ABSTRACT
Endoleaks are unique complications of endovascular aneurysm repair (EVAR) that necessitate lifelong imaging surveillance for the patient. Several imaging modalities may be used to monitor the patient for endoleaks and other complications related to the stent graft. At present, computed tomographic angiography remains the gold standard for the detection of endoleaks. Other modalities that can be used to detect endoleaks include magnetic resonance, ultrasonography, nuclear medicine techniques, and pressure monitoring. In addition, follow-up imaging with digital subtraction angiography is important for endoleak classification and to guide decisions regarding therapy. In this article, we review the classification of endoleaks and discuss the different imaging strategies available for post-EVAR surveillance.
Keywords: Endovascular aneurysm repair, endoleak, computed tomography, ultrasound, magnetic resonance imaging, angiography
Since its introduction nearly 20 years ago,1 endovascular aneurysm repair (EVAR) has emerged as an increasingly utilized minimally invasive alternative to surgical repair of abdominal aortic aneurysms. Although EVAR has been associated with reduced mortality compared with open surgical intervention,2,3 patients undergoing EVAR encounter unique complications such as endoleaks. This complication has been estimated to occur in up to 25% patients undergoing EVAR.4 As endoleaks are most often asymptomatic, patients must undergo lifelong imaging surveillance following EVAR. This article reviews several of the imaging techniques used for post-EVAR surveillance.
ENDOLEAK CLASSIFICATION
Endoleaks are typically classified according the origin of blood flow.5 Table 1 summarizes the five categories of endoleaks. Type 1 endoleaks are defined as extraluminal flow that originates at the attachment of site of the stent graft with the artery. This can be further classified as a proximal (type 1A) or distal (type 1B) endoleak.
Table 1.
Endoleak Classification
| Type of Endoleak | Location of Leak |
|---|---|
| Adapted and modified from Shah A, Stavropoulos SW. Treatment strategies for type 2 endoleaks after endovascular aneurysm repair. Acta Chir Belg 2007;107:356–360. | |
| Type 1 | Attachment site |
| A | Proximal |
| B | Distal |
| C | Illiac occluder |
| Type 2 | Collateral vessel |
| A | Single vessel |
| B | Multiple vessels |
| Type 3 | Graft failure |
| A | Midgraft puncture |
| B | Junctional |
| C | Other (e.g., suture hole) |
| Type 4 | Porosity of graft wall |
| Type 5 | Endotension |
Type 2 endoleaks, the most common type, occur when then there is retrograde flow from anastomotic connections of the aortic or iliac arteries, most often the inferior mesenteric or lumbar artery, directly into the aneurysm sac. Type 2 endoleaks are further divided into those resulting from a single feeding and draining vessel (type 2A) versus those resulting from multiple vessels (type 2B). Although type 2 endoleaks often resolve spontaneously, increased flow and pressure in the aneurysm sac can result in enlargement and, potentially, rupture.6
Structural stent-graft failure resulting in an endoleak is known as a type 3 endoleak. This can occur as a result of a puncture hole in the graft (type 3A), due to junctional gaps resulting from modular devices (type 3B), and due to other causes of graft failure such as a suture hold (type 3C). It is thought that the prevalence of these endoleaks will increase with stent-graft age, together with increased imaging surveillance.7
Type 4 endoleaks result from porosity in the wall of the stent graft. These are typically detected on angiogram immediately following stent-graft insertion, although some authors have defined type 4 endoleaks as occurring within 30 days of stent-graft placement.4 Type 4 endoleaks usually resolve once coagulation parameters normalize following the EVAR procedure. Considered by many to be a diagnosis of exclusion, type 4 endoleaks are typically of no clinical consequence, although they can mimic other types of endoleaks.
Some consider endotension as representing a type 5 endoleak. Endotension refers to an enlargement of the aneurysm without a detectable endoleak following successful EVAR. It is thought that persistently elevated post-EVAR pressure in the sac results in its enlargement. Although endotension could represent an endoleak that is undetectable using current imaging techniques, it is likely that endotension explains why many patients have a persistently dilated aneurysm sac, despite successful abdominal aortic aneurysm repair.
IMAGING TECHNIQUES FOR SURVEILLANCE
Plain Radiograph
Although computed tomography (CT) is more widely used, the plain radiograph is an important adjunct in detecting structural changes in the stent graft that may be missed on CT. Plain radiographs can be useful in detecting kinking and deformity in the stent graft.8 Radiographs can also be used to detect movement of the stent graft, at a lower cost and radiation dose compared with CT. With proper positioning, plain radiographs can achieve a high level of accuracy in detecting stent-graft migration.9 Specifically, anteroposterior and lateral radiographs are useful for detecting migration and separation of the stent grafts, and oblique films can detect wire fractures.10 If scheduled in the same day, patients should undergo plain radiographs prior to CT, as contrast material in the renal collecting tubules may result in a nondiagnostic radiograph.10
Ultrasound
Ultrasound (US) is used at some centers for the detection of endoleaks following EVAR. The quality of US imaging for endoleaks can be affected by body habitus, operator experience, and technique. Compared with CT angiography (CTA), US offers patients a potential cost savings and decreased radiation dose. Studies to date have inconclusively demonstrated the utility of US in endoleak detection, although recent data have demonstrated the value of US. A recent prospective study of 132 patients compared CTA with duplex US in aneurysm surveillance following EVAR. In the 117 patients for whom follow-up was possible, duplex US was found to have only 45% positive predictive value for endoleak detection. However, US demonstrated all endoleaks found on CTA; US was 86% sensitive in endoleak detection.11 Studies have demonstrated Duplex US with contrast agent to be as sensitive as CTA in detecting endoleaks.12 Notably, contrast-enhanced US has been shown to be more specific than CTA in classifying endoleaks.13
Other studies, however, have been less convincing for the use of US surveillance, especially the detection of type 2 endoleaks.14,15 Although color Doppler US demonstrated larger endoleaks, CT was more useful in demonstrating outflow vessels and the origin of the endoleak.16 Moreover, US provides less accurate aneurysm diameter measurements compared with CT.17,18 Nevertheless, recent evidence suggests that US performed with newer-generation contrast agents, such as those composed of sulfur hexafluoride gas, provides endoleak visualization that is often superior to CT.19
Magnetic Resonance Imaging
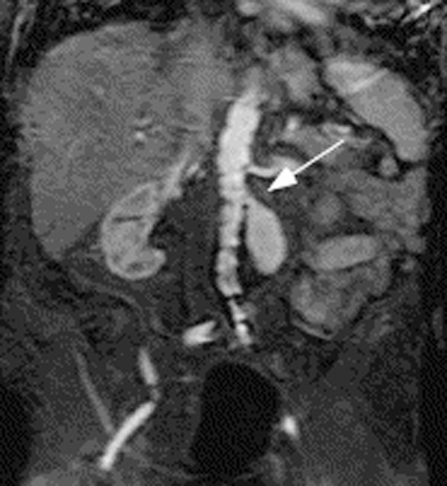
Gadolinium-enhanced magnetic resonance angiography (MRA) can be used to detect endoleaks and may be particularly useful in those patients who have contraindications to contrast agents due to severe allergy. However, the image quality depends on the material composition of the stent graft. Patients with nitinol stents are the best candidates for MRA, and those with stainless steel or elgiloy stents may experience significant artifact (Fig. 1). MRA is as sensitive as CT in detecting endoleaks,20,21,22 and in one study was shown to be superior to CT in detecting type 2 endoleaks in patients with nitinol stent grafts.23
Figure 1.
Coronal image from magnetic resonance imaging following gadolinium administration showing a type 1 endoleak (white arrow).
More recently, time-resolved MRA (TR-MRA) has been increasingly used as alternative technique to detect and classify endoleaks. By demonstrating the direction of blood flow in the aneurysm sac, TR-MRA appears to be a promising alternative to angiography.24 Cohen and colleagues recently demonstrated a 97% concordance in endoleak classification when comparing TR-MRA with angiography.25 Although these results are encouraging, the use of MRA for endoleak classification is limited by its spatial resolution.
Computed Tomography
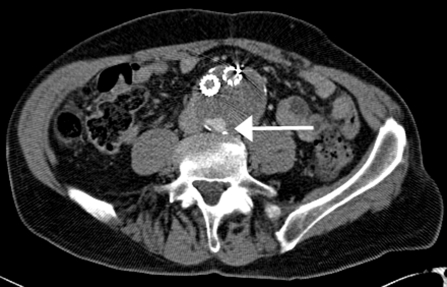
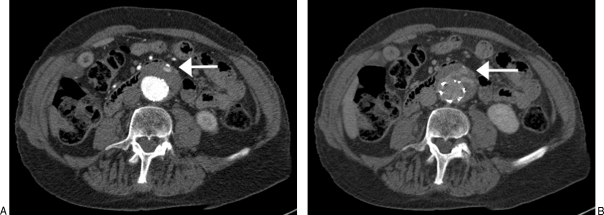
CTA remains the most widely used modality for the detection of endoleaks (Fig. 2). Compared with conventional angiography, CTA is more sensitive (92% versus 63%) in detecting endoleaks following EVAR.26 Multiphasic CT is recommended for endoleak surveillance because the inconsistent flow rates of endoleaks result in variable detection after contrast injection. At our institution, this includes precontrast and postcontrast arterial phase and a postcontrast delayed-phase imaging (i.e., triple-phase imaging; Table 2). Unenhanced images are useful in differentiating endoleaks from calcification. Postcontrast delayed-phase images may demonstrate other endoleaks not seen in arterial phase imaging27 including low-flow endoleaks28; in multidetector-row CTA, a delay of 300 seconds has been suggested for most accurate endoleak detection29 (Fig. 3). Importantly, CTA is effective in the surveillance of aortic aneurysm diameter and recurrent endoleaks following coil repair of type 2 endoleaks.30
Figure 2.
Delayed computed tomography image revealing endoleak posteriorly. This was shown to be a type 2 endoleak supplied by a lumbar artery on diagnostic arteriogram (white arrow).
Table 2.
Computed Tomographic Angiography Protocol in Post–Endovascular Aneurysm Repair Surveillance
| Precontrast | Arterial Phase | Delayed Phase | |
|---|---|---|---|
| Adapted and modified from Stavropoulos SW, Charagundla SR. Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology 2007;243:641–655. | |||
| Tube voltage (kV) | 120 | 100 | 120 |
| Tube current (mA) | 90 | 150 | 180 |
| Pitch | 1.0 | 1.2 | 1.2 |
| Section thickness (mm) | 5 | 2 | 3 |
| Section increment (mm) | 5 | 1.2 | 2 |
| Timing | N/A | Bolus tracking plus 8 s | 60 s after end of arterial phase |
Figure 3.
(A) Computed tomographic angiography demonstrating small endoleak anteriorly (white arrow). (B) Delayed computed tomography image showing how the same endoleak appears on a delayed-phase image (white arrow).
Although CTA remains the gold standard for imaging surveillance following EVAR, one must consider the cumulative radiation dose that patients receive. Dual-energy dual-source CT has recently emerged as an alternative to single-energy CT procedures.31,32 A study of 118 patients demonstrated that delayed-phase imaging with dual-energy dual-source CT, together with virtual nonenhanced data, was highly accurate in detecting endoleaks following EVAR.32 Importantly, this approach was associated with significantly lower radiation doses compared with triple-phase imaging.32 Alternatively, CT without arterial phase imaging has also been demonstrated to be effective in mitigating radiation exposure, with minimal effect on diagnostic accuracy.33
Angiography
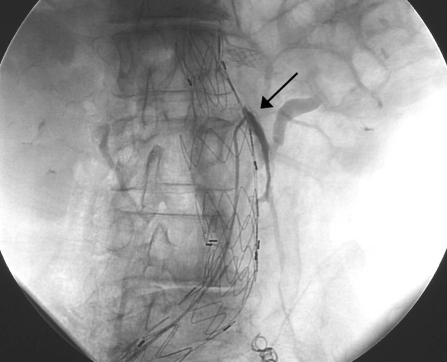
Endoleaks can be detected and classified with CTA; however, digital subtraction angiography (DSA) is more accurate in classifying endoleaks because the flow of blood into and out of the endoleak can be seen in detail. A recent study demonstrated only 86% agreement in endoleak classification when comparing CTA with DSA.34 Endoleaks detected using CTA will have contrast media in the lumbar and inferior mesenteric arteries. It is difficult, if not impossible, to determine whether this is a result of egress flow from a type 1 or type 3 endoleak or, alternatively, inflow from a type 2 endoleak (Fig. 4). Importantly, DSA allows operators to determine the direction of blood flow and thus more accurately determine endoleak classification. Although we routinely employ CTA in the surveillance of EVAR patients at our institution, endoleaks detected on CTA are classified using DSA. Accurate classification of endoleaks using DSA is critical to guiding patient management.
Figure 4.
Diagnostic arteriogram revealing a type 3 endoleak. This was thought to be a type 2 endoleak on computed tomographic angiography (black arrow).
Nuclear Medicine
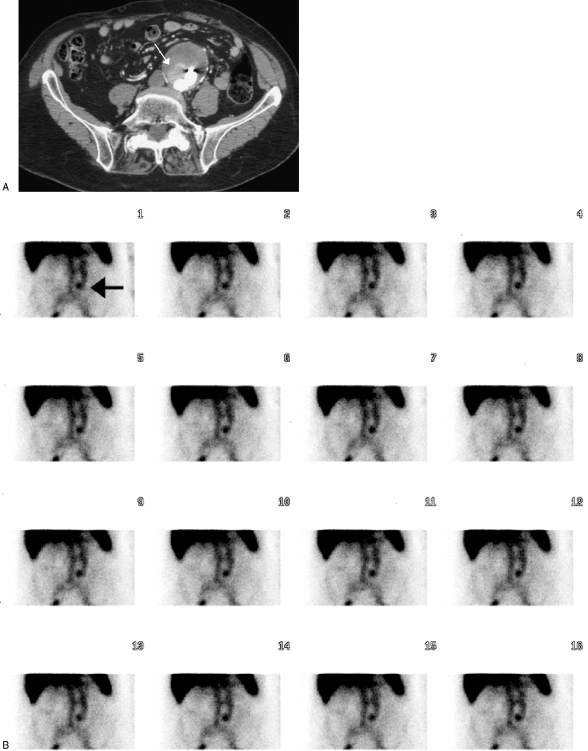
Radionuclide scanning with 99mTc-labeled red blood cells and technetium-99m sulfur colloid is often used to identify the source of occult lower gastrointestinal bleeding. These techniques have been evaluated for the detection of endoleaks following EVAR. Thus far, the results have been somewhat disappointing. One study found that although endoleaks were detected using nuclear medicine techniques, CT was more sensitive in endoleak detection35 (Fig. 5). However, Hovsepian and colleagues were unable to detect slow-filling or rapid-flow endoleaks using scintigraphy with Tc-99m sulfur colloid.36
Figure 5.
(A) Computed tomographic angiography showing an endoleak (white arrow). (B) This same endoleak is shown on a nuclear medicine tagged red blood cell study (black arrow).
Pressure Monitoring
Recently, pressure-monitoring sensors have been proposed as an alternative to imaging for post-EVAR surveillance.37,38 These pressure sensors are implanted into the aneurysm sac and monitored externally. Although this technology is largely investigational, early results have been encouraging for the detection of type 1 and type 3 endoleaks.37 Type 2 endoleaks have variable aneurysm sac pressures, and thus pressure monitoring may not been as effective with this type of endoleak.37
CURRENT RECOMMENDATIONS FOR IMAGING SURVEILLANCE
Results from the EUROSTAR trial demonstrated that EVAR performed using first- and second-generation stents were associated with late failure,39 although it is believed that newer stent-graft technology will be associated with improved outcomes.40 Nonetheless, the paucity of long-term clinical outcomes following EVAR currently warrants lifelong imaging surveillance for changes in aneurysm size and to detect endoleaks. Although there is growing evidence in support of techniques such as US, magnetic resonance, and pressure monitoring, we believe that currently, CT and DSA allow for the most accurate detection and classification of endoleaks. Most institutions utilize serial CTA: at our center, we recommend postprocedural CTA at 30 days, 6 months, and annually thereafter.
CONCLUSION
Endoleaks are a unique complication of EVAR that necessitate lifelong imaging surveillance for the patient. Several imaging modalities may be used to monitor the patient for endoleaks and other complications related to the stent graft. At present, CTA remains the gold standard for the detection of endoleaks. Compared with magnetic resonance and ultrasonography, CTA is the most sensitive modality available for endoleak detection. Nonetheless, follow-up imaging with DSA is critical for endoleak classification and to guide decisions regarding therapy.
REFERENCES
- Parodi J C, Palmaz J C, Barone H D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–499. doi: 10.1007/BF02015271. [DOI] [PubMed] [Google Scholar]
- Greenhalgh R M, Brown L C, Kwong G P, Powell J T, Thompson S G. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004;364:843–848. doi: 10.1016/S0140-6736(04)16979-1. [DOI] [PubMed] [Google Scholar]
- Schermerhorn M L, O'Malley A J, Jhaveri A, et al. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358:464–474. doi: 10.1056/NEJMoa0707348. [DOI] [PubMed] [Google Scholar]
- Veith F J, Baum R A, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002;35:1029–1035. doi: 10.1067/mva.2002.123095. [DOI] [PubMed] [Google Scholar]
- Stavropoulos S W, Baum R A. Imaging modalities for the detection and management of endoleaks. Semin Vasc Surg. 2004;17:154–160. doi: 10.1053/j.semvascsurg.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Schlosser F J, Gusberg R J, Dardik A, et al. Aneurysm rupture after EVAR: can the ultimate failure be predicted? Eur J Vasc Endovasc Surg. 2009;37:15–22. doi: 10.1016/j.ejvs.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Baum R A, Stavropoulos S W, Fairman R M, Carpenter J P. Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2003;14:1111–1117. doi: 10.1097/01.rvi.0000085773.71254.86. [DOI] [PubMed] [Google Scholar]
- Fearn S, Lawrence-Brown M MD, Semmens J B, Hartley D. Follow-up after endovascular aortic aneurysm repair: the plain radiograph has an essential role in surveillance. J Endovasc Ther. 2003;10:894–901. doi: 10.1177/152660280301000508. [DOI] [PubMed] [Google Scholar]
- Hodgson R, McWilliams R G, Simpson A, et al. Migration versus apparent migration: importance of errors due to positioning variation in plain radiographic follow-up of aortic stent-grafts. J Endovasc Ther. 2003;10:902–910. doi: 10.1177/152660280301000509. [DOI] [PubMed] [Google Scholar]
- Murphy M, Hodgson R, Harris P L, et al. Plain radiographic surveillance of abdominal aortic stent-grafts: the Liverpool/Perth protocol. J Endovasc Ther. 2003;10:911–912. doi: 10.1177/152660280301000510. [DOI] [PubMed] [Google Scholar]
- Manning B J, O'Neill S M, Haider S N, et al. Duplex ultrasound in aneurysm surveillance following endovascular aneurysm repair: a comparison with computed tomography aortography. J Vasc Surg. 2008 doi: 10.1016/j.jvs.2008.07.079. September 30 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Bendick P J, Bove P G, Long G W, et al. Efficacy of ultrasound scan contrast agents in the noninvasive follow-up of aortic stent grafts. J Vasc Surg. 2003;37:381–385. doi: 10.1067/mva.2003.17. [DOI] [PubMed] [Google Scholar]
- Carrafiello G, Lagana D, Recaldini C, et al. Comparison of contrast-enhanced ultrasound and computed tomography in classifying endoleaks after endovascular treatment of abdominal aorta aneurysms: preliminary experience. Cardiovasc Intervent Radiol. 2006;29:969–974. doi: 10.1007/s00270-005-0267-x. [DOI] [PubMed] [Google Scholar]
- AbuRahma A F, Welch C A, Mullins B B, Dyer B. Computed tomography versus color duplex ultrasound for surveillance of abdominal aortic stent-grafts. J Endovasc Ther. 2005;12:568–573. doi: 10.1583/05-1575MR.1. [DOI] [PubMed] [Google Scholar]
- Ashoke R, Brown L C, Rodway A, et al. Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review. J Endovasc Ther. 2005;12:297–305. doi: 10.1583/04-1479R.1. [DOI] [PubMed] [Google Scholar]
- Golzarian J, Murgo S, Dussaussois L, et al. Evaluation of abdominal aortic aneurysm after endoluminal treatment: comparison of color Doppler sonography with biphasic helical CT. AJR Am J Roentgenol. 2002;178:623–628. doi: 10.2214/ajr.178.3.1780623. [DOI] [PubMed] [Google Scholar]
- d'Audiffret A, Desgranges P, Kobeiter D H, Becquemin J P. Follow-up evaluation of endoluminally treated abdominal aortic aneurysms with duplex ultrasonography: validation with computed tomography. J Vasc Surg. 2001;33:42–49. doi: 10.1067/mva.2001.112215. [DOI] [PubMed] [Google Scholar]
- Elkouri S, Panneton J M, Andrews J C, et al. Computed tomography and ultrasound in follow-up of patients after endovascular repair of abdominal aortic aneurysm. Ann Vasc Surg. 2004;18:271–279. doi: 10.1007/s10016-004-0034-5. [DOI] [PubMed] [Google Scholar]
- Napoli V, Bargellini I, Sardella S G, et al. Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology. 2004;233:217–225. doi: 10.1148/radiol.2331031767. [DOI] [PubMed] [Google Scholar]
- Insko E K, Kulzer L M, Fairman R M, Carpenter J P, Stavropoulos S W. MR imaging for the detection of endoleaks in recipients of abdominal aortic stent-grafts with low magnetic susceptibility. Acad Radiol. 2003;10:509–513. doi: 10.1016/s1076-6332(03)80060-0. [DOI] [PubMed] [Google Scholar]
- der Laan M J van, Bartels L W, Viergever M A, Blankensteijn J D. Computed tomography versus magnetic resonance imaging of endoleaks after EVAR. Eur J Vasc Endovasc Surg. 2006;32:361–365. doi: 10.1016/j.ejvs.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Cejna M, Loewe C, Schoder M, et al. MR angiography vs CT angiography in the follow-up of nitinol stent grafts in endoluminally treated aortic aneurysms. Eur Radiol. 2002;12:2443–2450. doi: 10.1007/s00330-002-1429-8. [DOI] [PubMed] [Google Scholar]
- Ayuso J R, de Caralt T M, Pages M, et al. MRA is useful as a follow-up technique after endovascular repair of aortic aneurysms with nitinol endoprostheses. J Magn Reson Imaging. 2004;20:803–810. doi: 10.1002/jmri.20170. [DOI] [PubMed] [Google Scholar]
- Lookstein R A, Goldman J, Pukin L, Marin M L. Time-resolved magnetic resonance angiography as a noninvasive method to characterize endoleaks: initial results compared with conventional angiography. J Vasc Surg. 2004;39:27–33. doi: 10.1016/j.jvs.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Cohen E I, Weinreb D, Siegelbaum R, et al. Time-resolved MR angiography for the classification of endoleaks after endovascular aneurysm repair. J Magn Reson Imaging. 2008;27:500–503. doi: 10.1002/jmri.21257. [DOI] [PubMed] [Google Scholar]
- Armerding M D, Rubin G D, Beaulieu C F, et al. Aortic aneurysmal disease: assessment of stent-graft treatment—CT versus conventional angiography. Radiology. 2000;215:138–146. doi: 10.1148/radiology.215.1.r00ap28138. [DOI] [PubMed] [Google Scholar]
- Rozenblit A M, Patlas M, Rosenbaum A T, et al. Detection of endoleaks after endovascular repair of abdominal aortic aneurysm: value of unenhanced and delayed helical CT acquisitions. Radiology. 2003;227:426–433. doi: 10.1148/radiol.2272020555. [DOI] [PubMed] [Google Scholar]
- Iezzi R, Cotroneo A R, Filippone A, et al. Multidetector CT in abdominal aortic aneurysm treated with endovascular repair: are unenhanced and delayed phase enhanced images effective for endoleak detection? Radiology. 2006;241:915–921. doi: 10.1148/radiol.2413050959. [DOI] [PubMed] [Google Scholar]
- Iezzi R, Cotroneo A R, Filippone A, et al. Multidetector-row computed tomography angiography in abdominal aortic aneurysm treated with endovascular repair: evaluation of optimal timing of delayed phase imaging for the detection of low-flow endoleaks. J Comput Assist Tomogr. 2008;32:609–615. doi: 10.1097/RCT.0b013e31814b271d. [DOI] [PubMed] [Google Scholar]
- Stavropoulos S W, Marin H, Fairman R M, et al. Recurrent endoleak detection and measurement of aneurysm size with CTA after coil embolization of endoleaks. J Vasc Interv Radiol. 2005;16:1313–1317. doi: 10.1097/01.RVI.0000175900.61777.9A. [DOI] [PubMed] [Google Scholar]
- Chandarana H, Godoy M CB, Vlahos I, et al. Abdominal aorta: evaluation with dual-source dual-energy multidetector CT after endovascular repair of aneurysms—initial observations. Radiology. 2008;249:692–700. doi: 10.1148/radiol.2492080359. [DOI] [PubMed] [Google Scholar]
- Stolzmann P, Frauenfelder T, Pfammatter T, et al. Endoleaks after endovascular abdominal aortic aneurysm repair: detection with dual-energy dual-source CT. Radiology. 2008;249:682–691. doi: 10.1148/radiol.2483080193. [DOI] [PubMed] [Google Scholar]
- Macari M, Chandarana H, Schmidt B, et al. Abdominal aortic aneurysm: can the arterial phase at CT evaluation after endovascular repair be eliminated to reduce radiation dose? Radiology. 2006;241:908–914. doi: 10.1148/radiol.2413051571. [DOI] [PubMed] [Google Scholar]
- Stavropoulos S W, Clark T WI, Carpenter J P, et al. Use of CT angiography to classify endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2005;16:663–667. doi: 10.1097/01.RVI.0000152386.97448.F1. [DOI] [PubMed] [Google Scholar]
- Stavropoulos S W, Itkin M, Lakhani P, et al. Detection of endoleaks after endovascular aneurysm repair with use of technetium-99m sulfur colloid and Tc-99m-labeled red blood cell scans. J Vasc Interv Radiol. 2006;17:1739–1743. doi: 10.1097/01.RVI.0000241892.81074.1A. [DOI] [PubMed] [Google Scholar]
- Hovsepian D M, Siegel B A, Kimbiris G, et al. Tc-99m sulfur colloid scintigraphy for detecting perigraft flow following endovascular aortic aneurysm repair: a feasibility study. Cardiovasc Intervent Radiol. 1999;22:447–451. doi: 10.1007/s002709900430. [DOI] [PubMed] [Google Scholar]
- Springer F, Gunther R W, Schmitz-Rode T. Aneurysm Sac pressure measurement with minimally invasive implantable pressure sensors: an alternative to current surveillance regimes after EVAR? Cardiovasc Intervent Radiol. 2008;31:460–467. doi: 10.1007/s00270-007-9245-9. [DOI] [PubMed] [Google Scholar]
- Springer F, Schlierf R, Pfeffer J G, et al. Detecting endoleaks after endovascular AAA repair with a minimally invasive, implantable, telemetric pressure sensor: an in vitro study. Eur Radiol. 2007;17:2589–2597. doi: 10.1007/s00330-007-0583-4. [DOI] [PubMed] [Google Scholar]
- Harris P L, Vallabhaneni S R, Desgranges P, et al. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. J Vasc Surg. 2000;32:739–749. doi: 10.1067/mva.2000.109990. [DOI] [PubMed] [Google Scholar]
- Hinchliffe R J, Ivancev K. Endovascular aneurysm repair: current and future status. Cardiovasc Intervent Radiol. 2008;31:451–459. doi: 10.1007/s00270-008-9295-7. [DOI] [PubMed] [Google Scholar]