Abstract
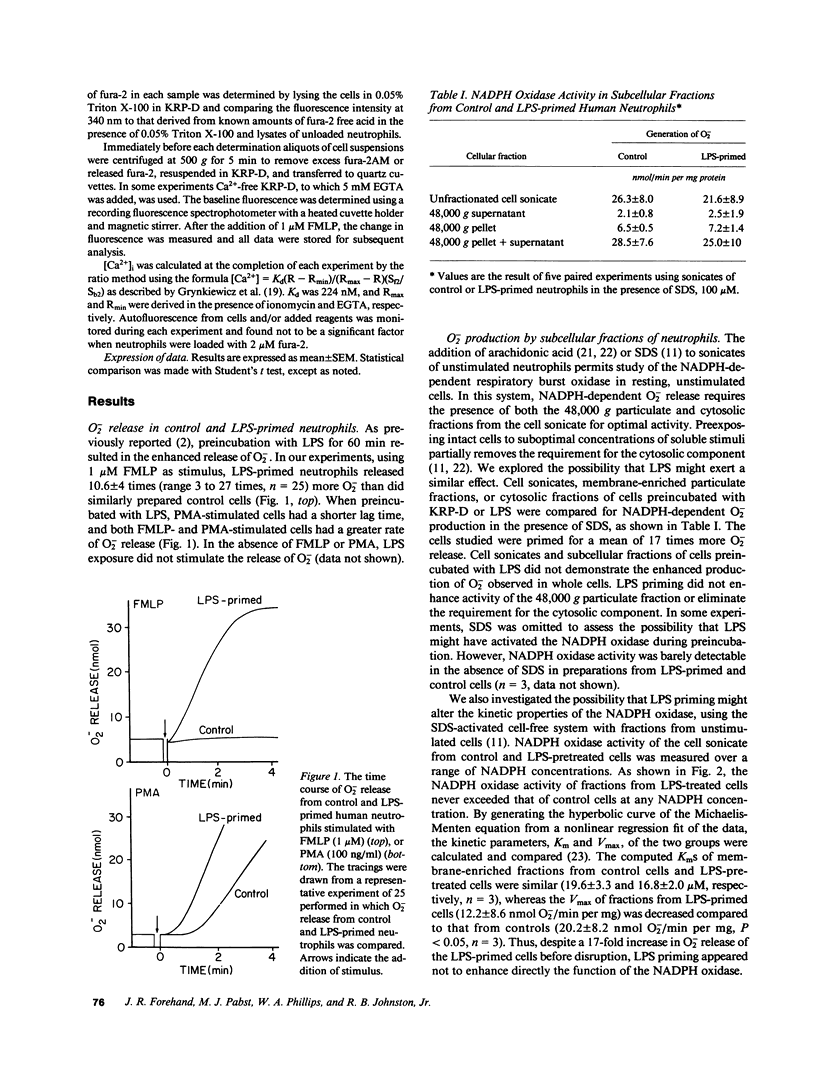
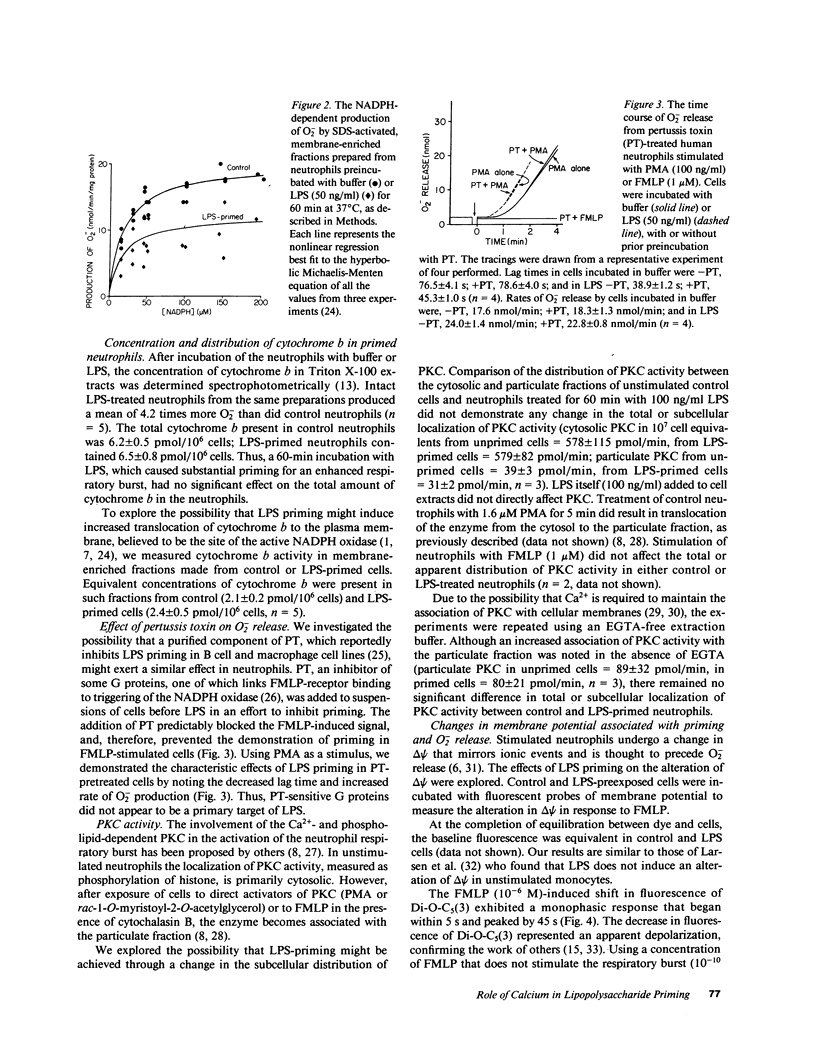
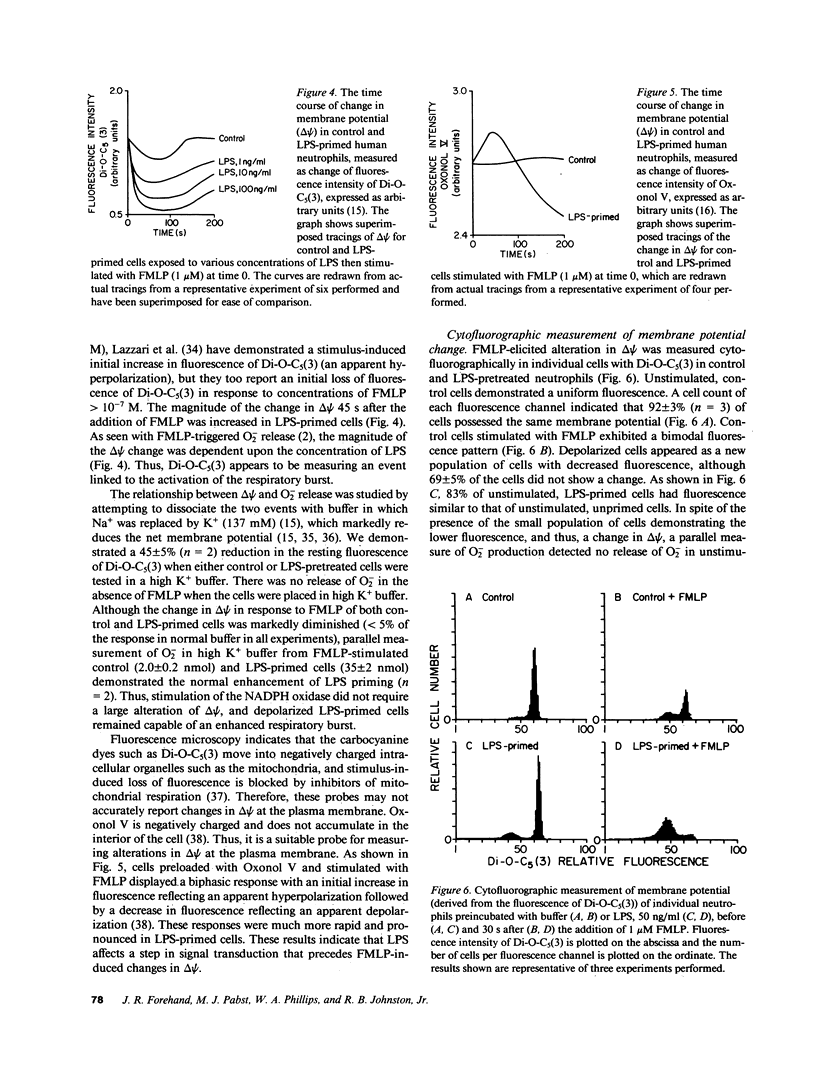
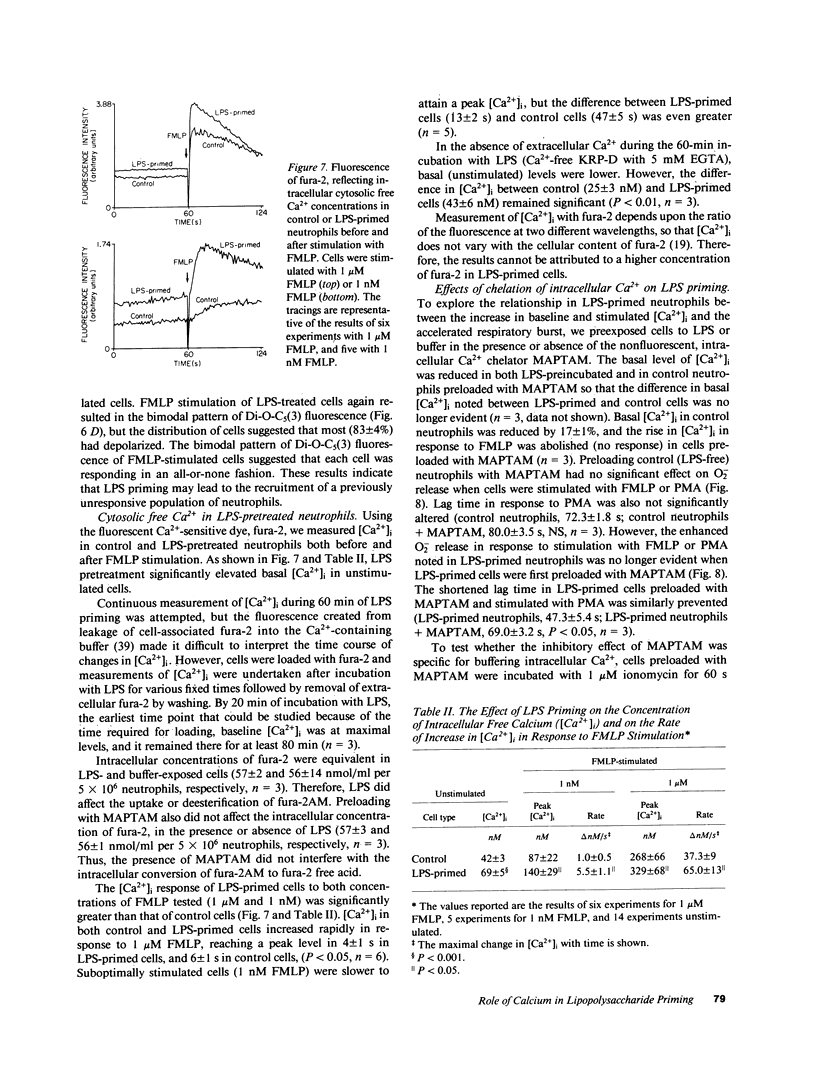
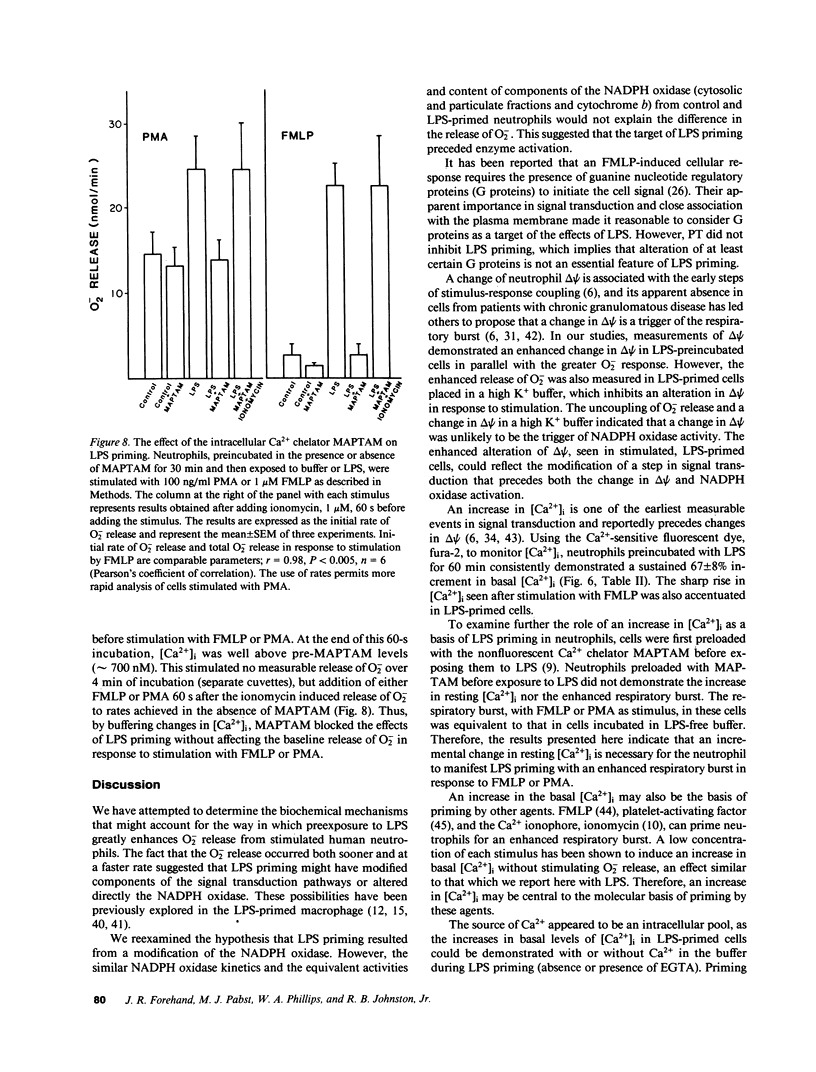
Lipopolysaccharide (LPS) pretreatment "primes" neutrophils to release increased amounts of superoxide anion (O2-) when stimulated. We investigated the molecular basis of this enhanced activity. Comparison of kinetic parameters of the respiratory burst NADPH oxidase in unstimulated LPS-primed and control neutrophils disclosed a similar Km for NADPH and no difference was seen in the content of cytochrome b. Pertussis toxin, which inhibits some G proteins, did not prevent priming. Change in membrane potential (delta psi) was five-fold greater in LPS-primed cells and paralleled the increased O2- release. Cytofluorographic analysis indicated that the increased change in delta psi was due to the creation of a new population of active cells. Changes in the concentration of intracellular free Ca2+ ([Ca2+]i) are believed to antecede changes in delta psi. There was a consistent increment (67 +/- 8%, n = 12) in resting [Ca2+]i in cells preincubated with LPS compared with control. When stimulated, the peak [Ca2+]i was significantly higher in LPS-primed cells. Ca2+-dependent protein kinase C activity was unaltered in resting and FMLP-stimulated neutrophils preexposed to LPS. Addition to cells of the intracellular Ca2+ chelator MAPTAM before preincubation with LPS blocked the changes in [Ca2+]i and the enhanced respiratory burst that characterize LPS priming. The increased resting [Ca2+]i in LPS-primed cells may enhance stimulus-induced cellular activity by modifying a Ca2+-dependent step in signal transduction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Cohen D. S., Wright S. D., Cohn Z. A. Bacterial lipopolysaccharides prime macrophages for enhanced release of arachidonic acid metabolites. J Exp Med. 1986 Jul 1;164(1):165–179. doi: 10.1084/jem.164.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. A., Olbrantz P., Szejda P., Seeds M. C., McCall C. E. Subpopulations of neutrophils with increased oxidative product formation in blood of patients with infection. J Immunol. 1986 Feb 1;136(3):860–866. [PubMed] [Google Scholar]
- Berkow R. L., Wang D., Larrick J. W., Dodson R. W., Howard T. H. Enhancement of neutrophil superoxide production by preincubation with recombinant human tumor necrosis factor. J Immunol. 1987 Dec 1;139(11):3783–3791. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg Y., Pick E. Activation of NADPH-dependent superoxide production in a cell-free system by sodium dodecyl sulfate. J Biol Chem. 1985 Nov 5;260(25):13539–13545. [PubMed] [Google Scholar]
- Cox J. A., Jeng A. Y., Sharkey N. A., Blumberg P. M., Tauber A. I. Activation of the human neutrophil nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase by protein kinase C. J Clin Invest. 1985 Nov;76(5):1932–1938. doi: 10.1172/JCI112190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T. Activation of human neutrophil nicotinamide adenine dinucleotide phosphate, reduced (triphosphopyridine nucleotide, reduced) oxidase by arachidonic acid in a cell-free system. J Clin Invest. 1985 May;75(5):1740–1743. doi: 10.1172/JCI111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C. A., Zingg J., Maly F. E., de Weck A. L. Leukotriene production in human neutrophils primed by recombinant human granulocyte/macrophage colony-stimulating factor and stimulated with the complement component C5A and FMLP as second signals. J Exp Med. 1988 Apr 1;167(4):1281–1295. doi: 10.1084/jem.167.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Steinberg T. H., Swanson J. A., Silverstein S. C. Fura-2 secretion and sequestration in macrophages. A blocker of organic anion transport reveals that these processes occur via a membrane transport system for organic anions. J Immunol. 1988 Feb 1;140(3):915–920. [PubMed] [Google Scholar]
- DiPersio J. F., Billing P., Williams R., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor and other cytokines prime human neutrophils for enhanced arachidonic acid release and leukotriene B4 synthesis. J Immunol. 1988 Jun 15;140(12):4315–4322. [PubMed] [Google Scholar]
- Finkel T. H., Pabst M. J., Suzuki H., Guthrie L. A., Forehand J. R., Phillips W. A., Johnston R. B., Jr Priming of neutrophils and macrophages for enhanced release of superoxide anion by the calcium ionophore ionomycin. Implications for regulation of the respiratory burst. J Biol Chem. 1987 Sep 15;262(26):12589–12596. [PubMed] [Google Scholar]
- Gallin E. K. Calcium- and voltage-activated potassium channels in human macrophages. Biophys J. 1984 Dec;46(6):821–825. doi: 10.1016/S0006-3495(84)84080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. L., Munford R. S. Enzymatic deacylation of the lipid A moiety of Salmonella typhimurium lipopolysaccharides by human neutrophils. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6671–6675. doi: 10.1073/pnas.80.21.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Jakway J. P., DeFranco A. L. Pertussis toxin inhibition of B cell and macrophage responses to bacterial lipopolysaccharide. Science. 1986 Nov 7;234(4777):743–746. doi: 10.1126/science.3095921. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Kitagawa S. Molecular basis for the enhanced respiratory burst of activated macrophages. Fed Proc. 1985 Nov;44(14):2927–2932. [PubMed] [Google Scholar]
- Kitagawa S., Johnston R. B., Jr Relationship between membrane potential changes and superoxide-releasing capacity in resident and activated mouse peritoneal macrophages. J Immunol. 1985 Nov;135(5):3417–3423. [PubMed] [Google Scholar]
- Kitagawa S., Ohta M., Nojiri H., Kakinuma K., Saito M., Takaku F., Miura Y. Functional maturation of membrane potential changes and superoxide-producing capacity during differentiation of human granulocytes. J Clin Invest. 1984 Apr;73(4):1062–1071. doi: 10.1172/JCI111291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivuranta-Vaara P., Banda D., Goldstein I. M. Bacterial-lipopolysaccharide-induced release of lactoferrin from human polymorphonuclear leukocytes: role of monocyte-derived tumor necrosis factor alpha. Infect Immun. 1987 Dec;55(12):2956–2961. doi: 10.1128/iai.55.12.2956-2961.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Rich A. M., Wilkenfeld C., Rutherford L. E., Weissmann G. A carbocyanine dye, DiOC6(3), acts as a mitochondrial probe in human neutrophils. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1495–1501. doi: 10.1016/s0006-291x(82)80076-4. [DOI] [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus response coupling in the human neutrophil. II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem. 1984 Apr 10;259(7):4076–4082. [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3818–3822. doi: 10.1073/pnas.75.8.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal B. A., Shak S., Maxfield F. R. Spreading of human neutrophils is immediately preceded by a large increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 May;83(9):2919–2923. doi: 10.1073/pnas.83.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki M., Kamo N., Kobatake Y., Okimasu E., Utsumi K. Measurement of membrane potential in polymorphonuclear leukocytes and its changes during surface stimulation. Biochim Biophys Acta. 1982 Dec 22;693(2):326–334. doi: 10.1016/0005-2736(82)90439-4. [DOI] [PubMed] [Google Scholar]
- Larsen N. E., Enelow R. I., Simons E. R., Sullivan R. Effect of bacterial endotoxin on the transmembrane electrical potential and plasma membrane fluidity of human monocytes. Biochim Biophys Acta. 1985 Apr 26;815(1):1–8. doi: 10.1016/0005-2736(85)90466-3. [DOI] [PubMed] [Google Scholar]
- Lazzari K. G., Proto P. J., Simons E. R. Simultaneous measurement of stimulus-induced changes in cytoplasmic Ca2+ and in membrane potential of human neutrophils. J Biol Chem. 1986 Jul 25;261(21):9710–9713. [PubMed] [Google Scholar]
- Light D. R., Walsh C., O'Callaghan A. M., Goetzl E. J., Tauber A. I. Characteristics of the cofactor requirements for the superoxide-generating NADPH oxidase of human polymorphonuclear leukocytes. Biochemistry. 1981 Mar 17;20(6):1468–1476. doi: 10.1021/bi00509a010. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. The NADPH oxidase of human polymorphonuclear leukocytes. Evidence for regulation by multiple signals. J Biol Chem. 1984 May 10;259(9):5768–5775. [PubMed] [Google Scholar]
- McPhail L. C., Shirley P. S., Clayton C. C., Snyderman R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system. Evidence for a soluble cofactor. J Clin Invest. 1985 May;75(5):1735–1739. doi: 10.1172/JCI111884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986 Oct 10;234(4773):203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- Nishihira J., McPhail L. C., O'Flaherty J. T. Stimulus-dependent mobilization of protein kinase C. Biochem Biophys Res Commun. 1986 Jan 29;134(2):587–594. doi: 10.1016/s0006-291x(86)80460-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Parks D. R., Herzenberg L. A. Fluorescence-activated cell sorting: theory, experimental optimization, and applications in lymphoid cell biology. Methods Enzymol. 1984;108:197–241. doi: 10.1016/s0076-6879(84)08086-1. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Prentki M., Wollheim C. B., Lew P. D. Ca2+ homeostasis in permeabilized human neutrophils. Characterization of Ca2+-sequestering pools and the action of inositol 1,4,5-triphosphate. J Biol Chem. 1984 Nov 25;259(22):13777–13782. [PubMed] [Google Scholar]
- Prpic V., Weiel J. E., Somers S. D., DiGuiseppi J., Gonias S. L., Pizzo S. V., Hamilton T. A., Herman B., Adams D. O. Effects of bacterial lipopolysaccharide on the hydrolysis of phosphatidylinositol-4,5-bisphosphate in murine peritoneal macrophages. J Immunol. 1987 Jul 15;139(2):526–533. [PubMed] [Google Scholar]
- Ransom J. T., Cambier J. C. B cell activation. VII. Independent and synergistic effects of mobilized calcium and diacylglycerol on membrane potential and I-A expression. J Immunol. 1986 Jan;136(1):66–72. [PubMed] [Google Scholar]
- Rasmussen H. The calcium messenger system (2). N Engl J Med. 1986 May 1;314(18):1164–1170. doi: 10.1056/NEJM198605013141807. [DOI] [PubMed] [Google Scholar]
- Roos D., Voetman A. A., Meerhof L. J. Functional activity of enucleated human polymorphonuclear leukocytes. J Cell Biol. 1983 Aug;97(2):368–377. doi: 10.1083/jcb.97.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff P. M., Cantley L. C. Lipopolysaccharide and phorbol esters induce differentiation but have opposite effects on phosphatidylinositol turnover and Ca2+ mobilization in 70Z/3 pre-B lymphocytes. J Biol Chem. 1985 Aug 5;260(16):9209–9215. [PubMed] [Google Scholar]
- Sasada M., Pabst M. J., Johnston R. B., Jr Activation of mouse peritoneal macrophages by lipopolysaccharide alters the kinetic parameters of the superoxide-producing NADPH oxidase. J Biol Chem. 1983 Aug 25;258(16):9631–9635. [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Scherman D., Henry J. P. Oxonol-V as a probe of chromaffin granule membrane potentials. Biochim Biophys Acta. 1980 Jun 20;599(1):150–166. doi: 10.1016/0005-2736(80)90064-4. [DOI] [PubMed] [Google Scholar]
- Seligmann B. E., Gallin J. I. Use of lipophilic probes of membrane potential to assess human neutrophil activation. Abnormality in chronic granulomatous disease. J Clin Invest. 1980 Sep;66(3):493–503. doi: 10.1172/JCI109880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. D., Lane B. C., Kusaka I., Verghese M. W., Snyderman R. Chemoattractant receptor-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate in human polymorphonuclear leukocyte membranes. Requirement for a guanine nucleotide regulatory protein. J Biol Chem. 1985 May 25;260(10):5875–5878. [PubMed] [Google Scholar]
- Suzuki H., Pabst M. J., Johnston R. B., Jr Enhancement by Ca2+ or Mg2+ of catalytic activity of the superoxide-producing NADPH oxidase in membrane fractions of human neutrophils and monocytes. J Biol Chem. 1985 Mar 25;260(6):3635–3639. [PubMed] [Google Scholar]
- Weening R. S., Corbeel L., de Boer M., Lutter R., van Zwieten R., Hamers M. N., Roos D. Cytochrome b deficiency in an autosomal form of chronic granulomatous disease. A third form of chronic granulomatous disease recognized by monocyte hybridization. J Clin Invest. 1985 Mar;75(3):915–920. doi: 10.1172/JCI111792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitin J. C., Chapman C. E., Simons E. R., Chovaniec M. E., Cohen H. J. Correlation between membrane potential changes and superoxide production in human granulocytes stimulated by phorbol myristate acetate. Evidence for defective activation in chronic granulomatous disease. J Biol Chem. 1980 Mar 10;255(5):1874–1878. [PubMed] [Google Scholar]
- Wightman P. D., Dahlgren M. E., Hall J. C., Davies P., Bonney R. J. Identification and characterization of a phospholipase C activity in resident mouse peritoneal macrophages. Inhibition of the enzyme by phenothiazines. Biochem J. 1981 Aug 1;197(2):523–526. doi: 10.1042/bj1970523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman P. D., Humes J. L., Davies P., Bonney R. J. [Identification and characterization of two phospholipase A2 activities in resident mouse peritoneal macrophages]. Biochem J. 1981 May 1;195(2):427–433. doi: 10.1042/bj1950427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., Cuatrecasas P., Sahyoun N. Interaction of protein kinase C with membranes is regulated by Ca2+, phorbol esters, and ATP. J Biol Chem. 1985 Dec 15;260(29):15718–15722. [PubMed] [Google Scholar]
- Wolfson M., McPhail L. C., Nasrallah V. N., Snyderman R. Phorbol myristate acetate mediates redistribution of protein kinase C in human neutrophils: potential role in the activation of the respiratory burst enzyme. J Immunol. 1985 Sep;135(3):2057–2062. [PubMed] [Google Scholar]
- von Tscharner V., Prod'hom B., Baggiolini M., Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. 1986 Nov 27-Dec 3Nature. 324(6095):369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]



