ABSTRACT
Available and improved options for the treatment of femoral-popliteal disease have increased over the last decade. Even though most patients suffering from claudication due to femoral-popliteal disease are treated with aggressive medical and noninvasive methods, patients with limb-threatening disease and severely lifestyle-limiting symptoms are treated with invasive treatments, which include endovascular treatment options. Unfortunately, the unique forces involved in this vascular segment have challenged the long-term patency and clinical effectiveness of these treatments. The purpose of this brief review is to discuss treatment strategies and options for patients with femoral-popliteal disease. Included in this discussion will be the review of data from recent published studies and late-breaking trials as it pertains to certain treatment strategies.
Keywords: Superficial femoral artery, angioplasty, stent, claudication, critical limb ischemia
The two main subsets of patients with femoral-popliteal disease include occlusive disease and aneurysmal disease. The endovascular treatment of both of these categories of patients remains an area of controversy. Historically, as in any rapidly advancing field of medicine, the endovascular treatment of these patients has been limited by the technologies available at the time. Over that last three decades, significant advancements in treatment methods have changed how physicians treat these patients. First percutaneous transluminal angioplasty (PTA) and then intravascular stents, beginning with balloon-expandable stents and then self-expanding stainless steel stents then leading to self-expanding nitinol stents and now to covered stents, drug-eluting stents (DES), and drug-eluting balloons.
The critical step in the evaluation of patients with infrainguinal disease is the ability to identify these patients, properly evaluate them, and decide when and how each patient should be treated. The most common symptom of infrainguinal peripheral artery disease is intermittent claudication (IC), which is described as an aching, pain, cramping, or numbness in the calf, less likely in the lower thigh or arch of the foot. These symptoms are induced by walking or exercise and are relieved by rest. Critical limb ischemia (CLI) is the most severe manifestation of peripheral arterial disease (PAD) and is due to severely reduced or absent oxygenation of the tissues of the leg due to a lack of arterial blood supply. These patients present with ischemic rest pain, nonhealing ischemic ulcerations, or gangrene.
The initial evaluation of patients with infrainguinal PAD includes a detailed and accurate history and physical. This allows the identification of risk factors and the documentation of atherosclerotic disease in other vascular beds. The risk factors for infrainguinal PAD are identical to cardiovascular atherosclerotic disease and the presence of infrainguinal PAD represents a marker for premature cardiovascular events. Even without a prior history of myocardial infarction or ischemic stroke, patients with PAD have approximately the same relative risk of death from cardiovascular causes as do patients with a history of coronary or cerebrovascular disease.1 Risk factors for peripheral vascular disease are well known and listed in Table 1.
Table 1.
Risk Factors for Peripheral Vascular Disease
| Adapted from TASC II document. J Vasc Surg 2007;45:S5–S67. ABI, ankle brachial indexes; IC, intermittent claudication; PAD, peripheral arterial disease. | |
| Race | ABI ≤0.90 is more common in non-Hispanic blacks (7.8%) than in whites (4.4%) |
| Gender | PAD (symptomatic or asymptomatic) is slightly greater in men than in women; this difference is most prevalent in younger age groups and in the more severe stages of disease |
| Age | Risk increase with age |
| Smoking | Heavy smokers have a fourfold higher risk of developing IC than nonsmokers25 |
| Diabetes mellitus | IC is about twice as common among diabetic patients than among nondiabetic patients; in patients with diabetes, for every 1% increase in hemoglobin A1c there is a corresponding 26% increased risk of PAD26 |
| Hypertension | Hypertension is associated with all forms of cardiovascular disease, including PAD |
| Dyslipidemia | The Framingham study states that a fasting cholesterol level greater than 7 mmol/L (270 mg/dL) was associated with a doubling of the incidence of IC, but the ratio of total to high-density lipoprotein cholesterol was the best predictor of occurrence of PAD |
| Inflammatory markers | C-reactive protein was raised in asymptomatic individuals who developed PAD in the subsequent 5 years |
| Hyperviscosity and hypercoagulable states | Raised hematocrit levels and hyperviscosity have been reported in patients with PAD |
| Hyperhomocysteinemia | The prevalence of hyperhomocysteinemia is high in the vascular disease population, compared with 1% in the general population |
| Chronic renal insufficiency | In the HERS study (Heart and Estrogen/Progestin Replacement Study), renal insufficiency was independently associated with future PAD events in postmenopausal women27 |
During the evaluation of patients with PAD, the distinction between CLI and IC needs to be properly determined. In the patient with claudication, the symptoms need to be properly characterized as far as severity specifically: How long have these symptoms been present? Are the symptoms lifestyle limiting? Has an exercise program and the proper medical management of lipid disorders, diabetes, and hypertension helped? With CLI, the patients are categorized as suffering from either acute or subacute/chronic disease. Patient with acute symptoms need to be properly evaluated for thrombectomy or thrombolytic therapy and the potential need for prompt endovascular or open revascularization. Subacute/chronic limb ischemia requires the existence of tissue loss (nonhealing ulcers or gangrene) or the presence of rest pain.2 The presence or absence of evidence of distal embolization also needs to be properly documented as it will broaden the scope of the treatment to include and treat the possible source of the embolization.
To properly treat these patients, imaging studies need to be properly utilized before performing an intervention. Pretreatment arterial noninvasive studies along with duplex imaging (include ankle brachial indexes before and after exercise, segmental plethysmography with pulse volume recordings, and toe pressures when appropriate) should be obtained to document the level and severity of disease. These studies also serve as a baseline for follow-up examinations to assess treatment success or failure objectively. Cross-sectional imaging such as with computerized tomographic angiography or magnetic resonance angiography yields additional information specific to the morphology and extent of disease that helps determine the best treatment access site and plan and hopefully will decrease procedure time and amount of contrast media used.3
Once a patient has been properly assessed and it has been determined that the patient needs treatment, how is the proper treatment selected? The first treatment decision usually involves the decision between endovascular and traditional (open) revascularization. The recently released TASC II4 recommendations addressed this question based on the target lesion (Table 2). The TASC II criteria demonstrate the improved outcomes since the release of the TASC I recommendations in the superficial femoral artery (SFA) by characterizing occlusions less than 15 cm as a class B lesion (formerly a class C lesion). According to TASC II, only lesions involving either the popliteal artery or common femoral artery are classified as class D lesions and recommended for surgery. Aside from several patient-specific factors that are also involved in forming the treatment decision, the patency and long-term clinical outcomes from endovascular treatments must be considered and the surgical alternatives should also be strongly considered particularly in class C and D lesions. The BASIL (Bypass versus Angioplasty in Severe Limb Ischaemia of the Leg) trial randomized patients in 27 UK centers with CLI between surgery and endovascular repair. There was no significant improvement between either of the two groups at 6 and 12 months, but the surgical first group had higher morbidity and underwent treatments that were about a third more expensive. After 2 years, the data suggested that patients were more likely to survive in the surgical group.5 These data have been used to recommend angioplasty and other endovascular techniques for short-term initial revascularization. However, if the patient has a reasonable life expectancy and proper anatomy for surgery, they will likely benefit from surgery due to its longer durability at a price of slightly higher initial morbidity.6 The study confirmed that regardless of the treatment arm, patients with CLI have extremely poor prognoses.
Table 2.
TASC II Classification of Femoral-Popliteal Lesions
| Classification | Lesion(s) | Recommendation |
|---|---|---|
| Adapted from TASC II document. J Vasc Surg 2007;45:S5–S67. CFA, common femoral artery; SFA, superficial femoral artery. | ||
| Type A lesions | Single stenosis ≤10 cm in length | Endovascular therapy is the treatment of choice for TASC A lesions |
| Single occlusion ≤5 cm in length | ||
| Type B lesions | Multiple lesions (stenoses or occlusions), each ≤5 cm | Endovascular therapy is the preferred treatment for type B lesions; the patient's comorbidities, fully informed patient preference, and the local operator's long-term success rates must be considered when making treatment recommendations for type B and type C lesions |
| Single stenosis or occlusion ≤15 cm not involving the infrageniculate popliteal artery | ||
| Single or multiple lesions in the absence of continuous tibial vessels to improve inflow for a distal bypass | ||
| Heavily calcified occlusion ≤5 cm in length | ||
| Single popliteal stenosis | ||
| Type C lesions | Multiple stenoses or occlusions totaling ≥15 cm with or without heavy calcification | Surgery is the preferred treatment for good-risk patients with type C lesions; the patient's comorbidities, fully informed patient preference, and the local operator's long-term success rates must be considered when making treatment recommendations for type B and type C lesions |
| Recurrent stenoses or occlusions that need treatment after two endovascular interventions | ||
| Type D lesions | Chronic total occlusions of the CFA or SFA >20 cm involving the popliteal artery | Surgery is the treatment of choice for TASC D lesions |
| Chronic total occlusion of the popliteal artery and proximal trifurcation vessels | ||
Once the decision to treat a lesion in the SFA using endovascular techniques has been made, the operator determines the best approach for the particular patient. The decision is based on several factors, which include lesion length and morphology, patient body habitus, previous surgical history, and available endovascular tools. Most operators perform a contralateral retrograde common femoral artery (CFA) puncture to address an SFA lesion. This approach allows proper evaluation and treatment of concomitant iliac disease as well as allow for a greater freedom to use devices that may require larger French sizes. The contralateral approach is the approach of choice for a flush occlusion of the SFA from its origin from the common femoral artery. However, a contralateral approach may not be successful due to the acuity of the native aortic bifurcation or in patients with prior aortobifemoral bypass. Additionally, it may be more difficult to generate sufficient torque and forward force on distal lesions from a contralateral approach. The ipsilateral antegrade approach can be utilized to treat patients with a nondiseased CFA, patent proximal SFA, favorable body habitus, and preserved iliac inflow. The antegrade approach on select patients with proper technique is safe and provides a more direct access to the SFA. Another approach may involve the use of an ipsilateral horizontal puncture to place a catheter in the ipsilateral iliac vessel to assess the aortoiliac segment and then reversing the puncture antegrade with a reverse curve catheter and sheath to treat the infrainguinal vessels. This technique can usually be avoided with proper preprocedural imaging evaluations.
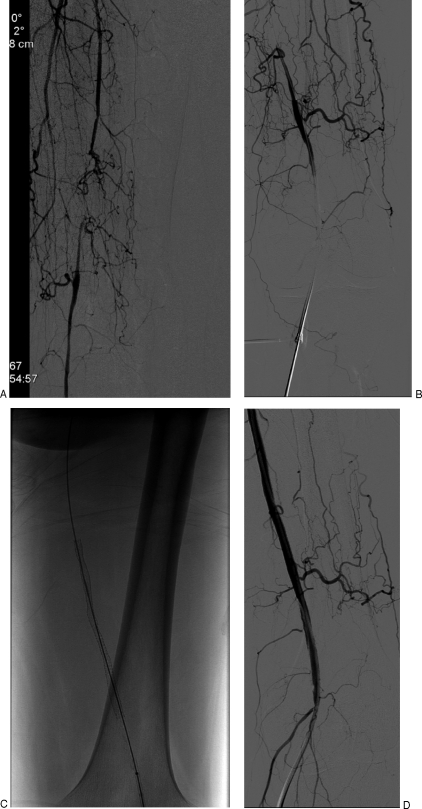
There are rare situations when retrograde access to the SFA is obtained from a popliteal or even dorsalis pedis approach (Fig. 1). The use of ultrasound guidance along with a microaccess needle has been helpful in obtaining a single wall arterial puncture and reducing complications.7 When there is disease of the popliteal artery, access via a dorsalis pedis artery cutdown at the dorsum of the foot can be utilized.8 Unfortunately, patients with CLI usually have associated tibial disease, and subsequent diminished healing of the surgical access may place the foot at risk. This situation occurs rarely but may be necessary in unique situations.
Figure 1.
Treatment of a superficial femoral artery (SFA) occlusion from a popliteal artery approach. A 66-year-old male presents with severe right leg claudication. The patient has failed several attempts from a contralateral approach due to a postsurgical changes that involve the aortic bifurcation and the right common femoral artery. The right lower-extremity angiogram demonstrates a right distal SFA occlusion with extensive collaterals (A). Patient is treated with a right popliteal artery approach. The puncture is performed with a 21-gauge needle and ultrasound guidance supplementing fluoroscopic imaging (B). The SFA occlusion was recanalized with a wire and catheter system. The occlusion was treated with the placement of a self-expanding stent (C). Final subtraction angiogram demonstrates patency of stent (D).
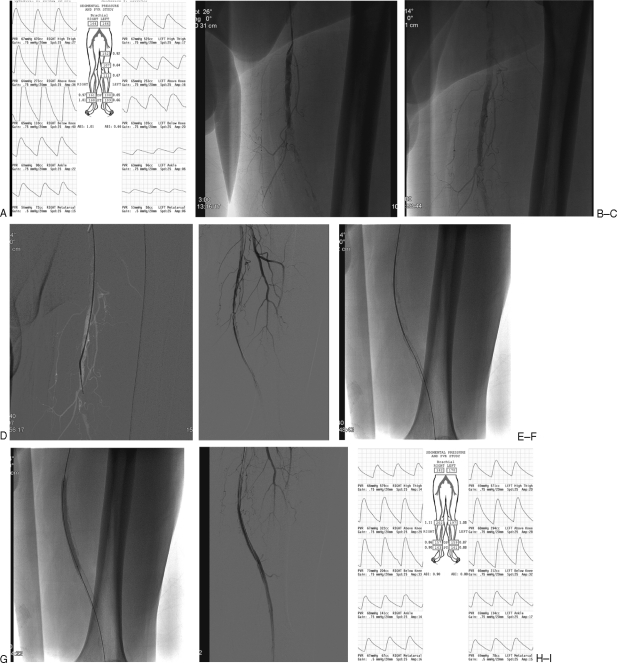
Prior to treatment, the lesion needs to be crossed with a wire. In the case of an arterial stenosis, a guide wire can usually be negotiated across the stenosis with fluoroscopic guidance. In the ideal situation, the guide wire finds an intraluminal path across the stenosis (Fig. 2). With an occlusion, a subintimal path may be created. In this situation, a wire can be looped on itself and advanced along the subintimal plane. A soft-tip angled hydrophilic wire is then usually utilized to attempt to reenter the lumen of the patent vessel.9,10 Extension of the subintimal plane should be minimized. It is desired to reenter the artery distal to the occlusion but above the popliteal artery or large collaterals. Gaining access to the vessel's lumen can be an arduous task that may require some patience. Fairly new needle-based reentry devices such as the Outback (Cordis, Bridgewater, NJ) or the Pioneer (Medtronics, Minneapolis, MN) catheters can be utilized. The Outback uses a small 21-gauge needle to aid in entering the vessel lumen. It relies on the use of fluoroscopy to relate the vessel lumen with the subintimal location (Fig. 3). The Pioneer catheter also uses a needle to aid access into the vessel lumen but it uses an intravascular ultrasound system (Volcano, San Diego, CA) for direct guidance. Once the needle allows access to the true lumen, a 0.014-inch wire is advanced into the distal vessel. There are several new technologies to aid in crossing an occluded vessel aside from the standard wires and needle-based devices. Controlled microdissection can be performed with several new technologies. These include the Frontrunner system (Cordis), which uses a pair of rounded hinged jaws that can be advanced across an occlusion and followed by passing a 4-French catheter over the device. There are several newer devices that are now available to aid in crossing occluded vessels, which will not be addressed in this article.
Figure 2.
Superficial femoral artery (SFA) recanalization with self-expanding stent placement. A 67-year-old man presents with severe left calf claudication. Preprocedure ankle brachial indexes (ABI) and segmental pressures demonstrate moderate to severe left lower extremity disease at rest. (A) Angiogram from a contralateral right common femoral approach demonstrates a left mid SFA occlusion early (B) and late (C). The occlusion was crossed using road map technique with a 5-French multipurpose angled catheter and an angled hydrophilic 0.035 in hydrophilic guide wire (D). Subtraction angiogram after predilation of the recanalized SFA with a 4-mm angioplasty balloon (E). Deployment of a 6-mm diameter by 15-cm length Lifestent (Bard, Tempe, AZ) self-expanding nitinol stent (F). Dilation of the stent with a 6-mm diameter angioplasty balloon (G). Final subtraction angiogram demonstrating adequate flow in the stent. (H) Preserved three-vessel runoff. (not shown) Arterial noninvasive study performed the day after intervention demonstrates normal ankle brachial indexes in left lower extremity (I).
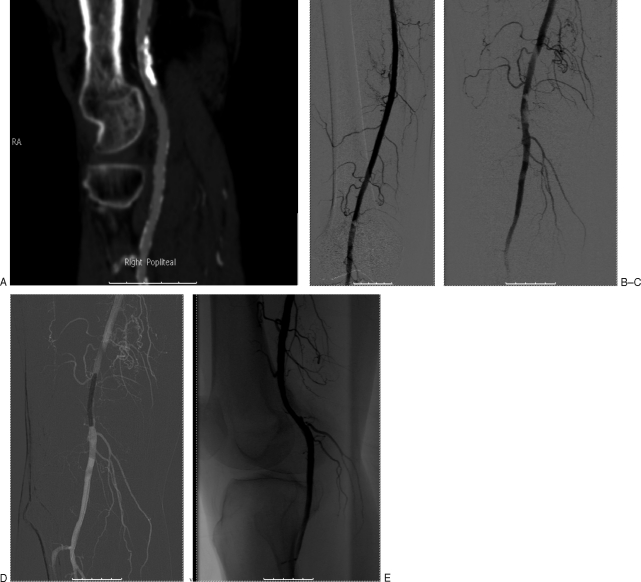
Figure 3.
Superficial femoral artery (SFA) recanalization using a needle reentry system. A 75-year-old male presents with a nonhealing ulcer in the right foot. Digital subtraction angiogram from a contralateral left common femoral approach demonstrates a long-segment right SFA occlusion (A). Attempts of recanalization of SFA using a catheter and wire system (B) results in subintimal course along the distal SFA (C). A needle-based reentry system, Outback catheter (Cordis, Bridgewater, NJ), is positioned and deployed toward the calcified but patent luminal vessel (D). The true lumen can be targeted using different fluoroscopic projections. The needle system is utilized until the 0.014-inch wire is advanced in an intraluminal location. (E). Final subtraction angiogram after dilatation and self-expanding stent placement in the SFA (F).
Even with all of the techniques and new technologies to aid in crossing chronic SFA occlusions, crossing techniques will be unsuccessful in some lesions. The crossing of these lesions maybe attempted on another day and possibly from a different approach. The risk of vessel perforation cannot be underestimated, but these perforations are usually self-limited especially if the occlusion is not crossed. The benefit of an intraluminal crossing is also unknown. Studies on subintimal angioplasty have documented long-term patency rates comparable to luminal angioplasty.11 There may be, however, a slightly higher risk of complications such as perforation and fistula formation. Even though not proven, most interventionalists believe that the patency is not related to how the occlusion was recanalized but on the length and the amount of calcium in the lesion.
In the setting of an acute occlusion, the guide wire will usually easily cross an occlusion, confirming the presence of acute thrombus. These acute thromboses usually warrant thrombolysis to best identify the underlying lesion, which is usually shorter in length than the acutely occluded length. Additionally, thrombolysis will minimize the risk of distal embolization during subsequent intervention. In patients who may not tolerate pharmaceutical thrombolysis, a mechanical or surgical thrombectomy may be necessary. Alternatively, the decision to minimize exchanges and manipulations to limit the possibility of distal embolization is paramount in the setting of acute occlusions. Covered stents have anecdotally been used in patients with acute thrombus who cannot tolerate pharmacological, mechanical, or surgical thrombectomy with the hope that the covered stents will trap the embolic material; however, this has never been proven.
Once the lesion has been successfully crossed and the appropriate guide wire has been placed for treatment, the operator must determine appropriate treatment. Consideration must be given to the use of anticoagulation, the use of a distal protection device, and the potential treatment strategy. Anticoagulation if desired can be accomplished with a heparin bolus (60 to 70 IU per kilogram) or bivalirudin (Angiomax, The Medicines Company, Parsippany, NJ) protocol. Distal embolization protection devices can be considered to hopefully minimize the risk of distal embolization. Unfortunately, the devices that are presently available would be used “off-label” as no embolization protection device has an indication for use in the femoral or popliteal segment.
ANGIOPLASTY OR STENT?
The ideal endovascular treatment method for femoral-popliteal disease has been the subject of many trials over the last decade. The STAR (SCVIR Transluminal Angioplasty) registry evaluated prospectively the long-term patency of patients who underwent femoral-popliteal angioplasty. It was a seven-institution registry that enrolled patients over a 3-year period with 5-year follow-up. Two hundred five patients (219 limbs) comprised the study cohort and demonstrated a 12-, 24-, and 36-month primary patency of 87, 80, and 69%, respectively. The mean lesion length was only 3.8 cm. Occlusions comprised 17% of the cohort with a mean occlusion length of 4.7 cm. The registry also concluded that diabetic and patients with poor tibial runoff had lower long-term patency.12
The role of PTA has been studied and compared with stenting. The FAST Trial (Femoral Artery Stenting Trial) randomized 244 patients with SFA lesions less than 10 cm in length (mean length 4.5 cm) to treatment with PTA or nitinol self-expanding stent (Luminexx 3, Bard, Flagstaff, AZ). The FAST trial did not demonstrate a significant benefit to stenting over angioplasty in these lesions using both ultrasound-assessed binary restenosis and clinical improvement (using Rutherford criteria improvement) at 1 year. The study did not find any benefit in stenting short SFA lesions.13 With the development of self-expanding stents, many endovascular specialists hoped to utilize these stents in the SFA to combat the restenosis and failure rate of angioplasty especially in long and calcified lesions. The initial 6-month data from the SIROCCO II trial (sirolimus eluting versus bare stents) was very positive for both drug-coated and non–drug-coated nitinol self-expanding stents (Smart Stent, Cordis).14 Unfortunately, the data at 24 months demonstrated significant restenosis with multiple fractures.15 Studies were then completed to assess the association between stent fractures and restenosis.16 In 2004, the ABSOLUTE trial published in the New England Journal of Medicine demonstrated the efficacy of a self-expanding nitinol stent (Dynalink/Absolute stent, Abbott, Abbott Park, IL) over angioplasty for SFA lesions. This trial randomized patients between angioplasty and nitinol stent placement with a mean lesion length greater than 10 cm and allowed the use of multiple overlapping stents. The trial had a much larger restenosis rate in the angioplasty group when compared with the FAST trial and STAR registry, suggesting that as the lesion length increases, the benefit from nitinol stenting increases over angioplasty.17 The RESILIENT trial was an international multicenter randomized control trial for SFA disease treatment between angioplasty and stenting with the Lifestent (Bard, Flagstaff, AZ) self-expanding helically designed nitinol stent, The initial results from this study demonstrated a significant improvement in patency in the patients who underwent stenting when compared with angioplasty in lesions greater than 5 cm. The study also found that angioplasty and stenting were equivalent in short lesions (<5 cm).18 These results are pending publication.
These studies suggest that PTA is the best option for short focal lesions in the SFA. Furthermore, nondiabetic patients with three-vessel tibial runoff vessels whose lesions were nonocclusive and noncalcified have the best long-term outcome with angioplasty. As lesion lengths increase, the patency from PTA worsens. In lesions greater than 5 cm, treatment with a self-expanding stent such as the Lifestent is supported by the RESILIENT trial. The patency of the self-expanding stents may be related to the amount of underlying calcification, lesion length, number of overlapping stents, and amount and length dilated before and after stent deployment. These factors, however, require further study.
Over the past several years, there has been much effort to improve the existing technology of traditional angioplasty to increase the efficacy and durability of PTA for SFA lesions. Cryoplasty uses liquid nitrous oxide to inflate modified angioplasty balloons. This technique claims to cause the same intimal disruption of angioplasty with the hope of creating apoptosis (cell death) and limiting intimal restenosis with the cryothermal energy balloons (Polarcath, Boston Scientific, Natick, MA). These balloons have been used in the femoral, popliteal, and tibial vessels. Cryoplasty may limit postangioplasty recoil and dissection due to a more homogenous inflation. In a multicenter registry with 102 patients with claudication and lesions less than 10 cm (mean length 4.7 cm), cryoplasty had a technical success rate of 85.3% (less than 30% residual) and a primary patency of 70.1% at 9 months using duplex ultrasonography systolic velocity criteria.19 Similarly, modified angioplasty variants include the use of cutting or scoring balloons. A Cutting balloon (Boston Scientific, Natick, MA) use four athertomes that function to create longitudinal incisions in the stenosis during the dilation of the cutting balloon. The scoring balloons (Angiosculpt, Angioscore Inc., Fremont, CA) feature a nitinol helical cage surrounding an angioplasty balloon that scores the lesion during the balloon dilation of the stenosis. Both of these devices are limited by the shorter balloon lengths (cutting balloons are usually 2 cm long, and newer scoring balloons are 4 cm in length) and the need for slightly larger access sheath than standard angioplasty balloons. Focal calcified lesions as well as lesions resistant to standard angioplasty may benefit from these technologies. Additionally, these technologies may serve as stand-alone treatment, avoiding the use of stents. The Angiosculpt scoring balloon has received FDA clearance for the treatment of infrainguinal lesions, and multicenter SFA trials are ongoing in Europe.
There are several devices that increase vessel patency by debulking the amount of atheromata at the site of stenosis. These devices include lasers and mechanical atherectomy devices. Laser techniques can be used not only to cross a focal occlusion but to remove tissue from a vessel wall. These devices may serve as stand-alone techniques or may require angioplasty to aid in dilating the vessel after debulking the plaque. Atherectomy devices include the directional atherectomy technology (Silverhawk, EV3, Plymouth, MN) where the plaque is shaved into a reservoir in the device nose cone or a rotational atherectomy, which sands the plaque (Diamondback device, Cardiovascular Systems Inc., St. Paul, MN). Additionally, there is a technology that combines rotational atherectomy with aspiration to debulk a lesion (Jetstream, Pathway Medical, Kirkland, WA). Even though there are numerous reports and studies, these techniques have not been studied in randomized controlled trials.
The VIABAHN (W.L. Gore and Associates, Flagstaff, AZ) is a covered stent that has been approved for use in the SFA. The VIABAHN is a nitinol stent skeleton supporting a polytetrafluoroethylene cover. These stents require slightly larger access sheaths when compared with self-expanding nitinol stents and rely upon accurate luminal measurements and complete luminal expansion of the stent to prevent infolding. These stents have rarely been associated with fracture.20,21 The VIBRANT trial is a multicenter study of long SFA occlusions (>12 cm) randomized between covered stents (VIABAHN) and self-expanding nitinol stents. This trial has closed enrollment (150 patients) and data for 3 years are being tabulated. The final data from this trial will be helpful in the assessment of the VIABAHN in the treatment of SFA occlusive disease, specifically evaluating long-term clinical effectiveness and patency. Additionally, it should evaluate and answer concerns about the rate and risk of acute stent thrombosis as well as restenosis at the edges of the stented length.
In 2004, the early 6-month data from the SIROCCO trial excited the endovascular community by reporting a high patency of drug-eluting self-expanding stents in the SFA.14 Unfortunately, the trend did not continue at 24 months, with a large number of fractures and clinical failures.15 The development of newer self-expanding stent designs along with several drug-eluting medication will hopefully bring longer patency to SFA stents. The ZILVER DES trial (Cook, Bloomington, IN) has finished enrollment and is presently in the follow-up phase. This trial will compare bare self-expanding stents in the SFA to self-expanding drug-coated stents. The manner in which the drug is applied to the stent on the Zilver platform is unique. Similarly, there is excitement surrounding the promise of drug-eluting balloons. Trials are presently underway in Europe involving the use of drug-eluting balloons that will hopefully maximize the benefits of angioplasty while the eluted drugs will limit restenosis. The technology may avoid the need for a stent and its associated risk of fracture. The THUNDER trial was recently published in the New England Journal of Medicine, which demonstrated the early efficacy of drug-eluting balloons in the SFA.22
Endovascular treatment of popliteal arterial occlusive disease is usually reserved for focal stenoses. As the popliteal artery serves as the solitary conduit for blood flow into the tibial vessels, treatment in this region carries more risk than the SFA. These lesions are usually treated with angioplasty. In lesions resistant to conventional PTA secondary to significant calcific plaque, the authors have had success using the previously mentioned cutting or scoring balloons (Fig. 4). Primary stenting for focal popliteal artery stenoses is not recommended due to the flexibility and bending that occurs at the knee joint; however, these devices are a valuable bailout option in flow-limiting dissection after angioplasty. The MELOPEE study is a five-center European study that placed self-expanding nitinol stents (Lifestents) in the popliteal fossa as a “bailout” for failed PTA.23 Even though the stents have performed well in these patients, most operators avoid bare stents in the popliteal fossa. Interestingly, covered stents such as the Viabahn have shown success for the treatment of femoral and popliteal aneurismal disease (Fig. 5). These stent grafts have shown a low rate of fracture and failure, particularly if the grafts are properly sized and fully expanded at deployment and placed in patients who can tolerate antiplatelet medications.24
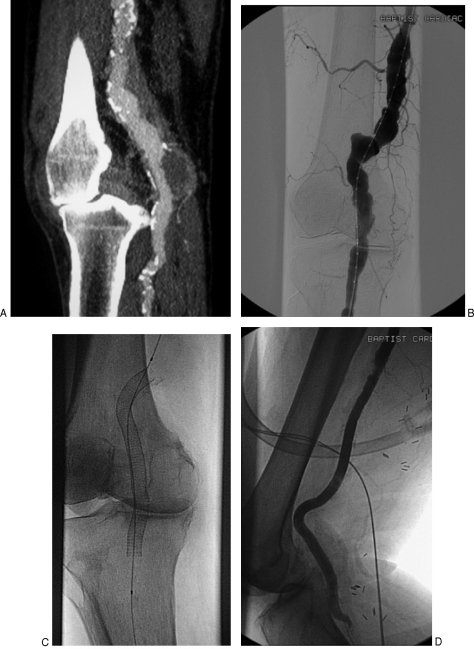
Figure 4.
Popliteal artery angioplasty using a scoring balloon. A 72-year-old male presents with progressive severe right calf and foot claudication over the last 3 months. Computerized tomographic angiography demonstrates a severe calcified stenosis within the proximal popliteal artery in the setting of moderate tibial disease (A). Subtraction angiogram performed from antegrade right common femoral artery puncture confirms calcified stenosis within the proximal popliteal artery (B), also with magnified views (C). The lesion was treated with a 5-mm diameter scoring balloon (Angiosculpt balloon, Angioscore, Fremont, CA) using road map technique (D). The final subtraction angiogram confirms improved patency without complication (E). Patient reported no further claudication at follow-up.
Figure 5.
Popliteal artery aneurysm treatment with a covered stent. A 75-year-old male presents with an incidentally discovered 2.5-cm partial thrombosed right popliteal artery aneurysm on computerized tomographic angiography. (A) From a right common femoral artery antegrade approach, the aneurysm was reidentified. (B) Two covered stents (VIABAHN, W L Gore and Associates, Flagstaff, AZ) were deployed. (C) Final angiograms in extension (not shown) and flexion (D) fail to demonstrate endoleak.
CONCLUSION
Proper treatment of patients with femoral-popliteal segment disease is challenging. The initial evaluation and proper assessment of the indications for treatment are equally important when compared with the actual treatment. Patients with minimal symptoms may be worsened long term with endovascular revascularization. When a patient has been deemed a candidate for endovascular treatment, the lesion and patient characteristics should be used to determine the ideal treatment. Lesions less than 5 cm should be initially treated with angioplasty, especially minimally calcified nonocclusive disease. Even though randomized controlled trials highlight the potential benefit of certain self-expanding stents over angioplasty, the long-term patency and clinical benefits warrant their judicious use. The role for covered stents (Viabahn) and drug-coated stents will likely increase with the release of the VIBRANT and ZILVER DES study data. Hopefully, drug-eluting balloons will allow treatment without the need for stents in the future.
REFERENCES
- Newman A B, Shemanski L, Manolio T A, et al. The Cardiovascular Health Study Group Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:538–545. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- Hirsch A T, Haskal Z J, Hertzer N R, et al. American Association for Vascular Surgery. Society for Vascular Surgery. Society for Cardiovascular Angiography and Interventions. Society for Vascular Medicine and Biology. Society of Interventional Radiology. ACC/AHA Task Force on Practice Guidelines. American Association of Cardiovascular and Pulmonary Rehabilitation. National Heart, Lung, and Blood Institute. Society for Vascular Nursing. TransAtlantic Inter-Society Consensus. Vascular Disease Foundation ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Sharafuddin M J, Stolpen A H, Dixon B S, Andresen K J, Sun S, Lawton W J. Value of MR angiography before percutaneous transluminal renal artery angioplasty and stent placement. J Vasc Interv Radiol. 2002;13(9 Pt 1):901–908. doi: 10.1016/s1051-0443(07)61773-4. [DOI] [PubMed] [Google Scholar]
- Norgren L, Hiatt W R, Dormandy J A, Nehler M R, Harris K A, Fowkes F G, TASC II Working Group Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Bradbury A, Gamgee S. Long term (5 year) results of BASIL Trial Show “Open Surgery First” to be Superior to “Endovascular Treatment First” for Critical Limb Ischemia. New York: Presented at: Veith Symposium; November 2008.
- Adam D J, Beard J D, Cleveland T, et al. BASIL trial participants Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- Yilmaz S, Sindel T, Lüleci E. Ultrasound-guided retrograde popliteal artery catheterization: experience in 174 consecutive patients. J Endovasc Ther. 2005;12:714–722. doi: 10.1583/05-1576MR.1. [DOI] [PubMed] [Google Scholar]
- Spinosa D J, Harthun N L, Bissonette E A, et al. Subintimal arterial flossing with antegrade-retrograde intervention (SAFARI) for subintimal recanalization to treat chronic critical limb ischemia. J Vasc Interv Radiol. 2005;16:37–44. doi: 10.1097/01.RVI.0000141336.53745.4A. [DOI] [PubMed] [Google Scholar]
- Bolia A, Brennan J, Bell P R. Recanalisation of femoro-popliteal occlusions: improving success rate by subintimal recanalisation. Clin Radiol. 1989;40:325. doi: 10.1016/s0009-9260(89)80231-4. [DOI] [PubMed] [Google Scholar]
- Nadal L L, Cynamon J, Lipsitz E C, Bolia A. Subintimal angioplasty for chronic arterial occlusions. Tech Vasc Interv Radiol. 2004;7:16–22. doi: 10.1053/j.tvir.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Met R, Lienden K P Van, Koelemay M J, Bipat S, Legemate D A, Reekers J A. Subintimal angioplasty for peripheral arterial occlusive disease: a systematic review. Cardiovasc Intervent Radiol. 2008;31:687–697. doi: 10.1007/s00270-008-9331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T W, Groffsky J L, Soulen M C. Predictors of long-term patency after femoropopliteal angioplasty: results from the STAR registry. J Vasc Interv Radiol. 2001;12:923–933. doi: 10.1016/s1051-0443(07)61570-x. [DOI] [PubMed] [Google Scholar]
- Krankenberg H, Schlüter M, Steinkamp H J, et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST) Circulation. 2007;116:285–292. doi: 10.1161/CIRCULATIONAHA.107.689141. [DOI] [PubMed] [Google Scholar]
- Duda S H, Pusich B, Richter G, et al. Sirolimus-eluting stents for the treatment of obstructive superficial femoral artery disease: six-month results. Circulation. 2002;106:1505–1509. doi: 10.1161/01.cir.0000029746.10018.36. [DOI] [PubMed] [Google Scholar]
- Duda S H, Bosiers M, Lammer J, et al. Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: the SIROCCO II trial. J Vasc Interv Radiol. 2005;16:331–338. doi: 10.1097/01.RVI.0000151260.74519.CA. [DOI] [PubMed] [Google Scholar]
- Scheinert D, Scheinert S, Sax J, et al. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J Am Coll Cardiol. 2005;45:312–315. doi: 10.1016/j.jacc.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–1888. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- Katzen B. Veith Symposium; November 23, 2008. New York. Update and new findings from the resilient trial comparing the life stent with POBA: improvement with stenting is durable beyond one year. Available at: http://www.medicalnewstoday.com/articles/130504.php Available at: http://www.medicalnewstoday.com/articles/130504.php
- Laird J, Jaff M R, Biamino G, et al. Cryoplasty for the treatment of femoropopliteal arterial disease: results of a prospective, multicenter registry. J Vasc Interv Radiol. 2005;16:1067–1073. doi: 10.1097/01.RVI.0000167866.86201.4E. [DOI] [PubMed] [Google Scholar]
- Saxon R R, Coffman J M, Gooding J M, Ponec D J. Long-term patency and clinical outcome of the Viabahn stent-graft for femoropopliteal artery obstructions. J Vasc Interv Radiol. 2007;18:1341–1349. quiz 1350. doi: 10.1016/j.jvir.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Saxon R R, Dake M D, Volgelzang R L, Katzen B T, Becker G J. Randomized, multicenter study comparing expanded polytetrafluoroethylene-covered endoprosthesis placement with percutaneous transluminal angioplasty in the treatment of superficial femoral artery occlusive disease. J Vasc Interv Radiol. 2008;19:823–832. doi: 10.1016/j.jvir.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358:689–699. doi: 10.1056/NEJMoa0706356. [DOI] [PubMed] [Google Scholar]
- Bosiers M. Melopee study 12 months results. Hollywood, FL: International Symposium on Endovascular therapy (ISET) Conference; January 22, 2008.
- Idelchik G M, Dougherty K G, Hernandez E, Mortazavi A, Strickman N E, Krajcer Z. Endovascular exclusion of popliteal artery aneurysms with stent-grafts: a prospective single-center experience. J Endovasc Ther. 2009;16:215–223. doi: 10.1583/08-2412.1. [DOI] [PubMed] [Google Scholar]
- Fowkes F G, Housley E, Cawood E H, Macintyre C C, Ruckley C V, Prescott R J. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384–392. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- O'Hare A M, Vittinghoff E, Hsia J, Shlipak M G. Renal insufficiency and the risk of lower extremity peripheral arterial disease: results from the Heart and Estrogen/Progestin Replacement Study (HERS) J Am Soc Nephrol. 2004;15:1046–1051. doi: 10.1097/01.asn.0000119574.27772.fd. [DOI] [PubMed] [Google Scholar]