during wire manipulation.
Figure 13.
Mechanically detached coil (hinge mechanism).
Electrolytic detachment was designed and first used by Dr. Guido Guglielmi in 1991. Guglielmi-detachable coils (GDCs) were originally designed for embolization of intracranial saccular aneurysms. These coils revolutionized endovascular treatment of intracranial aneurysms by offering a device that was safe and relatively effective compared with prior methods of endovascular therapy. The original GDC is a nonfibered, extremely soft, uncoated platinum coil affixed to a stainless steel delivery wire. A 1-mA current is used to detach the coil at the weld point. A 2-mA current can alternatively be used to detach the coil more quickly. Currently, the platinum coil is welded to platinum-tungsten alloy and has a highly successful deployment rate (Fig. 14).
Figure 14.
Electrolytic detachable coil setup: connecting cables and power supply (left to right).
Electrolytic coil detachment provides the ability to reposition the coil, change the coil configuration, or completely remove the coil before detachment. In addition, the coil is detached without motion, allowing precise positioning and controlled release. Other advantages of GDC include good trackability, softness, and visibility with fluoroscopy. The disadvantages are that they are relatively expensive, require more operator experience, and require an elaborate setup before delivery. The most common clinical uses are cerebral aneurysms and AVMs where precise deployment is mandatory. However, use in visceral artery aneurysms and pseudoaneurysms has been described.
Technical problems that have been described include premature coil detachment without supplying current, proximal end slipping back into the microcatheter after deployment, and delivery catheter tip melting and adhering to the coil due to heat generated.
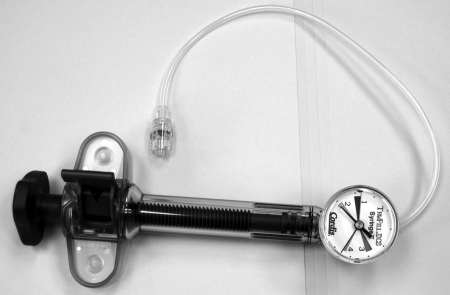
Hydrostatic detachment uses balloon technology. A platinum microcoil is attached to the end of the micro delivery tube. The tip of the tube has a microballoon that holds the coil in place. To detach the coil, an inflator syringe (Fig. 15) is connected to the hub and up to 700 psi is applied, expanding the microballoon, and the coil is released. A disadvantage is potential for coil motion during release.
Figure 15.
Graded syringe for detachment of hydrolytic detachable coils.
Hydrogel Coils
Hydrogel coils are detachable platinum coils coated with an expandable polymer. When detached in the vasculature, the polymer expands and coil diameter increases from 0.014 to 0.027 inches (up to a ninefold increase in volume). Complete expansion occurs within 20 minutes (Fig. 16). Hydrogel is a liquid reaction mixture that contains a monomer, a cross-linker, and a polymerization initiator (porosigen). Expansion occurs through the incorporation of unsaturated monomers with ionizable functional groups causing disentanglement of polymer chains and swelling,19,20 Hydrogel is physiologically stable and unaffected by natural thrombolytic processes that may cause recanalization.
Figure 16.

Hydrogel coil. Top image is initial coil, and lower image is after 20 minutes of hydration showing the swollen gel coating.
Hydrogel coils provide a greater volume occlusion than regular coils and do not depend on thrombus formation. This can theoretically confer more complete aneurysm fill. Disadvantages of hydrogel coils include the potential for a coil to become stuck in the catheter if the coil is not compatible with the delivery system.
DETACHABLE SACK VASCULAR OCCLUSION DEVICE
First described by Dr. Ronald G. Grifka and Prof. Cesare Gianturco, the detachable sack Grifka-Gianturco vascular occlusion device (GGVOD) incorporates a flexible nylon sack in varying diameters attached to a 4.5F sack catheter (Fig. 17). Coils are advanced into the sack and then the filled sack is released from the catheter by advancing a release catheter up against the neck of the sack. The sack catheter is the withdrawn firmly, which releases the sack. The GGVOD allows repositioning of the device before release; coils can be pulled out of the sack and the sack can be pulled back into the sack catheter. This is not FDA approved and not commercially available in United States.
Figure 17.
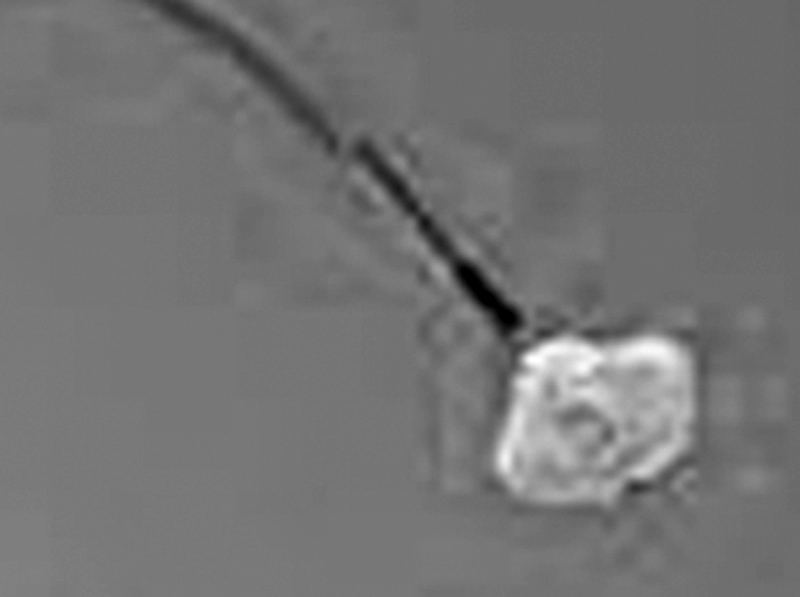
The Grifka-Gianturco vascular occlusion device. The sack contains a wire mesh for bulk. The sack is pushed off to occlude the intended vessel.
DETACHABLE BALLOON
The detachable balloon is not currently FDA approved for use in the United States. It was first used in 1974 and was originally designed for carotid cavernous fistulas.21 Its advantages are the ability to occlude large vessels and the possibility of being repositioned. Disadvantages are rupture risk, deflation, migration, and premature detachment. The balloon is made of latex or silicon. Sizes available are: latex, 6 to 14 mm; silicon, 4 to 10 mm. They would be ideal in cavernous carotid fistulas, pulmonary AVMs, and large-vessel occlusion.
AMPLATZER VASCULAR PLUGS
Amplatzer Vascular Plugs (AGA Medical, Plymouth, MN) are relatively new and include a family of expandable nitinol mesh vascular occlusion devices that are derived from septal occluders commonly used in cardiology. The first device marketed, the Amplatzer I, is a simple thick disk that ranges from 4 to 16 mm in diameter in 2 mm increments. The 4-,6-,8-mm plugs are 7 mm long and require a deployment catheter with minimum inner diameter (ID) of 5F. The 10- and 12-mm diameter devices are 7 and 8 mm long, respectively, and require a 6F guiding sheath; the 14- and 16-mm diameter ones are 8 mm long and require 8F guide or 6F sheath catheter for deployment (Fig. 18).
Figure 18.
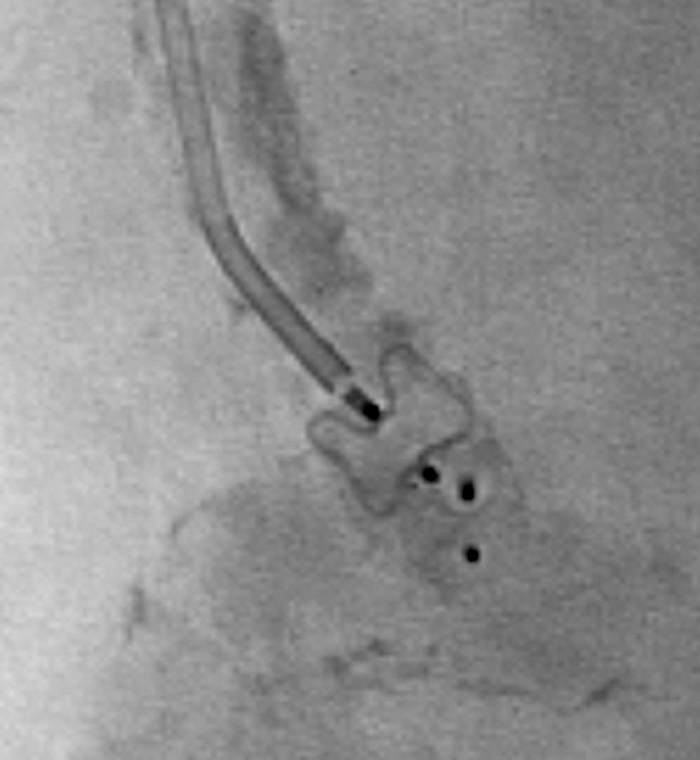
Amplatzer Vascular Plug, used to occlude the inflow to a popliteal aneurysm. This image shows two devices within the vessel.
The second device of the same family, the Amplatzer II, includes an additional thinner disk attached at either end for additional cross-sectional material and ranges in size from 3 to 22 cm in diameter and 6 to 18 cm in length. These devices have a stainless steel screw attachment to a delivery wire and radio-opaque marker bands at both ends. The delivery wire allows for precise placement, and the deformability of the plug allows it to be recaptured by advancing the delivery catheter over the plug. Although relatively expensive, vascular plugs may reduce the need for multiple individual coils and hence potentially save money and time in some areas.
Disadvantages to these plugs include a need to be deployed in a relatively straight segment of vessel that does not taper. The Amplatzer does not cause instantaneous thrombosis, and in high-flow situations may take several minutes.17 Also, as they are nonfibered and rely on the patient's ability to form thrombus, their use may be limited in patients that are significantly coagulopathic. Many uses have been described including occlusion of the internal iliac artery (before aortoiliac endograft repair to prevent endoleak), mesenteric arteries, renal artery, portal vein, splenic artery, transjugular intrahepatic portosystemic shunt (TIPS), and so on.22,23
Amplatzer plugs should be oversized by 30 to 50% of the vessel diameter at the occlusion site. They are prepackaged with a loader and attached to a delivery wire. The loader is inserted into the delivery catheter, which should be less than 100 cm in length. It is not recommended for use with a braided sheath due to excess friction. The Amplatzer is pushed through the catheter with the deliver wire to the level of desired occlusion. The catheter is then withdrawn to unsheathe the device and allow it to expand. If the device is satisfactorily in position, a plastic vise or clamp is attached to the wire and the plug is released by rotating the delivery wire counterclockwise. Contrast can be injected through the guiding sheath to confirm position before deployment. The device can be resheathed if not in proper position.
N-BUTYL-2 CYANOACRYLATE
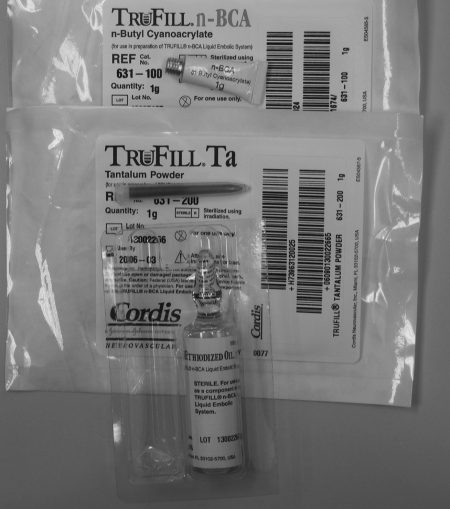
N-butyl-2 cyanoacrylate (NBCA) (TruFill, Cordis, Miami Lakes, Fl; Glubran 2, Gem, Viareggio, Lucca, Italy) has several forms. TruFill is a cyanoacrylate-based synthetic glue that was approved by the FDA in 2000 for cerebral AVM embolization. It is supplied as one or two 1-g tubes of NBCA, 10 mL of ethiodized oil, and 1 g of tantalum powder, which are mixed together immediately before use.24 TruFill has been reported in the treatment of extracerebral and spinal tumors and spinal AVMs and AVFs. Glubran 2 is a similar agent approved for use in Europe, and has been used for embolization of brain and spinal cord tumors, cerebral AVMS, and brain and spinal cord dural AVFs.25
N-butyl-2 cyanoacrylate is supplied as a free monomer, which is clear and free flowing. When exposed to an anionic environment such as blood or water, polymerization occurs.24 Ethiodol is used as a vehicle and acts as a polymerization retardant. Tantalum powder is included to provide radiographic opacification, but also slows initiation of polymerization. Before use, adequate visualization should be confirmed with fluoroscopy (Figs. 19 and 20).
Figure 19.
TruFill n-butyl-2 cyanoacrylate (glue). Image shows the glue vial on top with the tantalum powder below and the Ethiodol at the bottom. These come together as a package or separately.
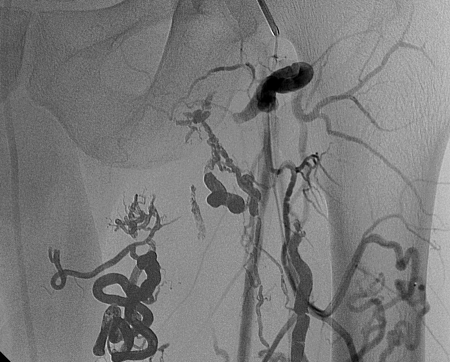
Figure 20.
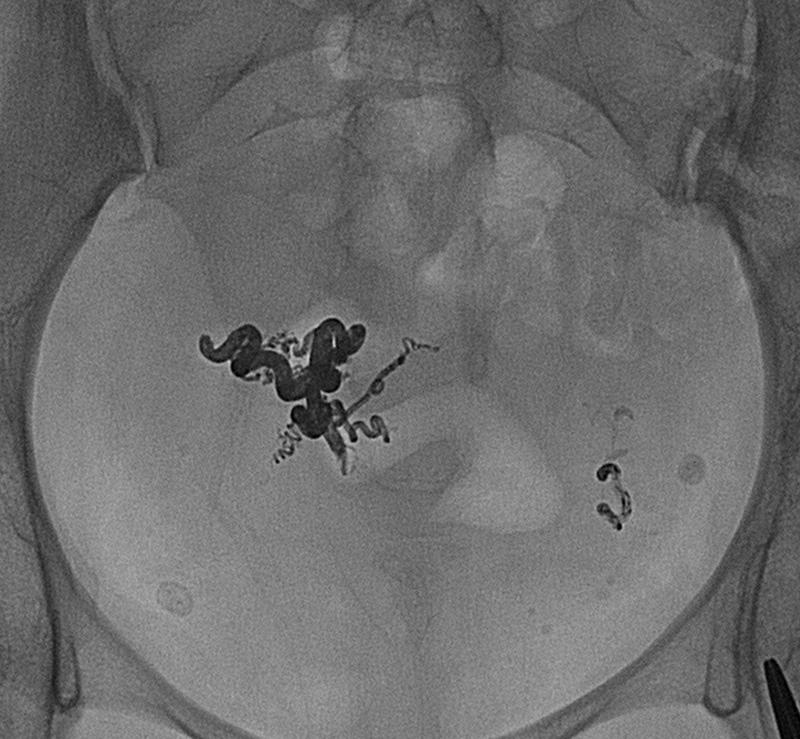
Glue cast in a pelvic arteriovenous malformation. Opacity is due partly to Ethiodol and partly to the tantalum.
Polymerization of NBCA starts immediately on contact with anions, at a rate dependent on the concentration of the NBCA. Polymerization rate can be altered by varying the NBCA concentration with Ethiodol (Savage Laboratories, Melville, NY, USA) or glacial acetic acid. To avoid unintended polymerization by premature contact with anions, catheter flushes should be performed with dextrose 5% (D5W) in water. In addition, cyanoacrylate can destroy polycarbonate, so polypropylene syringes should be used.2
N-butyl-2 cyanoacrylate embolization requires a special setup that includes attaching a syringe of NBCA and a syringe of D5W to the catheter via a three-way stopcock. Initial contrast injection is performed to determine the volume of glue injection required. Immediately after glue injection, the catheter tip is retracted to avoid catheter adherence to the vessel lumen. The catheter often occludes after one to two glue injections.
N-butyl-2 cyanoacrylate makes a permanent cast of the vessel, independent of inherent coagulation. It incites an acute inflammatory reaction in the vessel wall, which progresses to chronic inflammation and fibrosis.24 Recanalization can occur if only partial embolization is achieved. Advantages of NBCA are that it works instantly, completely occludes vessels, and is permanent. Disadvantages are that use of NBCA requires expertise. The catheter can become entrapped in the occluded vessel. Polymerization can spread distally or reflux proximally to the intended location. Finally, as part of embolization, catheter position must be abandoned.
ETHYLENE VINYL ALCOHOL COPOLYMER
Ethylene vinyl alcohol copolymer (EVOH) (Onyx, Micro Therapeutics, Inc., Irvine, CA) was first used as an embolic agent by Dr. Taki in 199026 and was granted FDA approval in July 2005 as an embolic agent for cerebral AVM. It is a copolymer of ethylene vinyl alcohol prepared with dimethyl sulfoxide (DMSO) as solvent. Tantalum powder is added for opacity. On contact with blood, the DMSO diffuses away allowing polymerization of the EVOH, which forms a cast of blood vessels. In the United States, EVOH is available under the trade name Onyx, and comes prepared as Onyx 18 and Onyx 34, which differ in viscosity. Onyx 500 is available outside the United States and is indicated for the treatment of cerebral aneurysms. Lower concentration of Onyx results in a lower viscosity solution that can be injected into smaller vessels.27
Onyx comes packaged with a separate vial of DMSO, and special DMSO-compatible catheters must be used for the procedure. Once the catheter is placed in desired position, a small amount of DMSO is injected to fill the catheter dead space and to inhibit in-catheter Onyx polymerization. Once the catheter is in position, Onyx is delivered through the catheter under fluoroscopy (Figs. 21 and 22).
Figure 21.
Onyx components: dimethyl sulfoxide (DMSO) vial, Onyx vial, and shaker. The Onyx needs to be shaken for ~20 minutes before use.
Figure 22.
Onyx cast in thigh arteriovenous malformation. Note similar appearance to glue cast.
Histologic analysis of AVMs on a few patients embolized with Onyx demonstrated filling of the vessel lumen either partially or completely. Specimens resected 1 day postembolization showed with mild inflammation or angionecrosis. No specimen demonstrated extravasated Onyx.28 Others have reported successfully using Onyx alone or in combination with coils to treat peripheral vascular malformations.29
Advantages of EVOH include that it is nonadhesive. This allows for longer injection times and the ability to temporarily suspend embolization and proceed with further angiography mid procedure if necessary. Although, there is a risk of embedding the catheter in Onyx if it refluxes around the catheter tip, the material is not adhesive and will not adhere to the catheter as does NBCA glue. Disadvantages of EVOH include the need for DMSO-compatible catheters and syringes. DMSO is toxic, and rapid injection of DMSO can cause vasospasm and necrosis. The safe injection rate is < 0.3 mL injected longer than 40 seconds. The lowest toxic dose of Onyx in humans (that is, the maximum safe allowable dose) is 600 mg/kg (personal communication, regional representative of Micro Therapeutics, Inc.).
CALCIUM ALGINATE GEL
Calcium alginate gel (ALGEL) (Neural Intervention Technologies, Ann Arbor, MI) is a relatively new embolic agent being evaluated primarily for neuroendovascular procedures. It is comprised of a polymer of alginic acid, a natural polysaccharide gel found in brown algae. When exposed to a cation such as calcium chloride, alginic acid immediately polymerizes into an alginate gel matix.30 Calcium alginate is delivered via a concentric catheter approach. Liquid alginate, which is available premixed with contrast for visibility, is injected by hand through an inner catheter. Calcium chloride is infused through the concentric catheter. On contact, a nonadhesive gel forms.31
Calcium alginate gel occlusion of AVMs and aneurysms in a swine model has been reported.31,32 Histological analysis of aneurysms occluded with ALGEL in a swine model demonstrates calcium alginate filling of the aneurysm with fibrous tissue growth across the aneurysm neck, excluding the aneurysm from the circulation.31 Evaluation of ALGEL in a swine model of AVM demonstrates occlusion for up to 6 months without downstream embolization.32 Histological al analysis at 6 months showed vessel fibrosis around alginate. No significant immune response in either application was noted.
Advantages of ALGEL include rapid formation of occlusive alginate gel on injection of the two components. Unlike coils, the gel fills the entire structure to be occluded and does not depend on thrombosis. Because the two components are injected separately, catheter occlusion should not occur. Both the alginic acid and calcium carbonate components are liquid, nontoxic, and nonocclusive, and the occlusive gel does not form until the two are mixed. This limits the risk of inadvertent downstream embolization. The main disadvantage of this system is the lack of clinical experience with this technique.
ABSOLUTE ALCOHOL
Ethanol causes tissue infarction by inciting denaturation of proteins with resultant acute thrombosis and subsequent fibrosis. Use of absolute ethanol is generally limited to cases in which nontarget embolization is unlikely, such as in the kidney. It is generally used with flow control using balloons either in the arterial or venous portions or in some cases by using coils to slow the flow to acceptable levels. Venous outflow occlusion may be required if there is rapid systemic drainage of the lesion.
Disadvantages of absolute alcohol embolization include difficulty to control placement, lack of opacity, and rapid dilution by vascular inflow. There are additional risks of damaging normal adjacent tissues including skin, mucosa, and nerves.
CONCLUSION
To conclude, there is a wide plethora of embolic agents, each with its own particular characteristics that makes it ideal for certain situations. Familiarity with these and their modes of use and action can help in selecting the correct agent depending on the goal of embolization.
REFERENCES
- Speakman T J. Internal occlusion of a carotid-cavernous fistula. J Neurosurg. 1964;21:303–315. doi: 10.3171/jns.1964.21.4.0303. [DOI] [PubMed] [Google Scholar]
- Patel A A, Soulen M C. In: Baum S, Pentecost MJ, editor. Abrams' Angiography Interventional Radiology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2006. Agents for small vessel/tissue embolization and transcatheter tissue ablation. pp. 169–175.
- Tadavarthy S M, Moller J H, Amplatz K. Polyvinyl alcohol (Ivalon)–a new embolic material. Am J Roentgenol Radium Ther Nucl Med. 1975;125(3):609–616. doi: 10.2214/ajr.125.3.609. [DOI] [PubMed] [Google Scholar]
- Tadavarthy S M, Knight L, Ovitt T W, Snyder C, Amplatz K. Therapeutic transcatheter arterial embolization. Radiology. 1974;112:13–16. doi: 10.1148/112.1.13. [DOI] [PubMed] [Google Scholar]
- Derdeyn C P, Moran C J, DeWitte T C, Dietrich H H, Dacey J, Ralph G. Polyvinyl alcohol particle size and suspension characteristics. AJNR Am J Neuroradiol. 1995;16:1335–1343. [PMC free article] [PubMed] [Google Scholar]
- Derdeyn C P, Graves V B, Salamat M S, Rappe A. Collagen-coated acrylic microspheres for embolotherapy: in vivo and in vitro characteristics. AJNR Am J Neuroradiol. 1997;18:647–653. [PMC free article] [PubMed] [Google Scholar]
- Quisling R G, Mickle J P, Ballinger W B, Carver C C, Kaplan B. Histopathologic analysis of intraarterial polyvinyl alcohol microemboli in rat cerebral cortex. AJNR Am J Neuroradiol. 1984;5(1):101–104. [PMC free article] [PubMed] [Google Scholar]
- Spies J B, Cornell C, Worthington-Kirsch R, Lipman J C, Benenati J F. Long-term outcome from uterine fibroid embolization with tris-acryl gelatin microspheres: results of a multicenter study. J Vasc Interv Radiol. 2007;18(2):203–207. doi: 10.1016/j.jvir.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Laurent A, Beaujeux R, Wassef M, et al. Trisacryl gelatin microspheres for therapeutic embolization, I: development and in vitro evaluation. AJNR Am J Neuroradiol. 1996;17:533–540. [PMC free article] [PubMed] [Google Scholar]
- Beaujeux R, Laurent A, Wassef M, et al. Trisacryl gelatin microspheres for therapeutic embolization II: preliminary clinical evaluation in tumors and arteriovenous malformations. AJNR Am J Neuroradiol. 1996;17(3):541–548. [PMC free article] [PubMed] [Google Scholar]
- Gianturco C, Anderson J H, Wallace S. Mechanical devices for arterial occlusion. Am J Roentgenol Radium Ther Nucl Med. 1975;124(3):428–435. doi: 10.2214/ajr.124.3.428. [DOI] [PubMed] [Google Scholar]
- Goldstein H M, Wallace S, Anderson J H, Bree R L, Gianturco C. Transcatheter occlusion of abdominal tumors. Radiology. 1976;120(3):539–545. doi: 10.1148/120.3.539. [DOI] [PubMed] [Google Scholar]
- Wallace S, Gianturco C, Anderson J H, et al. Therapeutic vascular occlusion utilizing steel coil technique: clinical applications. AJR Am J Roentgenol. 1976;127(3):381–387. doi: 10.2214/ajr.127.3.381. [DOI] [PubMed] [Google Scholar]
- Chuang V P, Wallace S, Gianturco C, Soo C S. Complications of coil embolization: prevention and management. AJR Am J Roentgenol. 1981;137(4):809–813. doi: 10.2214/ajr.137.4.809. [DOI] [PubMed] [Google Scholar]
- Vrachliotis T G, Falagas M E. Infections after endovascular coil embolization. J Endovasc Ther. 2007;14(6):805–806. doi: 10.1583/07-2219C.1. [DOI] [PubMed] [Google Scholar]
- Osuga K, Mikami K, Higashihara H, et al. Principles and techniques of transcatheter embolotherapy for peripheral vascular lesions. Radiat Med. 2006;24(4):309–314. doi: 10.1007/s11604-006-2411-1. [DOI] [PubMed] [Google Scholar]
- Cook Cook peripheral vascular. Available at: http://wwwcookmedicalcom/di/familyListingActiondo?family=Embolization + and + Occlusion. Accessed Nov. 9, 2007. Available at: http://wwwcookmedicalcom/di/familyListingActiondo?family=Embolization + and + Occlusion
- Ha-Kawa S K, Kariya H, Murata T, Tanaka Y. Successful transcatheter embolotherapy with a new platinum microcoil: the Berenstein liquid coil. Cardiovasc Intervent Radiol. 1998;21(4):297–299. doi: 10.1007/s002709900264. [DOI] [PubMed] [Google Scholar]
- Kallmes D F, Fujiwara N H. New expandable hydrogel-platinum coil hybrid device for aneurysm embolization. AJNR Am J Neuroradiol. 2002;23(9):1580–1588. [PMC free article] [PubMed] [Google Scholar]
- Ding Y H, Dai D, Lewis D A, Cloft H J, Kallmes D F. Angiographic and histologic analysis of experimental aneurysms embolized with platinum coils, Matrix, and HydroCoil. AJNR Am J Neuroradiol. 2005;26(7):1757–1763. [PMC free article] [PubMed] [Google Scholar]
- Serbinenko F A. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974;41(2):125–145. doi: 10.3171/jns.1974.41.2.0125. [DOI] [PubMed] [Google Scholar]
- Tuite D J, Kessel D O, Nicholson A A, et al. Initial clinical experience using the Amplatzer Vascular Plug. Cardiovasc Intervent Radiol. 2007;30(4):650–654. doi: 10.1007/s00270-007-9044-3. [DOI] [PubMed] [Google Scholar]
- Ha C D, Calcagno D. Amplatzer Vascular Plug to occlude the internal iliac arteries in patients undergoing aortoiliac aneurysm repair. J Vasc Surg. 2005;42(6):1058–1062. doi: 10.1016/j.jvs.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Pollak J S, White R I., Jr The use of cyanoacrylate adhesives in peripheral embolization. J Vasc Interv Radiol. 2001;12:907–913. doi: 10.1016/s1051-0443(07)61568-1. [DOI] [PubMed] [Google Scholar]
- Raffi L, Simonetti L, Cenni P, Leonardi M. Use of Glubran 2 acrylic glue in interventional neuroradiology. Neuroradiology. 2007;49:829–836. doi: 10.1007/s00234-007-0238-9. [DOI] [PubMed] [Google Scholar]
- Taki W, Yonekawa Y, Iwata H, et al. A new liquid material for embolization of arteriovenous malformations. AJNR Am J Neuroradiol. 1990;11(1):163–168. [PMC free article] [PubMed] [Google Scholar]
- In: Micro Therapeutics Inc. 2003. Onyx Liquid Embolic System. Available at: http://www.ev3.net/neuro/us/liquid-embolics/onyx-liquid-embolic-system.htm. Accessed Mar 8, 2008. Available at: http://www.ev3.net/neuro/us/liquid-embolics/onyx-liquid-embolic-system.htm
- Jahan R, Murayama Y, Gobin Y, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48(5):984–995. discussion 995–987. doi: 10.1097/00006123-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Vanninen R L, Manninen I. Onyx, a new liquid embolic material for peripheral interventions: preliminary experience in aneurysm, pseudoaneurysm, and pulmonary arteriovenous malformation embolization. Cardiovasc Intervent Radiol. 2007;30(2):196–200. doi: 10.1007/s00270-006-0071-2. [DOI] [PubMed] [Google Scholar]
- Becker T A, Kipke D R, Brandon T. Calcium alginate gel: a biocompatible and mechanically stable polymer for endovascular embolization. J Biomed Mater Res. 2001;54(1):76–86. doi: 10.1002/1097-4636(200101)54:1<76::aid-jbm9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Becker T A, Preul M C, Bichard W D, Kipke D R, McDougall C G. Preliminary investigation of calcium alginate gel as a biocompatible material for endovascular aneurysm embolization in vivo. Neurosurgery. 2007;60:1119–1128. doi: 10.1227/01.NEU.0000255447.90106.12. [DOI] [PubMed] [Google Scholar]
- Becker T A, Preul M C, Bichard W D, Kipke D R, McDougall C G. Calcium alginate gel as a biocompatible material for endovascular arteriovenous malformation embolization: six-month results in an animal model. Neurosurgery. 2005;56:793–801. doi: 10.1227/01.neu.0000156494.94675.bb. [DOI] [PubMed] [Google Scholar]