The first clinical transcatheter embolization procedure was reported in 1972, utilizing autologous clot as the embolic agent. Since then, embolization procedures have become progressively more sophisticated and the number, variety, and complexity of embolic agents has exponentially increased. Hence, one of the most difficult decisions an operator must make before any given embolization procedure is what embolic agent to use.
A systematic approach can be used to determine the best agent for most clinical scenarios encountered. The first decision that must be made by the operator is whether or not a vessel can safely be sacrificed without putting the downstream organ(s) at significant risk. Once it is decided that the vessel can be sacrificed, to determine the best embolic agent to use, the operator should answer three questions:
Is the vessel to be embolized large or small?
How long is the vessel to be occluded?
Is the tissue supplied by the vessel to remain viable after the embolization?
After these three questions are answered, the decision regarding which embolic agent to use is simplified.
The purpose of this article is threefold: to provide a brief historical perspective on embolization procedures, to describe a systematic algorithm applicable to most clinical situations requiring embolization, and to give a brief description of the commonly used embolic agents.
HISTORICAL PERSPECTIVE
Catheter directed embolization was first performed by Charles Dotter's group at the Oregon Health Sciences Center in 1970, where an autologous clot was used as a transcatheter embolic agent to control acute upper gastrointestinal bleeding by selective embolization of the right gastroepiploic artery. This particular procedure was performed due to the patient being a poor surgical candidate. The procedure was soon applied to other organs.
Since the 1970s, embolization procedures have become progressively more complex. Improvements in imaging (high-quality digital subtraction angiography) and catheter technology (the advent of microcatheters and coaxial catheter techniques) have allowed for significant advances in embolization procedures. Additionally, the range of available embolic agents has greatly expanded from autologous clots, Gelfoam (Upjohn; Kalamazoo, MI), and human tissue to polyvinyl alcohol, coils, glue, microspheres, balloons, and many others.
APPROACH AND ALGORITHM
The vast number of agents at an operator's disposal may lead to confusion in determining which agent to use for any given clinical scenario. For example, the most appropriate embolic agent would differ for each of the following clinical scenarios: postcatheterization femoral artery pseudoaneurysm; traumatic injury of the hepatic artery; peripheral arteriovenous malformation; and treatment of hepatoma. The goal of each of these procedures is different, and the embolic agent used is also different.
To best determine which agent to use, a systematic approach may be employed. Before choosing an agent the operator must ask themselves three questions:
How large is the vessel to be embolized? For the authors, any vessel that can be seen angiographically is considered large. Vessels smaller than the resolution of digital subtraction angiography are considered small.
How long should the vessel remain occluded following embolization? Temporary occlusion implies occlusion of the vessel for hours to weeks; permanent occlusion is self-explanatory.
Should the embolized tissue remain viable after embolization? Ischemia is often a result (intended or unintended) of embolization. Intentional organ or cellular death implies using specific embolic agents.
It is vital to recognize the important characteristic of all embolic agents: regardless of the makeup of the agent, the smaller the agent, the greater the likelihood of ischemia of the organ being supplied by the embolized vessel. This is due to the fact that most organs have some duplication of vascular supply; this collateralization is vital to organ survival following a proximal vascular event. If very small embolic agents are used, the effective level of the embolization is distal to where the collateral vessels join the main feeding artery, thereby effectively occluding inflow from both the primary and collateral circulations. Therefore, for most organ systems, small agents cause much greater ischemia than larger agents.
Before determining which agent is best to use in a given clinical scenario, a brief description of commonly used embolic agents is in order.
COMMONLY USED LARGE-VESSEL EMBOLIC AGENTS
Coils
Coils range in diameter from submillimeter to several centimeters (Table 1). Configurations are also widely variable, including straight, helical, spiral, and various three-dimensional shapes. In addition to a thrombogenic coating, most coils have small fibers attached to the metal component. The fibers elicit a thrombogenic response, causing superimposed thrombosis and vessel occlusion. Without this superimposed thrombus, coils are much less effective. Scaffolding is the act of building an adequately tight coil pack, as multiple coils are typically needed to cause vascular occlusion. Due to the wide range in coil characteristics, coils are one of the most widely used embolic agents.
Table 1.
Technical Pearls for Coil Embolization
| Issue | Pearl |
|---|---|
| PAVM, pulmonary arteriovenous malformations. | |
| Scaffolding | Adequate scaffolding can be obtained by initially deploying a larger coil, followed by smaller ones. |
| An Amplatzer Vascular Obstruction Device may be used for vessels that do not taper; it has legs that penetrate the walls of the vessel, and it acts as a cone into which coils may be placed. | |
| Coils can be used as a scaffold in combination with Gelfoam when patient is coagulopathic (trauma/shock); such a “coil-Gelfoam sandwich” causes a complete, permanent mechanical occlusion. | |
| Stiffer coils are generally deployed first and are used as a “backstop.” Softer and pliable coils with unpredictable coil shapes that tend to conform to vessel anatomy, such as the Nestor coil (Cook, Inc., Bloomington, IN), may be deployed following a stiffer “backstop” coil deployment. | |
| Microcoils | Microcatheters should be used when the coil is <0.018 inches (microcoil) to prevent catheter occlusion; care should be taken to be certain that “high-flow” catheters are used with caution when deploying coils, as the larger inner lumen may allow the coils to partially form in the catheter, leading to catheter blockage. |
| Undersizing | Undersizing may lead to distal embolization and must be avoided especially in cases of PAVM; as a rule of thumb, the coil should be ∼20% larger than the vessel diameter. |
| Oversizing | Too much oversizing will prevent the coil from achieving its shape; this leads to inadequate occlusions, as well as a markedly longer coil (which may in turn lead to proximal coil malposition). |
| Deployment | When deployment precision is required (i.e., intracranial procedures, PAVM), coils may be deployed using the floppy end of a “pusher wire”; when precision of deployment is not a concern (i.e., filling a pseudoaneurysm), the coil may be deployed using a saline bolus. |
| Retrievable coils | Retrievable coils allow the coil to be pulled back in the delivery catheter just before deployment; this technique is also helpful when precise placement is essential. |
| Composition | As a rule of thumb, steel coils tend to be stiffer while platinum coils tend to be softer. |
Balloons
Detachable balloons were removed from the market in the United States due to potential migration issues.
Amplatzer Vascular Plug
A self-expandable device made of nitinol mesh, Amplatzer Vascular Plugs are used when a permanent large-vessel occlusion is required (Table 2). Initially composed of a single biconcave disc, the current device is composed of three adjacent discs connected to one another. This stacked configuration leads to a more rapid and complete vessel occlusion. Devices are available ranging from 4 to 16 mm in diameter.
Table 2.
Technical Pearls for Amplatzer Vascular Plug Embolization
| Issue | Pearl |
|---|---|
| Oversizing | Oversize the device by 15–30% (30–50% for the single disc device) relative to the vessel to prevent distal migration. |
| Positioning | Two platinum bands serve as radiopaque markers. |
| Deployment | A simple unscrewing mechanism allows for precise deployment of the device. |
Guide Wires
Movable core guide wires are reserved for very large aneurysms and pseudoaneurysms (Table 3). The outer coiled spring with Teflon wrapping is the part of the guide wire used for embolization; the inner mandril and safety wire are discarded. Guide wires offer little to no thrombogenic potential, but rather are used as a cheap and easy method to “take up space” in a very large vascular space.
Table 3.
Technical Pearls for Guide Wire Embolization
| Issue | Pearl |
|---|---|
| Deployment | Repositioning the wires is extremely difficult. |
| Positioning | Once deployed, the wires tend to conform to the outer contour of the aneurysm while the inner portion of the sac remains patent. |
| Thrombotic properties | Guide wires by themselves are generally insufficient for complete thrombosis, and combination with other devices is generally necessary. |
Suture Material
Sutures may be used in conjunction with other agents (Table 4). 2–0 or 3–0 silk suture is cut into 5- to 10-mm pieces prior to embolization. The suture fragments are delivered hydrostatically by injecting with a 1- to 3-mL syringe.
Table 4.
Technical Pearls for Suture Embolization
| Issue | Pearl |
|---|---|
| Radiopacity | Sutures are radiolucent—use a syringe filled with contrast for injection followed by a saline flush. |
| Hydrostatic deployment | Hydrostatic deployment confers little to no control, making nontarget embolization a distinct possibility. |
Gelfoam
Gelfoam is derived from subcutaneous porcine adipose tissue (Table 5). It is available either as a sponge (block) or as a powder. During embolization procedures, the sponge can be used in combination with contrast to form a thick slurry or paste. Additionally, the sponge can be cut into small cubes or torpedoes and injected directly into the vessel to be embolized. With either of these preparations, a proximal (large) vessel occlusion will result. Gelfoam powder, in contrast, has a mean diameter of only 10 to 100 μm. Although the particles tend to clump during embolization procedures, the small size of the particles causes a more distal occlusion, increasing the likelihood of tissue ischemia. Vessels embolized with Gelfoam typically recanalize within 3 weeks to 3 months. Recanalization is notoriously unpredictable, however, with regards to both degree and timing.
Table 5.
Technical Pearls for Gelfoam Embolization
| Issue | Pearl |
|---|---|
| Slurry | Gelfoam slurry is prepared using two syringes with a three-way stopcock; one syringe contains contrast material and the other contains Gelfoam sponge; by using a to-and-fro motion between the two syringes, a homogenous slurry is formed; the consistency of pudding is desired, which will allow a Gelfoam “cast” of the proximal embolized vessel. |
| Torpedoes | Gelfoam torpedoes or cubes may also be used for larger vessels. |
| Air trapping | Gelfoam traps a large amount of air during preparation; postprocedural cross-sectional imaging often demonstrates this air, which should not be confused with infection. |
| Combination therapy | Gelfoam may also be used for permanent occlusions in coagulopathic patients, where coils are used as scaffolding and Gelfoam is used to cause the mechanical occlusion. |
Autologous Clot
Clot is a rarely used embolic today. Clot often lyses too quickly for clinical success. The authors now typically only use autologous clot as a blood patch for embolizing tracts post–lung biopsy.
CLINICAL SCENARIOS FOR LARGE-VESSEL EMBOLIZATION
Large-Vessel Permanent Occlusion
Clinical scenarios in which large-vessel permanent occlusion is required are situations in which angiographically visualized vascular abnormalities are present and a permanent occlusion of these abnormalities is required for patient's treatment. In these situations, the end organ supplied by the embolized vessel has a good collateral blood supply, preventing organ death following successful embolization. Example clinical scenarios include: gastrointestinal bleeding; pulmonary arteriovenous malformations, traumatic pseudoaneurysms, carotid or vertebral artery sacrifice, and visceral artery aneurysms. For this purpose, coils and Amplatzer plugs are the usual embolic agents used (Fig. 1). Such a proximal large-vessel occlusion is the equivalent of surgical ligation of the vessel.
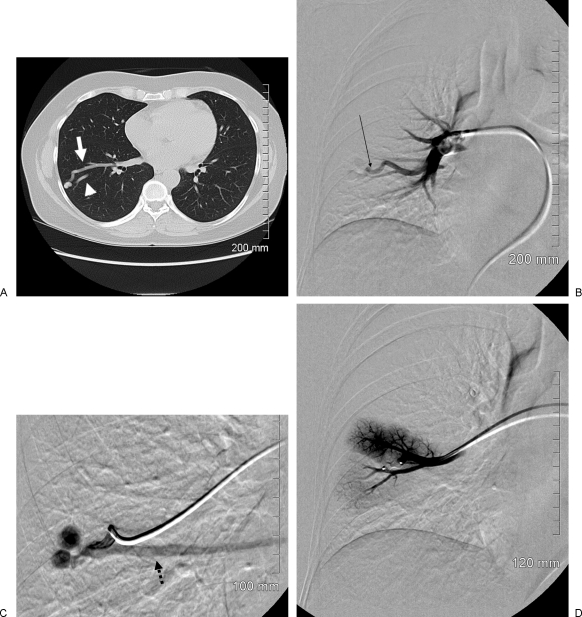
Figure 1.
Large-vessel permanent occlusion. A 51-year-old woman presented with a 6-month history of dyspnea on exertion and intermittent chest pain. She was on continuous oxygen therapy. A screening chest radiograph demonstrated a masslike lesion in her right midlung field. (A) Thin-section axial computed tomography through the midright lung field demonstrates a pulmonary nodule with a solitary feeding artery (white arrow) and a single draining vein (arrowhead). (B) Nonselective right pulmonary angiography demonstrates an enlarged and ecstatic feeding artery and early filling of the pulmonary peripheral arteriovenous malformation (AVM) (black arrow). (C) Superselective angiography via the large feeding artery demonstrates the bilobed pulmonary AVM with an early draining vein (black dashed arrow). (D) Selective angiography following deployment of a single Amplatzer Vascular Plug (white arrow) demonstrates complete occlusion of the large feeding artery.
Large-Vessel Temporary Occlusion
Clinical scenarios in which a large-vessel temporary occlusion is desired are situations where temporary arrest of hemorrhage is necessary with subsequent recanalization and vessel healing. The classic situation for such an agent is a large-vessel traumatic injury, such as hemorrhage following pelvic trauma. For these situations, Gelfoam sponge is used, either as a slurry or torpedoes (Fig. 2).
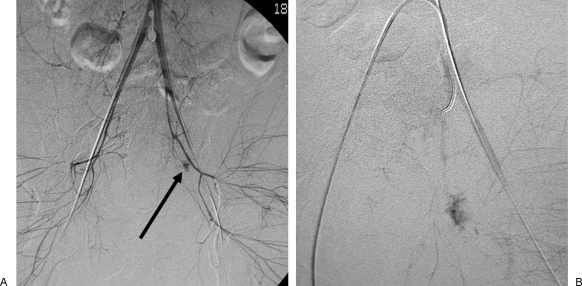
Figure 2.
Large-vessel temporary embolization. A 15-year old woman pinned under a truck for several hours following a rollover motor vehicle collision. She was hemodynamically unstable (systolic blood pressure <60 mm Hg), and her core temperature was 27°C. She was brought immediately to the angiography suite for embolization of vascular injuries sustained from her pelvic fracture. (A) Digital subtraction angiography from a distal aortic injection demonstrating contrast extravasation from the left internal iliac artery distribution (black arrow). (B) Delayed arterial phase imaging from a selective internal iliac artery injection demonstrating contrast pooling in the left internal iliac artery distribution. A nonselective Gelfoam embolization of the entire left internal iliac artery was performed with a good angiographic and clinical response.
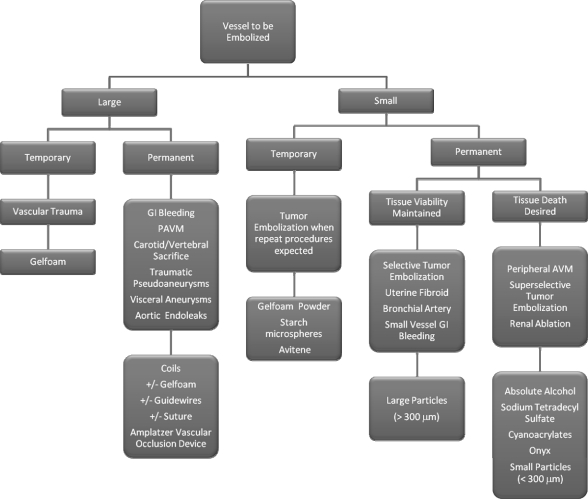
The algorithm we use when choosing embolic agents is given in Fig. 3. To reiterate the point made above, three questions must be answered before choosing the appropriate embolic agent:
Figure 3.
Embolic agent decision algorithm.
What is the vessel size?
Is a permanent or temporary occlusion desired?
Is cell death a desirable end point?
In the second part of this article, to be published at a later date, the use of small-vessel embolic agents will be discussed.
SUGGESTED READINGS
- Bauer J R, Ray C E., Jr In: Mauro M, Murphy K, Thomson K, Venbrux A, Zollikofer C, editor. Image-Guided Interventions. Philadelphia: Saunders Elsevier; 2008. Embolization agents. pp. 131–139.
- Funaki B. Microcatheter embolization of lower gastrointestinal hemorrhage: an old idea whose time has come. Cardiovasc Intervent Radiol. 2004;27:591–599. doi: 10.1007/s00270-004-0243-x. [DOI] [PubMed] [Google Scholar]
- Kickuth R, Rattunde H, Gschossmann J, Inderbitzin D, Ludwig K, Triller J. Acute lower gastrointestinal hemorrhage: minimally invasive management with microcatheter embolization. J Vasc Interv Radiol. 2008;19:1289–1296, e2. doi: 10.1016/j.jvir.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Pollak J S, Saluja S, Thabet A, Henderson K J, Denbow N, White R I., Jr Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radiol. 2006;17:35–44, quiz 45. doi: 10.1097/01.RVI.0000191410.13974.B6. [DOI] [PubMed] [Google Scholar]
- Rösch J, Dotter C T, Brown M J. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1972;102:303–306. doi: 10.1148/102.2.303. [DOI] [PubMed] [Google Scholar]
- Velmahos G C, Toutouzas K G, Vassiliu P, et al. A prospective study on the safety and efficacy of angiographic embolization for pelvic and visceral injuries. J Trauma. 2002;53:303–308. discussion 308. doi: 10.1097/00005373-200208000-00019. [DOI] [PubMed] [Google Scholar]