ABSTRACT
Portal vein embolization (PVE) is used to induce preoperative liver hypertrophy in patients with anticipated marginal future liver remnant (FLR) volumes who are otherwise potential candidates for resection. PVE can be performed utilizing the transhepatic contralateral and ipsilateral approaches. The transhepatic contralateral approach is the most commonly used technique worldwide, largely owing to its technical ease. However, the contralateral approach risks injuring the FLR, thereby compromising the planned surgical resection. The transhepatic ipsilateral approach offers a potentially safer alternative because the complications associated with this approach affect only the hepatic lobe that will be resected and are usually not serious enough to preclude surgery. This article discusses PVE using the transhepatic ipsilateral and contralateral approaches, including patient selection criteria, anatomical and technical considerations, and patient complications and outcomes.
Keywords: Portal vein embolization, liver cancer, liver resection, liver hypertrophy
Surgical resection remains the mainstay of curative treatment for liver cancer, and many recent improvements in perioperative care have increased the number of major hepatic resections that could be performed for primary and metastatic liver cancer. However, many of these patients have concomitant underlying liver disease. In patients with large or widely distributed tumor burden and/or underlying liver disease, the risk of perioperative hepatic failure remains. Although many factors contribute to this risk, recent studies emphasized an association between the volume of the residual liver and its postoperative function.1,2 In fact, many patients are considered to be at high risk for postoperative complications due to an initially deficient future liver remnant (FLR) volume. An insufficient functional liver mass may result in postoperative cholestasis, fluid retention, and impaired synthetic function, among other complications, and ultimately preclude the surgical resection altogether.
Because of the liver's regenerative capacity, preoperative portal vein embolization (PVE) has been advocated to increase the functional mass of the liver segments that will remain in situ postoperatively. PVE redirects portal flow to the intended FLR, initiating hypertrophy of the nonembolized liver segments and increasing the functional reserve of the FLR before hepatic resection. Recent studies confirmed improvements in the postoperative course after PVE,1,3 enabling many patients previously considered to have unresectable disease to become candidates for potentially curative hepatic resection. For this reason, PVE is now considered the standard of care prior to major hepatectomy in many comprehensive hepatobiliary centers worldwide.
PVE can be performed by one of four approaches. Most commonly used are the percutaneous transhepatic approaches; the transhepatic contralateral approach accesses the portal vein through the FLR, and the transhepatic ipsilateral approach accesses the portal vein through the liver to be resected. The transhepatic contralateral approach is the most commonly used technique worldwide, largely owing to its technical ease, but this approach risks injuring the FLR and compromising the planned hepatic resection.4 The transhepatic ipsilateral approach offers a potentially safer alternative because its complications affect the hepatic lobe that will be resected and are usually not serious enough to preclude surgery.5 Given this, the ipsilateral approach is gaining favor. The percutaneous transhepatic approaches comprise the vast majority of PVEs that interventional radiologists perform.
The other two approaches to PVE, the percutaneous transjugular and intraoperative transileocolic venous approaches, are less common. The transjugular approach (in which the portal vein is accessed via the hepatic vein), has been described, but reports of experience with this technique are scarce.6 The intraoperative transileocolic venous approach (in which the portal vein is accessed at laparotomy) was the first technique used for PVE, but because it is more invasive and requires anesthesia support, it is rarely used,7 except when combined with other synchronous surgical procedures.
Thus this article focuses on the use of percutaneous transhepatic PVE using the more common ipsilateral and contralateral transhepatic approaches, including information on patient selection criteria, anatomical and technical considerations, and patient complications and outcomes.
PATHOPHYSIOLOGICAL RATIONALE FOR PVE
The liver's regenerative response is typically mediated by the proliferation of surviving hepatocytes within the acinar architecture of the hepatic remnant. The molecular and cellular events that arise during hepatic regeneration result from injury-induced growth-factor stimulation.8 Importantly, extrahepatic growth factors are transported primarily from the intestines to the liver via the portal vein, not the hepatic artery. The degree of hepatocyte proliferation is directly proportional to the degree of stimulus, with injury to > 10% of the liver resulting in proliferation of cells throughout the liver.9
A diseased liver has a lower capacity to regenerate than a healthy liver. This may be because of the hepatocytes' diminished capacity to respond to hepatotropic factors or because parenchymal damage, such as fibrosis, leads to slower portal blood flow rates.8,10
In 1986, Kinoshita et al11 were the first to report that PVE limits extension of portal tumor thrombi from hepatocellular carcinoma. Then in 1990, Makuuchi et al7 published their experience with preoperative PVE performed solely to induce hypertrophy in the left lobe of the liver preceding right lobe hepatectomy. Their rationale for using PVE was to minimize the abrupt increase in portal pressure at resection that can lead to hepatocellular damage to the FLR and to improve overall tolerance to major resection by increasing the hepatic mass before resection to reduce the risk of postresection metabolic changes.
Doppler ultrasonography can be used to partially assess these postresection changes in the hepatic circulation. Doppler ultrasonography shows that after PVE, portal blood flow to the nonembolized hepatic segments increases rapidly and then decreases gradually. The increased portal flow to the nonembolized liver is a result of the unchanged splanchnic flow being redistributed through the altered portal circulation after PVE. There is a corresponding abrupt increase in arterial flow to the embolized liver segments, which is secondary to intrahepatic blood flow regulation. Parallel to the hemodynamic changes, upregulation of various humoral mediators and subsequent activation of intrahepatocyte signal transduction occur during hepatic regeneration following PVE. These mediators include cytokines, vasoregulators, growth factors, eicosanoids, and various hormones.8 Despite all these hemodynamic and humoral changes, the liver function tests typically show only mild and transient alterations, if any.12,13
A variety of conditions may interfere with the regulation of the mediators just mentioned and inhibit regeneration. These conditions include biliary obstruction, diabetes, chronic ethanol consumption, malnutrition, and infection. Whenever possible, treatment of these conditions prior to PVE is essential for optimizing hepatic hypertrophy.8
PATIENT SELECTION
To select patients who will benefit from PVE, the surgeon must consider several important factors. After determining that the patient is a candidate for major surgery, the presence of underlying hepatic disease should be assessed to help determine whether the FLR volume will be adequate for hepatic function after major hepatectomy. Other important considerations include patient weight and body surface area (large patients require larger FLRs), the presence or absence of portal hypertension, and extrahepatic disease. The surgeon must also consider the extent and complexity of the planned resection and the probability that an associated extrahepatic surgery will be performed at the time of hepatic resection, particularly if concomitant pancreatectomy will be performed.14,15 For example, in a study by Kawarada et al,16 dogs subjected to a 70% hepatectomy combined with a pancreatectomy had delayed recovery of hepatic function and more limited regenerative capacity than dogs that underwent hepatectomy alone. The reduction in hepatic regeneration was proportional to the extent of the pancreatectomy.
As is commonly known, a normal liver has a greater regenerative capacity, functions more efficiently, and tolerates injury better than a cirrhotic liver. Lethal postresection hepatic failure is more common in patients with cirrhosis, and other complications of the poorly functioning liver remnant, such as ascites, fluid retention, and wound breakdown from poor protein synthesis, occur with greater frequency after resection in patients with cirrhosis. Therefore, most surgeons only consider patients with Child-Pugh classification A cirrhosis for major hepatectomy. In addition, a retrospective study has suggested an increased risk of postoperative complications in patients who previously received systemic or local neoadjuvant chemotherapy, which may be associated with hepatic injury.17
The true FLR limit required prior to major hepatic resection has yet to be determined. However, guidelines have evolved based on the presence or absence of underlying hepatic disease and patient size. Most authors agree that patients with cirrhosis and an FLR < 40% should undergo PVE prior to major hepatectomy. Recent studies recommended PVE for patients with hepatic fibrosis or a severe hepatic injury and an FLR < 30%. Patients without underlying hepatic disease should undergo PVE only when the FLR is < 20%.1,18 Failure to adhere to these guidelines often results in unnecessary overuse of PVE.19
It is important to individualize PVE for each patient according to intrahepatic segmental variability. A liver volume analysis revealed that the lateral left liver (segments II/III) makes up ≤ 20% of the total liver volume in > 10% of patients.20 Therefore, an FLR-to-total estimated liver volume (TELV) ratio < 20% can be expected in most patients who do not develop compensatory hypertrophy from tumor growth and require an extended right hepatectomy. In this subset of patients, right PVE with extension to segment IV is indicated. In contrast, left PVE is rarely necessary, as demonstrated by Nagino et al.21
Estimating an adequate FLR for patients with cirrhosis is particularly challenging. In these patients, estimating volume alone may not be sufficient, and a concomitant functional assessment is advocated by some groups. The most common test utilized to evaluate FLR function is indocyanine green (ICG) dye retention. Fifteen minutes after ICG dye injection at a dose of 0.5 mg/kg, ≤ 10% of the dye should remain in the FLR.3
There are few contraindications to PVE. The only absolute contraindication is overt clinical portal hypertension, which contraindicates major hepatic resection. Also, PVE is not appropriate when tumor has invaded the portal vein because portal flow is already redistributed. However, in this setting, individualized patient care is needed because tumor thrombus of small segmental portal branches will not divert considerable portal flow and PVE may be beneficial. Relative contraindications to PVE include extrahepatic metastatic disease (including portal adenopathy), uncorrectable coagulopathy, a tumor precluding safe transhepatic access, biliary dilation in the FLR (preprocedural biliary drainage is required), mild portal hypertension, and renal failure. Although tumor extension to the FLR has been considered a contraindication to PVE, aggressive resection approaches such as two-stage hepatectomy and thermal ablation therapy within the FLR (if the tumor burden is small) permit PVE in this scenario. Finally, as we discuss in detail later, if it is not possible to access an adequate portal vein branch for PVE (e.g., because of large tumor burden with use of the ipsilateral approach), the contralateral approach can be considered.
VOLUMETRIC COMPUTED TOMOGRAPHY
Volumetric computed tomography (CT) is essential for planning hepatic resection.14,20,22 Three-dimensional volumetric estimates are calculated by outlining the hepatic segmental contours and estimating surface measurements from each slice. To estimate FLR size accurately, the measurements have to be standardized to individual patient size because larger patients need a larger FLR than smaller patients. CT is used directly to measure the FLR, which is by definition disease free. The total liver volume is then estimated by the following formula:
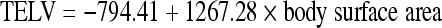 |
This formula is derived from the close relationship between liver size and patient size based on a patient's body weight and body surface area. The FLR-to-TELV ratio is then calculated, and this ratio is referred to as the standardized FLR (sFLR) measurement. The sFLR then can be correlated with surgical outcome.18,22 Most protocols obtain CT images immediately before PVE and ~3 to 4 weeks after the procedure to assess the degree of FLR hypertrophy, which is discussed in more detail elsewhere in this volume and beyond the scope of this article.
TECHNICAL ASPECTS OF PERCUTANEOUS TRANSHEPATIC PVE
Access Routes to the Portal Venous System
Note that the approach to the portal vein is chosen at the discretion of the operator, and the decision may be based on multiple factors, including the extent of the embolization and surgery, the operator's preference for a specific embolic agent, tumor burden within the liver, and the operator's level of experience with one technique over another.
THE IPSILATERAL APPROACH
With the ipsilateral approach, access to the portal vein is obtained through the portal venous branches within the tumor-bearing liver (Fig. 1). The main advantage of the ipsilateral approach is that it avoids puncturing through healthy FLR parenchyma. It also allows for straightforward catheterization of segment IV branches during a planned extended right hepatectomy (i.e., resection of the entire right lobe and segment IV). When planning a percutaneous puncture, the anterior segment of the right portal vein is preferred because its use is associated with a lower rate of complications.5 However, catheterizing the right portal branches can be difficult owing to the sharp angulation of the veins, which often necessitates using reverse-curve catheters or balloon occlusion catheters with multiple lumina.23 Choosing a particular catheter for PVE also depends on the embolic agent used, operator preference, and catheter availability (e.g., three- and four-lumen balloon catheters are not readily available worldwide). Depending on the embolic agent used, a final portogram can be obtained to evaluate the extent and completeness of embolization, but care must be taken to avoid dislodging the embolic material. Operators also need to be aware that the ipsilateral approach carries the risk of puncture through tumor tissue when multiple or large tumors are present, which theoretically could lead to tumor seeding.
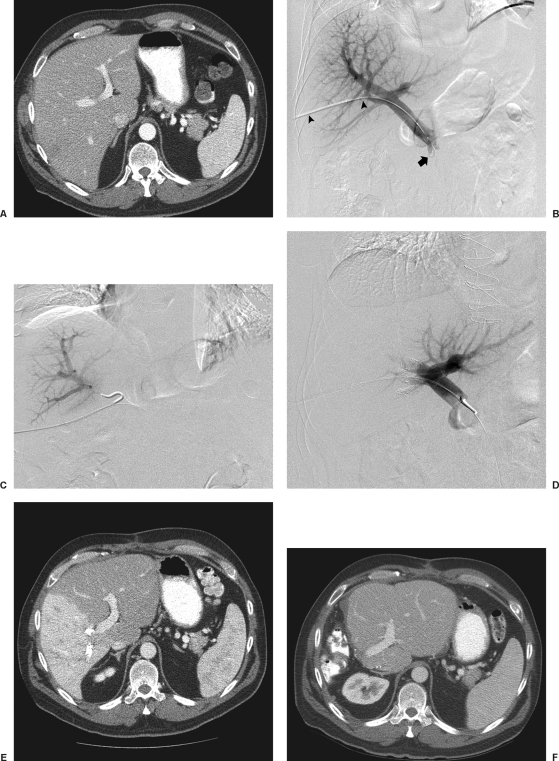
Figure 1.
Transhepatic ipsilateral right PVE using tris-acryl particles and coils performed in a 49-year-old with colon cancer metastatic to the liver. (A) Computed tomography (CT) scan obtained prior to portal vein embolization (PVE) shows marginal future liver remnant (FLR) (FLR-to-TELV (total estimated liver volume) ratio = 25%). (B) Anteroposterior flush portogram shows a 6F vascular sheath in a right portal vein branch (arrowheads) and a 5F flush catheter within the main portal vein (arrow). (C) Selective right portogram with use of reverse-curve catheter during right PVE. (D) Final portogram shows occlusion of the portal vein branches to segments V through VIII with continued patency of the veins supplying the left lateral lobe. (E) CT scan obtained 1 month after right PVE shows substantial FLR hypertrophy (FLR-to-TELV ratio = 50%). The degree of hypertrophy is 25%. (F) CT scan after right hepatectomy shows hypertrophy of remnant liver.
Nagino et al23 first described the ipsilateral approach in 1996. Under sonographic guidance, the right anterior portal vein was punctured, and a 6F sheath was introduced into the portal vein. To make this procedure feasible, the authors designed two types of 5.5F triple-lumen balloon catheters. The type 1 catheter was designed with one lumen connected to the balloon and two lumina connected to the tip. The type 2 catheter had two separate lumina open proximal to the balloon. The balloons were used to prevent any backflow of embolic material. Both catheters had two separate lumina so fibrin glue and iodized oil could be injected simultaneously. To facilitate hepatic resection, the authors advocated that a proximal right portal vein segment of ~1 cm remain patent. Depending on the portal vein anatomy and the need to spare the proximal right portal vein, type 1 or type 2 catheters were used for embolization. The type 1 catheter was used for embolization of branches distal to the catheter tip, whereas the type 2 catheter was used for embolization of branches proximal to the catheter tip, as mandated by each patient's portal vein anatomy.
In a recent study, Gibo et al24 reported on a modified technique utilizing a four-lumen balloon catheter in eight patients. The authors recommended using this modified catheter because of its larger occlusion balloon and lumina, which allowed for safer and easier embolization using fibrin glue.24 Unfortunately, neither the original nor the modified catheters are available in the United States. Thus the original ipsilateral technique has been modified using standard angiographic catheters. At The University of Texas M. D. Anderson Cancer Center, we use standard angiographic catheters. To optimize FLR growth, the operator should aggressively embolize the entire liver parenchyma to be resected. Therefore, embolization of the right portal vein extended to segment IV should be considered for all patients scheduled for an extended right hepatectomy.25
Before performing this procedure, we obtain informed written consent. Patients receive a single dose of intravenous antibiotic (1 g ceftriaxone sodium; Roche, Basel, Switzerland) immediately before the procedure. Procedures are performed with intravenous conscious sedation consisting of midazolam hydrochloride (ESI Lederle, Philadelphia, PA) and fentanyl citrate (Abbott Laboratories, Abbott Park, IL) and a local anesthetic (1% lidocaine hydrochloride).
The portal venous system is accessed percutaneously under sonographic and fluoroscopic guidance. A 22-gauge Chiba needle (Neff Percutaneous Access Set; Cook Medical, Bloomington, IN) is placed into a distal branch of the right portal venous system. The Seldinger technique is then used to place a 5F or 6F vascular sheath (Boston Scientific, Natick, MA) into the right portal vein branch to aid subsequent catheter exchanges. Flush portography is performed with a 5F angiographic flush catheter (Royal Flush Plus, Cook Medical; Omni Flush, Angiodynamics, Inc., Queensbury, NY) placed within the main portal vein or splenic vein. Anteroposterior and craniocaudal projections are obtained as needed to delineate the portal venous anatomy. Selective left and right portal venography is also performed. Interestingly, Nishio et al25 studied the different projections obtained during portography and determined that the most informative projection for PVE is in the right anterior caudal oblique position, with the image intensifier tilted 30 degrees to the patient's right and 20 degrees caudally. More recently, C-arm CT has been used to facilitate understanding of complex portal venous anatomy, especially when PVE is to be performed.26
When embolization of the right portal vein extended to segment IV is required, segment IV embolization should be performed first,5 due to the difficulty of exchanging catheters through an already embolized right portal vein system and the potential for dislodging embolic material from the right liver during the subsequent treatment of segment IV. At M. D. Anderson Cancer Center, segment IV is embolized with a 3F microcatheter (Tracker 325; Boston Scientific) placed coaxially through a 5F selective angiographic catheter (Kumpe Access Catheter; Cook Medical). Previously, polyvinyl alcohol (PVA) particles ranging from 355 μm to 1000 μm in diameter (Contour SE Microspheres; Boston Scientific) were the embolic agents of choice. PVA was administered in a stepwise fashion: The smaller particles (355 to 500 μm) were used first to occlude the distal branches, and the larger particles (500 to 1000 μm) were subsequently used to occlude more proximal branches. The larger particles were not used until the forward portal blood flow was substantially reduced. Additional embolization with the larger particles was performed until near-complete stasis was achieved. Later, with the advent of spherical embolic agents, tris-acryl microspheres (EmboGold Microspheres; Biosphere Medical, Rockland, MA) became our embolic agent of choice. EmboGold Microspheres ranging from 100 μm to 700 μm in diameter are administered in a stepwise fashion, similar to the method used for the PVA particles. After particulate embolization is complete, platinum microcoils (Boston Scientific) are placed within the proximal segment IV branches to further reduce the portal inflow that could lead to recanalization (Fig. 2).
Figure 2.
Transhepatic ipsilateral right portal vein embolization (PVE) extended to segment IV using tris-acryl particles and coils in a 59-year-old woman with a history of gallbladder carcinoma. (A) Computed tomography (CT) scan obtained prior to PVE shows marginal future liver remnant (FLR) (FLR-to-TELV (total estimated liver volume) ratio = 17%). (B) Anteroposterior flush portogram shows a 6F vascular sheath in a right portal vein branch (arrowheads) and a 5F flush catheter within the main portal vein (arrow). (C) Selective left portogram prior to segment IV embolization shows the veins that supply segments II, III, and IV. (D) Selective portography via a 3F microcatheter (arrowheads) during embolization of segment IVa. (E) Selective left portogram via a 5F catheter (arrow) after segment IV embolization (arrowheads show coils within proximal branches of segment IV). (F) Selective venogram via a 5F reverse-curve catheter with tip in the right portal vein (arrow). Note multiple previously placed coils within segment IV branches (arrowheads). (G) Final portal venogram shows occlusion of the portal vein branches to segments IV through VIII with continued patency of the veins supplying the left lateral lobe. (H) CT scan obtained 1 month after right PVE shows substantial atrophy of right liver and FLR hypertrophy (FLR-to-TELV ratio = 26%). The degree of hypertrophy is 9%. The patient underwent successful extended hepatectomy.
For right PVE, the working catheter system is exchanged for a 5F reverse-curve catheter (Simmons 2, Cook Medical; SOS Omni 2, Angiodynamics) to enable delivery of the particulate embolic agent. The 5F reverse-curve catheters were chosen for their ease of manipulation into the right portal branches, given the severe angulation of the right portal tree with the ipsilateral approach. As with segment IV embolization, smaller particles are used first to occlude the distal, smaller portal branches, and the larger particles are used later to occlude the more proximal, larger branches. After near-complete stasis is achieved, 0.035-inch or 0.038-inch detaching embolization coils (Gianturco; Cook Medical, Bloomington, IN) are placed within the secondary portal branches to further reduce the portal inflow that could lead to recanalization. A final portogram is obtained with the flush catheter positioned within the main portal vein to assess completeness of the embolization. At the completion of the procedure (i.e., during withdrawal of the catheter), the access tract is embolized with coils and/or Gelfoam to minimize the risk of bleeding at the liver puncture site.
THE CONTRALATERAL APPROACH
Kinoshita et al first described the contralateral approach to PVE in 1986,11 and the modified versions of this technique were subsequently developed in France and are currently used worldwide.12,13 The contralateral approach requires ultrasonography-guided percutaneous puncture (using an 18- or 22-gauge needle with an echogenic tip) of a peripheral portal branch, preferably segment III accessed via the subxiphoid route. Because access is gained through the FLR, great care is taken to limit the number of punctures, and as with the ipsilateral approach, the most peripheral branch possible is targeted to avoid damage to the central branches. Depending on the embolic agent used, an introducer sheath may or may not be required. A standard 5F polyethylene catheter is then inserted over the guidewire and placed with its distal tip within the main portal trunk to allow for portography, which allows for easy access to the right portal venous branches. Depending on where the procedure is performed, embolization is performed using either standard angiographic or balloon occlusion catheters. A short catheter between 25 and 30 cm in length is used because it is easier to handle and provides a smaller so-called dead space, which is especially important when using cyanoacrylate for embolization (Fig. 3).
Figure 3.
Technique of transhepatic contralateral right portal vein embolization using n-butyl cyanoacrylate mixed with ethiodized oil. (A) Ultrasound view of needle puncture of a segment III portal branch (arrowheads). (B) Anteroposterior flush portogram shows a 5F flush catheter within the main portal vein (arrow). Note portal vein access through the left portal vein (arrowheads). (C) Single image obtained during fluoroscopy shows cast of embolic material within right portal vein branches (arrowheads). (D) Final portogram shows occlusion of the portal vein branches to segments V through VIII with continued patency of the veins supplying the left lateral lobe. Cast of embolic material visualized in the treated right portal branches (arrows).
In terms of accessing the portal vein, the contralateral approach can be challenging when segment IV embolization is also needed. To ensure an adequate distance between the portal entry point and the segment IV branches, the operator must perform the puncture in a branch of the left lateral lobe, usually from the Rex recess. Usually, segment IV embolization is performed after embolization of the right portal branches has rendered the segment IV branches more easily visible and dilated. However, a puncture in the Rex recess will render catheterization extremely difficult owing to the sharp angle of the vein and the risk of losing access to the portal vein. Segment III is often easier to access than segment II. Ultrasonography guidance in the axial plane makes it easy to differentiate segment II from segment III and the Rex recess. Because segment IV branches arise in front of segment III branches in the Rex recess, catheterization of segment IV branches may be easier after puncture of segment III branches. After achieving complete occlusion of the targeted portal branches, the 5F catheter is removed. Because the catheter entry site traverses the FLR, embolic material cannot be used to seal the puncture tract.
The major advantage of the contralateral approach over the ipsilateral approach is that catheterization of the right portal vein branches is easier and does not have to contend with awkward angles, making the contralateral procedure technically easier and faster. In addition, the catheters used tend to be smaller, and embolization is performed with the catheter pointed toward the direction of flow. Some authors report that the contralateral approach allows better visualization of the embolized portal branches during the final portogram27; however, different operators have found that in using the modified ipsilateral approach, the image quality of the final postembolization portography is equivalent. In addition, with the use of reverse-curve catheters, ipsilateral PVE in the right hepatic lobe can also be performed safely in the direction of portal venous flow.28
The main drawback of the contralateral approach is that it requires instrumentation of the left hepatic parenchyma during catheterization of the portal venous branches supplying the FLR, potentially causing injury to the FLR parenchyma and/or the left portal vein. If there are complications after PVE, these will most likely involve the FLR and make the planned surgical resection more difficult or, at times, impossible.
Potential Complications of the Ipsilateral and Contralateral Approaches
Kodama et al5 compared the complication rate between the ipsilateral and contralateral approaches in 47 patients who underwent PVE. They found that in 11 patients who underwent contralateral PVE, 2 (18.1%) experienced complications, and in 36 patients who underwent ipsilateral PVE, 5 (13.9%) experienced complications. This difference was not statistically significant. The rate of technical complications associated with percutaneous PVE using either approach was 14.9%. Most complications reported were similar to those associated with any other percutaneous transhepatic procedure and included subcapsular hematoma, hemobilia, pneumothorax, pseudoaneurysm, arteriovenous fistula, arterioportal shunt, and sepsis. Complications more specific to percutaneous PVE included portal vein thrombosis and portal hypertension resulting in esophageal variceal hemorrhage (Fig. 4).5 However, the authors emphasized that given the potential for injury to the FLR when using the contralateral approach, the ipsilateral approach should be tried first.
Figure 4.
Left portal vein thrombosis in a 67-year-old woman with history of islet call carcinoma metastatic to the liver. (A) Computed tomography (CT) scan obtained prior to portal vein embolization (PVE) shows marginal future liver remnant (FLR) and right hepatic metastasis. (B) Anteroposterior flush portogram shows a 6F vascular sheath in a right portal vein branch and a 5F flush catheter within the main portal vein. (C) Selective left portogram shows the veins that supply segments II, III, and IV. (D) Selective left portogram after segment IV embolization (arrowheads show coils within proximal branches of segment IV). (E) Selective left portogram after segment IV embolization shows filling defects within the left portal vein branches (arrowheads). (F) Late-phase selective left portogram shows patchy enhancement throughout the left hepatic parenchyma with large defect in segment III (arrowhead) consistent with thrombosis. (G) Single image obtained during fluoroscopy shows infusion catheter placed within the left portal vein (arrowheads). (H) Flush main portogram after 30-hour infusion of recombinant tissue plasminogen activator (Activase; Genentech, South San Francisco, CA) (infusion rate = 0.5 mg/hour in the left portal vein) shows patency of the vein that supply segment II and III. (I) CT scan obtained 1 month after right PVE shows substantial FLR hypertrophy. The patient underwent successful extended right hepatectomy 1 month after PVE.
Di Stefano et al27 conducted a study of 188 patients who underwent PVE using the contralateral approach. They reported that only one patient experienced a major complication (complete portal vein thrombosis) directly related to the contralateral approach that precluded the planned surgical resection. Two other patients experienced inadvertent migration of embolic material into the FLR requiring intervention; one needed a portoportal graft during hepatic resection because of portal vein thrombosis. On CT imaging, another 10 patients were found to have embolic material in nontargeted portal venous branches.
Ribero et al1 conducted a study of 112 patients who underwent PVE using the ipsilateral approach. In this study, only one patient was found to have nontargeted embolization of the FLR. However, the overall complication rate was 8.9%, which was not substantially different than the rate reported by Di Stefano et al.27 In particular, if one takes into account the fact that Di Stefano et al considered clinically occult incidental CT findings in their complication rate, the studies reported remarkably similar numbers.
EMBOLIC AGENTS USED FOR PVE
Many embolic materials have been used for PVE, with no remarkable differences reported in the degree or rate of hypertrophy. The agents include, but are not limited to, fibrin glue, n-butyl cyanoacrylate (NBCA) mixed with ethiodized oil, gelatin sponge, thrombin, metallic coils, microparticles (e.g., PVA particles or tris-acryl gelatin microspheres), and absolute alcohol. Choosing a particular embolic agent is at the discretion of the operator, and the decision is based on the extent of the embolization and surgery, the operator's preference for a particular catheter and approach, and the operator's experience with a specific agent.
In the early experiences with PVE, gelatin sponge was widely used as an embolic agent. However, frequent portal recanalization was observed 2 weeks after the procedure,7,11,13 and when compared with other embolic agents, gelatin sponge seemed less efficient at 4 weeks in terms of hypertrophy. Some authors prefer NBCA mixed with ethiodized oil because the mixture leads to fast, reliable hypertrophy and minimizes the delay between PVE and definitive resection. NBCA ensures portal vein occlusion that persists > 4 weeks. Because polymerization time can be modulated by varying the lipiodol volume added to the NBCA, distal and proximal branches can be aggressively embolized. Typically, NBCA is mixed with lipiodol at a ratio of 1:1 to 1:2.5. Because it is a liquid, NBCA can be quickly delivered throughout the entire right portal system, which greatly decreases procedure time. De Baere et al13 reported that NBCA embolization led to a 90% increase in liver volume after 30 days. However, NBCA embolization has a few drawbacks. For instance, the NBCA injections have to be precise because of the risk of nontargeted embolization associated with NBCA, which requires that the operator have a high level of experience. In addition, NBCA induces an inflammatory process that may make hepatectomy more difficult.13 NBCA is also difficult to use in patients with reduced hepatopetal flow, as is commonly seen in patients with chronic hepatic disease. These altered flow dynamics are usually associated with an increased risk of procedural complications.27
Absolute ethanol is another effective embolic agent. Osagawara et al29 demonstrated near doubling of the left lobe volume within 4 weeks for patients with chronic hepatic disease and hepatocellular carcinoma who underwent PVE with absolute ethanol. Unfortunately, marked changes in hepatic function tests and subsequent poor patient tolerance are associated with absolute ethanol.30 Fibrin glue combined with ethiodized oil is a commonly used mixture for PVE. This mixture usually induces < 75% portal occlusion at 2 weeks and < 25% portal occlusion at 4 weeks.31
Madoff et al28,32 demonstrated that using spherical particles and coils is safe and effective for PVE. As described earlier, particles ranging in size from 100 to 700 μm are usually delivered in a stepwise fashion. The smaller particles (100 to 300 μm) are infused first until substantial reduction in forward flow is observed. This type of distal embolization is thought to limit development of collateral circulation that may potentially reduce hypertrophy. Metallic coils are then used proximally to block venous inflow and further reduce the possibility of recanalization.
OUTCOMES AFTER PVE
Since the initial description of preoperative PVE in 1986, many authors have studied the safety and effectiveness of this technique. Two studies in particular focused on PVE-related complications. In 2004, Di Stefano et al27 reported on the adverse events of PVE in 188 patients. In their study, adverse events occurred in 12.8% of the patients. Half of the adverse events were detected solely by imaging studies, with no clinical repercussions seen. In their study of 46 patients, Kodama et al5 reported a complication rate of 14.9% after PVE. The most common complications were subcapsular hematoma, pneumothorax, hemobilia, transient hepatic failure, nontargeted embolization, and portal vein thrombosis. In the series by Di Stefano's group, one patient did not undergo hepatic resection due to a complication of PVE. In another larger study of 240 patients (the largest PVE study to date), Nagino et al3 reported that no complications occurred that required blood transfusions or radiologic or surgical interventions. In that study, one patient developed extensive portal and mesenteric thrombosis, but after thrombolytic therapy, the patient was able to undergo exploratory laparotomy. A second patient developed hypersplenism with splenomegaly.
Because smaller FLRs are associated with an increase in postoperative hepatic dysfunction and longer hospital stays, different studies have assessed the degree of hepatic hypertrophy after PVE. Ribero et al1 reported a study of 112 patients who underwent PVE prior to major hepatectomy. In 85 patients without cirrhosis who underwent embolization of the right portal vein extended to segment IV, the absolute FLR volume increased from an average of 290 cm3 before PVE to 440 cm3 after PVE. The difference between the sFLR before and after PVE was defined as the degree of hypertrophy (DH). The sFLR increased from 16.6 to 25.8%, with a median DH of 8.8%. In 21 patients without cirrhosis who underwent right PVE, the sFLR increased from 28.1 to 43.7%, with a median DH of 10.9%. The higher DH in patients who underwent right PVE was due to substantial growth of segment IV. Of note, among patients without cirrhosis, the response to PVE was similar in patients who received preoperative chemotherapy and those who did not. In 31 patients with fibrosis or liver injury, the absolute FLR and the sFLR substantially increased after PVE, from 435 to 707 cm3, on average. The median DH was 9.6%. There was no difference in DH between patients with and without underlying hepatic disease. The complication rates were similar for patients undergoing right PVE alone compared with embolization of the right portal vein extended to segment IV.
In the Ribero et al study,1 the volumetric data obtained from the patients showed a substantial increase in the FLR volume in the first 3 weeks after PVE. After the initial increase, the DH reached a plateau phase of minimal regeneration. Similarly, in the Nagino et al study,3 the calculated volume of the nonembolized hepatic lobe increased from an average of 361 cm3 before PVE to 460 cm3 after PVE. The volumetric ratio calculated by the authors, which was equivalent to the sFLR, increased from 33 to 43% on average. These numbers are concordant with the data from several recent studies.
In general, 2 to 20% of patients with hepatic cirrhosis or fibrosis do not experience hepatic hypertrophy despite successful PVE. The lack of an increase of the FLR after technically adequate PVE suggests the hepatic parenchyma is unable to regenerate and these patients may not be able to tolerate major hepatic resection.
The question remains of how PVE impacts patient outcomes. To date, no randomized clinical trials have studied the efficacy of PVE, and such trials are unlikely in the future given the ethical dilemma of denying preoperative PVE to patients who are not surgical candidates because of inadequate FLR size or function. However, a recent meta-analysis conducted by Abulkhir et al studied the outcome of 1088 patients after PVE using both percutaneous and transileocolic approaches. The procedure was successful in > 95% of the patients. The increase in FLR volume was significantly greater in patients who underwent PVE using a percutaneous approach (11.9%), compared with those patients who underwent PVE using the transileocolic approach (9.7%). Eighty-five percent of the patients underwent laparotomy for attempted hepatic resection. Major complications were observed in only 2.2% of the patients and included liver abscess, portal vein thrombosis, subcapsular hematoma, portal hypertension, and septic necrosis from hepatic artery injury.33 Farges et al34 published a prospective study comparing the outcomes of 55 patients who underwent right hepatectomy with (n = 28) or without (n = 27) preoperative PVE. Patients who underwent PVE before hepatectomy had a mean FLR of 35% compared with a mean FLR of 39% for those patients who underwent immediate hepatectomy. There was no substantial difference in the postoperative course for the subset of patients without underlying hepatic disease. In contrast, patients with underlying hepatic disease benefited from PVE, with a decreased number of postoperative complications and shorter hospital stays.. The study was limited by the lack of randomization and the relatively large FLR in several of the patients without underlying hepatic disease who underwent PVE. The importance of FLR size was again highlighted by Ribero et al, who showed that both sFLR after PVE (≤ 20%) and DH (≤ 5%) are correlated with postoperative hepatic dysfunction.1 Combining the sFLR and DH values predicted hepatic dysfunction with high sensitivity and was associated with clinical outcome. PVE will potentially allow more patients with previously unresectable disease to become surgical candidates and decrease postoperative morbidity.
CONCLUSION
Preoperative PVE is a safe and effective method of inducing hypertrophy in the FLR before major hepatectomy. Currently, the percutaneous transhepatic ipsilateral and contralateral PVE approaches are the safest and most commonly used by interventional radiologists worldwide. The complication rates appear to be similar for both approaches. Because PVE is a complex procedure, strict patient selection is critical. Patients with underlying hepatic disease, such as fibrosis or cirrhosis, benefit the most from PVE. In most patients without underlying hepatic disease, PVE should only be performed when the FLR is < 20%. Recent retrospective studies and meta-analysis strongly suggest improved postsurgical outcomes after PVE, but randomized clinical trials are needed to prove its effectiveness.
REFERENCES
- Ribero D, Abdalla E K, Madoff D C, Donadon M, Loyer E M, Vauthey J N. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- Ferrero A, Vigano L, Polastri R, et al. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007;31:1643–1651. doi: 10.1007/s00268-007-9123-2. [DOI] [PubMed] [Google Scholar]
- Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364–372. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madoff D C, Abdalla E K, Vauthey J N. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol. 2005;16:779–790. doi: 10.1097/01.RVI.0000159543.28222.73. [DOI] [PubMed] [Google Scholar]
- Kodama Y, Shimizu T, Endo H, Miyamoto N, Miyasaka K. Complications of percutaneous transhepatic portal vein embolization. J Vasc Interv Radiol. 2002;13:1233–1237. doi: 10.1016/s1051-0443(07)61970-8. [DOI] [PubMed] [Google Scholar]
- Perarnau J M, Daradkeh S, Johann M, Deneuville M, Weinling P, Coniel C. Transjugular preoperative portal embolization (TJPE): a pilot study. Hepatogastroenterology. 2003;50:610–613. [PubMed] [Google Scholar]
- Makuuchi M, Thai B L, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- Yokoyama Y, Nagino M, Nimura Y. Mechanisms of hepatic regeneration following portal vein embolization and partial hepatectomy: a review. World J Surg. 2007;31:367–374. doi: 10.1007/s00268-006-0526-2. [DOI] [PubMed] [Google Scholar]
- Bucher N L, Swaffield M N. The rate of incorporation of labeled thymidine into the deoxyribonucleic acid of regenerating rat liver in relation to the amount of liver excised. Cancer Res. 1964;24:1611–1625. [PubMed] [Google Scholar]
- Lee K C, Kinoshita H, Hirohashi K, Kubo S, Iwasa R. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg. 1993;17:109–115. doi: 10.1007/BF01655721. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803–808. doi: 10.1007/BF01655244. [DOI] [PubMed] [Google Scholar]
- de Baere T, Roche A, Vavasseur D, et al. Portal vein embolization: utility for inducing left hepatic lobe hypertrophy before surgery. Radiology. 1993;188:73–77. doi: 10.1148/radiology.188.1.8511321. [DOI] [PubMed] [Google Scholar]
- de Baere T, Roche A, Elias D, Lasser P, Lagrange C, Bousson V. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology. 1996;24:1386–1391. doi: 10.1053/jhep.1996.v24.pm0008938166. [DOI] [PubMed] [Google Scholar]
- Abdalla E K, Hicks M E, Vauthey J N. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165–175. doi: 10.1046/j.1365-2168.2001.01658.x. [DOI] [PubMed] [Google Scholar]
- Abdalla E K, Barnett C C, Doherty D, Curley S A, Vauthey J N. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–680. discussion 680–671. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- Kawarada Y, Sanda M, Kawamura K, Suzaki M, Nakase I, Mizumoto R. Simultaneous extensive resection of the liver and the pancreas in dogs. Gastroenterol Jpn. 1991;26:747–756. doi: 10.1007/BF02782863. [DOI] [PubMed] [Google Scholar]
- Elias D, Lasser P, Spielmann M, et al. Surgical and chemotherapeutic treatment of hepatic metastases from carcinoma of the breast. Surg Gynecol Obstet. 1991;172:461–464. [PubMed] [Google Scholar]
- Vauthey J N, Abdalla E K, Doherty D A, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- Denys A L, De Baere T, Doenz F. Portal vein embolization: a plea for strict patient selection. AJR Am J Roentgenol. 2006;187:W125. doi: 10.2214/AJR.06.5049. author reply 126. [DOI] [PubMed] [Google Scholar]
- Abdalla E K, Denys A, Chevalier P, Nemr R A, Vauthey J N. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404–410. doi: 10.1016/j.surg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Nagino M, Nimura Y, Kamiya J, et al. Right or left trisegment portal vein embolization before hepatic trisegmentectomy for hilar bile duct carcinoma. Surgery. 1995;117:677–681. doi: 10.1016/s0039-6060(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Vauthey J N, Chaoui A, Do K A, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- Nagino M, Nimura Y, Kamiya J, Kondo S, Kanai M. Selective percutaneous transhepatic embolization of the portal vein in preparation for extensive liver resection: the ipsilateral approach. Radiology. 1996;200:559–563. doi: 10.1148/radiology.200.2.8685357. [DOI] [PubMed] [Google Scholar]
- Gibo M, Unten S, Yogi A, et al. Percutaneous ipsilateral portal vein embolization using a modified four-lumen balloon catheter with fibrin glue: initial clinical experience. Radiat Med. 2007;25:164–172. doi: 10.1007/s11604-007-0120-z. [DOI] [PubMed] [Google Scholar]
- Nishio H, Nagino M, Kamiya J, et al. Most informative projection for portography: quantitative analysis of 47 percutaneous transhepatic portograms. World J Surg. 2003;27:433–436. doi: 10.1007/s00268-002-6655-3. [DOI] [PubMed] [Google Scholar]
- Madoff D C, Abdalla E K, Wallace M J, Ng C S, Ribero D, Vauthey J N. Portal vein embolization: a preoperative approach to improve the safety of major hepatic resection. Curr Med Imaging Rev. 2006;2:385–404. [Google Scholar]
- Di Stefano D R, de Baere T, Denys A, et al. Preoperative percutaneous portal vein embolization: evaluation of adverse events in 188 patients. Radiology. 2005;234:625–630. doi: 10.1148/radiol.2342031996. [DOI] [PubMed] [Google Scholar]
- Madoff D C, Abdalla E K, Gupta S, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215–225. doi: 10.1097/01.RVI.0000147067.79223.85. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Uchino J, Une Y, Fujioka Y. Selective portal vein embolization with absolute ethanol induces hepatic hypertrophy and makes more extensive hepatectomy possible. Hepatology. 1996;23:338–345. doi: 10.1053/jhep.1996.v23.pm0008591861. [DOI] [PubMed] [Google Scholar]
- Yamakado K, Takeda K, Nishide Y, et al. Portal vein embolization with steel coils and absolute ethanol: a comparative experimental study with canine liver. Hepatology. 1995;22:1812–1818. [PubMed] [Google Scholar]
- Matsuoka T. Experimental studies of intrahepatic portal vein embolization and embolic materials. Nippon Igaku Hoshasen Gakkai Zasshi. 1989;49:593–606. [in Japanese] [PubMed] [Google Scholar]
- Madoff D C, Hicks M E, Abdalla E K, Morris J S, Vauthey J N. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness—study in 26 patients. Radiology. 2003;227:251–260. doi: 10.1148/radiol.2271012010. [DOI] [PubMed] [Google Scholar]
- Abulkhir A, Limongelli P, Healey A J, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]