ABSTRACT
Comprehension of the structural and functional characteristics of the hepatic microcirculation can help improve the design, planning, and practice of imaging-guided treatment for hepatic tumors and for portal vein embolization (PVE). The hepatic microcirculation derives dual blood supply from the portal vein and the hepatic artery. The terminal portal venules directly connect to the hepatic sinusoids, but the terminal hepatic arterioles connect to arterioportal communications before entering the sinusoids: the peribiliary plexus, the terminal arteriosinus twigs, the vasa vasorum on the portal vein, and the direct arterioportal anastomosis. These communications play important roles in the balance of blood perfusion to the liver parenchyma and in controlling the blood supply to hepatic tumors and the anticipated remnant liver (in cases of PVE). At the microcirculatory level, various embolic agents present different distribution patterns. To further our understanding, iodized oil has been found to pass into the portal vein after hepatic arterial administration through the peribiliary plexus and subsequently traverses the sinusoids to enter the lungs and then the systemic circulation. Ultimately, a thorough knowledge of the host environment at the microcirculatory level is essential in developing strategies for both tumor treatment and for inducing liver regeneration.
Keywords: Hepatic microcirculation, in vivo microscopy, iodized oil, Kupffer cell, transhepatic arterial chemoembolization
The hepatic microcirculation is composed of the terminal blood vessels, which are < 0.1 mm (100 μm) in diameter and cannot be clearly observed using current clinical imaging techniques. In vivo microscopy, however, allows the visualization and quantification of real-time activities in the microcirculation of live animals. At low magnifications, in vivo microscopy shows the direction, rate, and magnitude of the blood flow in capillaries, and at high magnifications, it clearly depicts the microvasculature at the cellular level (Fig. 1). This technique has been widely used in basic studies of the activities and mechanisms involved in the development of diseases such as cancer in various organs and tissues. In our laboratory, in vivo microscopy has been used, supplemented by other techniques, in studies of blood supply to the normal liver and hepatic tumors and to monitor the distribution, effects, and clearance of intravascular embolic or targeted agents, host reactions, and the fate of metastatic tumor cells in the hepatic microvasculature. This article reviews the normal anatomy within the hepatic microcirculation, the changes that occur in the presence of tumors, and the effects of embolic agents as they relates to tumor therapy and liver regeneration resulting from portal vein embolization (PVE).
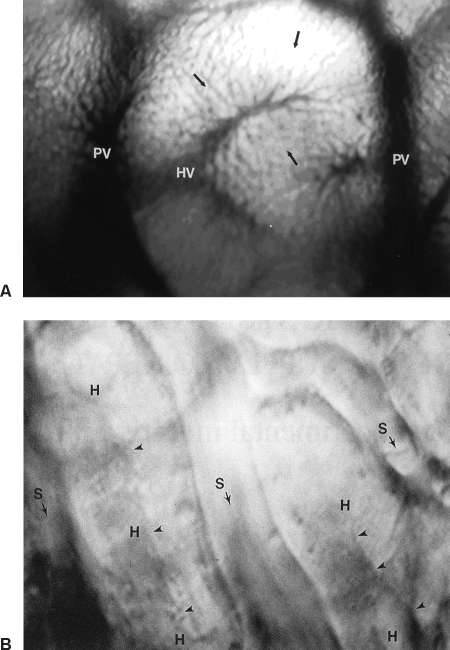
Figure 1.
(A) In vivo photomicrograph of a hepatic lobule (×125) with blood flow from the portal venule (PV) to a hepatic venule (HV) in the center through the sinusoids. (B) High-resolution in vivo photomicrograph (×1500) depicts detailed structure of the hepatic microcirculation. S, sinusoids; H, hepatocytes; arrowheads, bile canaliculi. Arrows point the direction of blood flow.
HEPATIC MICROCIRCULATION IN THE NORMAL LIVER
The liver is the largest visceral organ in the body, receiving approximately a quarter of the cardiac output. The normal liver has a dual blood supply, deriving 70 to 80% of its blood, rich in nutrients, from the portal vein and the other 20 to 30%, rich in oxygen, from the hepatic artery. These two vessels course and branch together in the portal tract, with the hepatic artery spiraling around the portal vein. After repeated branching, the terminal branches of the vessels supply blood together to the hepatic sinusoids. These sinusoids, which compose the capillary bed in the hepatic parenchyma, are characterized by the presence of fenestrae, sieve-like pores on the endothelium, and by the lack of a basement membrane, facilitating transvascular exchange between the blood and liver cells.
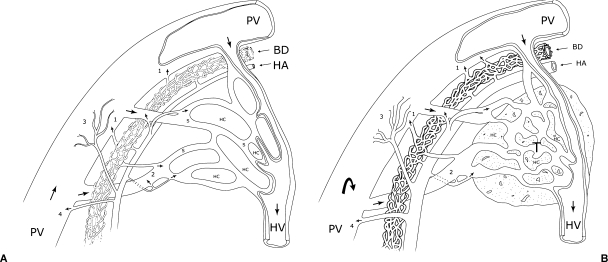
Structurally, the terminal portal venules continue with the sinusoids. The lumen of the portal venule is covered with endothelial cells, and there is no pore or gap within or between the endothelial cells. The blood from the portal vein pours directly into the hepatic sinusoids. The termination of the hepatic arterioles, however, is complex. Studies using scanning electron microscopy and in vivo microscopy have revealed four major types of communication between the terminal hepatic arterioles and the terminal portal venules:1 (1) the peribiliary plexus, (2) the terminal arteriosus twigs, (3) the vasa vasorum of the portal vein, and (4) the direct arterioportal anastomosis (Fig. 2). The peribiliary plexus is a dense vascular network around the bile ducts whose inner layer derives from the hepatic arterioles and whose outer venous layer drains into either the portal venules via the “internal roots” or into the sinusoids directly via the “radicular roots.” Hepatic arterial blood supplies the peribiliary plexus, nourishing the bile duct and collecting the products of the bile duct, playing the same function as the portal splanchnic circulation of the biliary system. The peribiliary plexus is the most frequently observed type of termination of the hepatic arterioles in our investigations. The next type, the terminal arteriosinus twigs, are small branches arising from the hepatic arterioles to connect to the sinusoids at their origin. Through the arteriosinus twigs, blood from the hepatic artery enters and flows through the sinusoid. However, this arterial flow can reflux into the portal venule when the sphincter at the junction of the portal venule and the sinusoid is closed and the outlet resistance in the sinusoid is increased.2,3 Our experiments have demonstrated the existence of the arteriosus twigs and their role in the perfusion of liver parenchyma, especially when the portal flow is compromised.4 However, it is not clear whether the arteriosinus twigs or the peribiliary plexus is the main type of arterial termination and what proportion of the arterial blood flow enters the sinusoids through them. Whether the arteriosinus twigs are the terminal branches of the arterial system or in fact the outlet vessels of the peribiliary plexus (the radicular roots) has not been verified. The third type of communication between the terminal hepatic arterioles and portal venules, the vasa vasorum on the wall of the portal vein, drains into the portal vein after nurturing the vessel wall. Finally, the direct arterioportal anastomosis, a shortcut connection between the artery and the portal vein, is the least frequently reported of the four types of communication, with great interspecies variation. We did not see this type of communication in our studies in rats and mice.
Figure 2.
Sketches illustrate the four types of terminal arterioportal communication in (A) normal liver and (B) in liver with tumor: (1) peribiliary plexus, (2) terminal arteriosinus twigs, (3) vasa vasorum of the portal vein, and (4) direct arterioportal anastomosis. Arrows indicate the direction of blood flow. BD, bile duct; HA, hepatic arteriole; HC, hepatocytes; HV, hepatic venule; PV, portal venule; S, sinusoids; T, tumor.
The term “hepatic microcirculation” generally refers to the circulatory system beginning with the portal venule (50 to 100 μm in diameter), extending to the terminal portal venule (15 to 50 μm), then reaching the sinusoid (5 to 8 μm) network, followed by the postcapillary terminal hepatic venules (~25 μm) and the collecting venule (~40 μm), and ending with the muscular venule (~60 μm).3 The basic structural unit of the hepatic microcirculation is the hepatic lobule, in which a terminal hepatic venule is located at the center and several portal venules at the periphery, with the hepatic sinusoids running from the terminal portal venules to the terminal hepatic venule, forming a hexagonal vascular structure (Fig. 1A).5 The physiology of the liver is more accurately represented by a unit structure known as the hepatic acinus, as proposed by Rappaport.6 A hepatic acinus, which contains a terminal portal venule at the center and terminal hepatic venules at the periphery, is divided into three zones from the portal venule to the hepatic venule. The sinusoids in zone 1 have the narrowest lumen, the largest fenestrae, and the most Kupffer cells, where the active metabolic activities take place. Subsequently, zones 2 and 3 have larger lumen, smaller fenestrae, less Kupffer cells, and less active metabolic activities. Large endothelial cells at the junction of the sinusoids with the portal venules and at the junction of the sinusoids and the hepatic venules act as sphincters that control sinusoidal flow as they contract and occlude the sinusoidal lumen.2,3
HEPATIC MICROCIRCULATION IN HEPATIC TUMORS
Blood Supply to Hepatic Tumors
The dominant role of the hepatic artery in the vascularization of hepatic tumors has long been known. As early as 1923, Segall,7 in investigating the vasculature in liver tumors, found that only hepatic arteries were found in tumor tissue, and the portal vein took no part in nourishing hepatic tumors. Since then, the results of numerous studies have all supported the finding that hepatic tumors, primary or metastatic, are predominantly supplied by the hepatic artery and not or only minimally by the portal vein. This characteristic of tumor blood supply in the liver forms the basis of specific therapies using hepatic arterial infusion to deliver drugs selectively to the tumor, embolization to obstruct blood flow to the tumor while preserving adequate liver function, and chemoembolization as a combination of both.
As seen on in vivo microscopy, tumor nodules growing in the liver exhibit an irregular tortuous vascular network bordered by a vascular ring composed of compressed sinusoids. Blood flow in the tumor vasculature is active, with varied speed and direction. The exchange of blood occurs at the tumor border where blood flow enters the tumor from the portal venules and drains out into the hepatic venules through the microvasculature. Occasionally, tumor blood drains into the portal venule at the tumor interface and then into the hepatic venule. Injections of fluorescent tracer into the hepatic artery have demonstrated that the hepatic arterial flow enters hepatic tumors without resistance at the tumor border. When a tracer is injected into the portal vein, however, the portal blood stops at the tumor border, thereby accumulating in the sinusoids surrounding the tumor, with only a small volume entering the tumor. Further studies of the blood circulation in hepatic tumors have demonstrated that the hepatic artery and the portal vein are closely interrelated. The portal vein increases its blood flow into the tumor immediately after embolization of the hepatic artery. Interruption of either the hepatic arterial or the portal venous flow by ligation or embolization does not eliminate the blood circulation through the tumor. In fact, the average reduction in the flow rate in either vessel is statistically nonsignificant.8
Fluorescent tracer injected into the hepatic artery reveals that hepatic arterial blood enters tumors via the portal venules and the sinusoids surrounding the tumor, and no obvious dilation of the terminal arterioles is observed. Histological studies have also shown that the vascular network at the tumor border is formed mainly by dilated portal venules and that tumors derive arterial blood through the peribiliary plexus.9 As arterial flow shunts into the portal venules at the tumor border, the high arterial pressure created within those vessels impedes the portal flow from entering the tumor. As soon as the arterial flow is interrupted, the high pressure is relieved and the portal flow increases to supply the tumor (Fig. 2B).
Cellular Activities in the Hepatic Microcirculation
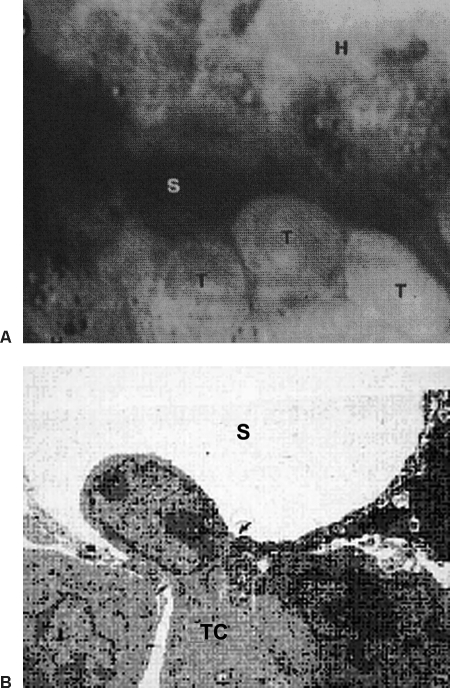
High-resolution in vivo microscopy elucidates cellular activities within tumor vessels. In the peripheral area of tumor growth, the tumor vessels preserve some structural characteristics of the hepatic sinusoids. Tumor cells circulating in these vessels tend to slow down and adhere to the vascular lining; these adherent tumor cells then flatten and begin passing through the endothelial fenestrae to enter the parenchyma and invade hepatocytes (Fig. 3). In the central part of a tumor, the vessels are severely altered, with no identifiable normal structure, and hepatic cells are replaced by tumor cells.10
Figure 3.
(A) In vivo photomicrograph (×1200) shows tumor cells (T) adhering to the sinusoidal wall. (B) Electron micrograph (×6800) shows a tumor cell passing through a fenestra (arrows) to enter the space of Disse. S, sinusoid.
Without labeling, tumor cells are difficult to detect in the circulation and to differentiate from background cells in live animal models. However, when tumor cells are labeled with green fluorescent protein (GFP), we can track the cellular activity, growth, and metastasis of tumors. Because the GFP gene is integrated into the cells' chromosomes, carrying GFP to subsequent generations, endogenously metastatic cells generated by the primary tumor inoculated with GFP-labeled tumor cells acquire equal fluorescence.11
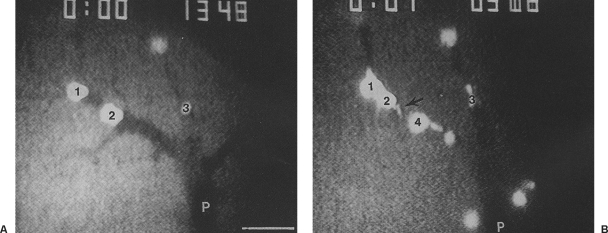
To study the fate of tumor cells in microcirculation, we introduced GFP-labeled tumor cells into the portal vein and the mesenteric artery. In vivo microscopy demonstrated that tumor cells injected into the portal vein were arrested in the terminal portal venules and killed within a few minutes by clasmatosis, a phenomenon in which a portion of the cytoplasm is torn off the cell body (Fig. 4). Tumor cells introduced into the mesenteric artery became severely deformed and were killed quickly in the small mesenteric capillary by the high-flow shearing force. Cells that survived this intravascular killing extravasated the microcirculation and migrated to the stromal tissue, where in vivo microscopy demonstrated that most of the extravasated tumor cells were killed, with only a small number of tumor cells surviving to develop metastatic lesions. Passage of tumor cells through the hepatic sinusoids into the lungs after portal introduction and through the mesenteric capillaries into the liver after mesenteric arterial injection was observed.
Figure 4.
Fluorescent in vivo photomicrographs (×250) of the liver in a rat after injection of tumor cells labeled with green fluorescent protein into the portal vein. (A) Tumor cells (1, 2, 3) were arrested in a portal venule (P) with fairly normal shape. (B) One minute later, the tumor cells became severely deformed, ruptured (arrow), and died.
DISTRIBUTION OF EMBOLIC AGENTS IN THE HEPATIC MICROCIRCULATION
The roles of embolic agents used in chemoembolization are to interrupt the blood supply to hepatic tumors and to carry and deliver anticancer drugs to the tumors. In vivo microscopy demonstrates that particulate embolic agents lodge in arterial branches of the same size as the particles. These particles accumulate, stopping or markedly reducing blood flow distal to the embolization site. Slow flow maintained in the embolized vessels comes from two sources: blood passing through the spaces between the particles, which have a regular round shape, and collateral circulation across the embolized site through the peribiliary plexus. In our studies with animal tumor models, particulate embolic agents as small as 40 μm in diameter failed to enter the tumor vasculature after hepatic arterial injection. No particulate agents appeared in the portal vessels after hepatic arterial introduction.
The iodized oil (Ethiodol; Savage Laboratories, Altana Inc., Melville, NY, or Lipiodol; Andre Guerbet, Aulnay-Sous-Bois, France) is an iodinated ester of the fatty acids of poppy seed oil that has been used in hysterosalpingography and lymphography. Since 1979, when Nakakuma12 reported that iodized oil selectively enters and is retained by liver tumors when it is injected into the hepatic artery, resulting in tumor shrinkage and necrosis, this agent has been used worldwide in chemoembolization of hepatic tumors, in particular for the hypervascular tumor hepatocellular carcinoma. In chemoembolization, iodized oil works not only as an embolic agent but also as a vehicle for chemotherapy drugs and as a contrast agent.
Iodized oil takes a different route in hepatic microcirculation than other embolic agents. When injected into the portal vein, iodized oil instantly flows into the terminal portal venules. When injected into the hepatic artery, iodized oil also appears and accumulates in the terminal portal venules before entering the sinusoids, but it takes a few minutes longer to arrive at the portal venules than when it is injected into the portal vein. The peribiliary plexus is the pathway that iodized oil passes through from the hepatic artery to the portal vein. As the oil arrives and accumulates in the terminal portal venules after either arterial or portal injection, it begins to slowly squeeze into and move through the sinusoids. After it has traversed the sinusoids, the oil drains into the hepatic venules and then flows to the lungs.13,14
Because the portal venules are occluded by the oil, the reopened hepatic artery becomes the only source of hepatic perfusion. At the site where the terminal arterioles connect to the sinusoids and terminal portal venules via the arteriosinus twigs, the resumed arterial flow pushes oil droplets into and through the sinusoids, taking hours to days depending on the volume injected. If secondary arterial embolization is applied using solid embolic agents, the resumed arterial flow stops immediately and the washing effect is interrupted.4,14
Unlike particulate embolic agents, which scarcely enter the tumor vasculature, iodized oil injected into the hepatic artery enters the tumor vessels in a larger quantity and is retained longer in tumor than in normal liver. Introduction into the hepatic artery of an emulsion prepared by mixing iodized oil with a water solution of anticancer drugs demonstrated that the iodized oil carried the drugs into the tumor vasculature.15 Nevertheless, iodized oil often failed to fill some tumors in the liver after arterial delivery, and changing techniques such as dosage, body position, and injection time and manner did not improve the results.
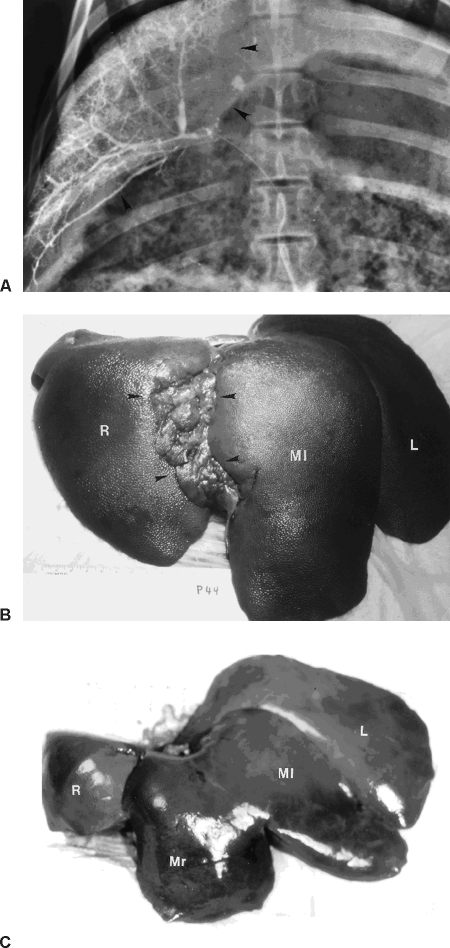
Its specific distribution pattern enables iodized oil to create dual hepatic arterial and portal venous embolization through a single hepatic arterial catheterization. In our experiments in rats and pigs, hepatic lobar and segmental ablations were achieved by selective injection of a mixture of iodized oil and absolute ethanol in proper ratio followed by a second embolization of the artery with solid embolic agents. Adding the absolute ethanol was done to enhance the blockage and reduce the oil's passage through the sinusoids. The second embolization was to prevent the recurrence of arterial circulation. The remnant liver became hypertrophied, and the ratio of liver weight to body weight recovered within 2 to 4 weeks (Fig. 5). Clinical application of this technique improves tumor growth inhibition and tumor resectibility.16 This less invasive procedure may also be used in percutaneous portal embolization17 or to replace surgical intervention to activate the replication of liver cells for retrovirus gene transfection in animal experiments.18
Figure 5.
(A) Radiograph of the liver of a pig treated with an infusion of a mixture of iodized oil and ethanol into the artery of right segment of middle lobe (arrowheads); the iodized oil filled the portal vein in the selected segment. (B) Complete atrophy of the right middle segment (arrowheads) and hypertrophied left middle segment (Ml) at the pig's euthanasia 4 weeks later. (C) Photograph of a normal pig liver shows the normal lobes for comparison. Ml, left middle segment; Mr, right middle segment; R, right lobe; L, left lobe.
Data are currently limited regarding specific alterations in the hepatic microcirculation after portal venule occlusion with other embolic agents. In a study by Shibayama and colleagues,19 performed in rats, 700,000 plastic beads (30 to 35 microns in diameter) were infused into the portal vein. When the occluded segments of the portal venule were short, infarction did not occur because of the rapid development of collateral vascular channels that bypassed the obstructed venules. However, when the occluded segment was long, infarction commonly occurred. In this situation, formation of collateral vessels began as dilation of the sinusoids in the vicinity of the occluded portal venules and increased in diameter and developed into new portal venules within 5 days. Increased portal vascular resistance after embolization was normalized after 5 days in parallel with the development of the new vascular channels. Furthermore, the reduced bile production returned to baseline levels within 3 days after embolization. Necrotic tissue was resorbed completely and replaced by fibroblasts 5 days after embolization. These findings indicate that the development of hepatic necrosis following portal venule occlusion largely depends on the length of the occluded segment. Hepatic microvasculature has a great ability for adaptation and rapid collateralization of vascular channels, which leads to quick recovery from hepatic circulatory disturbances and potentially resultant hepatic damage.
In a 2005 study by Liu and colleagues,20 the immediate alterations of intrahepatic microcirculation during the very early stages of portal venule obstruction were evaluated. After selective embolization of the terminal portal venules with plastic beads with a diameter of 30 to 35 μm, the hepatic microcirculation of 136 embolized areas on 15 mice was observed with epi-illuminated in vivo fluorescent microscopy. These investigators showed a reversal in blood flow within the sinusoids, resulting in three types of collaterals after embolization of the terminal portal venules. These collaterals channels developed from the proximal trunk of the embolized terminal portal venules, their adjacent branches, and from neighboring terminal portal venules, and they could appear concomitantly with a frequency of 59 of 136 (43.4%), 77 of 136 (56.6%), and 131 of 136 (96.3%), respectively. In addition, there was a strong correlation between collateral formation and the state of blood flow within the surrounding area (p < 0.01) but not with the extent of the embolized areas. These results indicated that after embolization of terminal portal venules, reverse blood flow occurred in sinusoids to form collaterals, and these collaterals could maintain the microcirculation of the embolized area.
Larger animal models have also been used to study PVE and its impact on the hepatic microcirculation. In a study by Yamakado and colleagues,21 two types of embolic materials, stainless-steel coils (n = 5) and absolute ethanol (n = 12), were compared in a canine model to assess the effectiveness between the two methods. Embolization effects of each embolic material on the hepatic vessels and the liver parenchyma were studied angiographically and histologically. In the steel coils group, no hepatic necrosis was observed in all dogs. However, in those treated with ethanol, venous occlusion was achieved immediately after embolization with ethanol of 0.4 mL/kg or more and remained occluded up to 4 weeks after embolization. Hepatic necrosis was observed in the segments where portal veins with ethanol and the degree of parenchymal damage was directly proportional to the injected ethanol dose. From these results, the authors concluded that ethanol is likely to be more suitable than coils as an embolic material for PVE, possibly owing to the more distal embolization with coils such that vascular collateralization is less likely. Thus the more distal embolization with the portal system that is performed, the better the liver regeneration, which has been shown clinically as well.22
THE ROLE OF KUPFFER CELLS IN THE HEPATIC MICROCIRCULATION
Kupffer cells are macrophages that reside in the hepatic sinusoids and constitute the largest number of fixed macrophages of the reticuloendothelial system in the body. In normal liver, Kupffer cells comprise 30% of the total hepatic nonparenchymal cell population and 3% of the weight of the liver. Kupffer cells are located in the lumens of the sinusoids, preferentially in zone 1, with long processes anchored to the fenestrae on the endothelium, which facilitates the Kupffer cells' filtration and removal of harmful substances from the microcirculation. As an important component in the host's defense system, Kupffer cells remove infective and foreign substances from the circulation by endocytosis. These cells also release toxic mediators involved in liver injury and cirrhosis.23,24 We have investigated the roles of Kupffer cells during tumor invasion of the liver and their role in clearing embolic agents introduced into the liver.
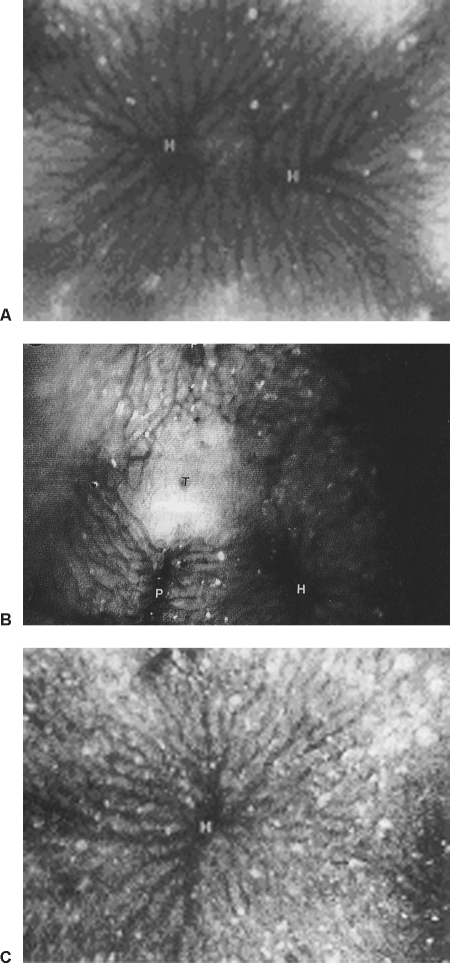
Our evaluation of Kupffer cell activation was performed by counting the number of Kupffer cells marked by fluorescein isothiocyanate–labeled latex beads (Fluoresbrite plain YG 1.0-μm microspheres; Polysciences Inc., Warrington, PA) introduced into the circulation and the number of the beads phagocytosed by individual Kupffer cells. In all tumor models we studied, the population of Kupffer cells inside the tumor and their phagocytotic capacity were significantly reduced compared with those in normal liver. However, the numbers in nontumorous areas in the tumor-bearing livers were significantly increased relative to those in normal liver, with the numbers even more significantly greater in the areas close to the tumors than in the areas remote from the tumors (Fig. 6). Activation of Kupffer cells improves their tumoricidal function, as evidenced by the fact that the Kupffer cells that captured and phagocytosed the circulating tumor cells were mostly observed in blood vessels adjacent to the tumor nodules. We rarely saw Kupffer cells inside tumors phagocytose tumor cells.10 In vivo microscopy demonstrated that debris from tumor cells killed in the hepatic circulation or in the mesenteric artery was mostly captured and cleared by Kupffer cells in the liver.
Figure 6.
In vivo photomicrographs (×150) of (A) a normal rat liver, (B) a tumor-bearing liver, and (C) a nontumorous area in a tumor-bearing liver, showing the lack of Kupffer cells, highlighted by fluorescent latex particles, in the tumor and the significant increase of Kupffer cells in the nontumorous areas of the tumor-bearing liver. H, hepatic venule.
In studies to evaluate the effect of embolization on Kupffer cells, the results suggested that activation of Kupffer cells occurs within hours of introduction of embolic agents into the liver via either the hepatic artery or the portal vein, in both normal and tumor-bearing livers, as evidenced by significant increases in the numbers of Kupffer cells and latex beads phagocytosed by each Kupffer cell. Their activation in tumor-bearing livers was sometimes statistically insignificant, probably due to saturation of activation by the tumor. The embolic agents tested included particulate agents polyvinyl alcohol particles (50 μm), Gelfoam powder, degradable starch microspheres (40 μm), and polylactic acid particles (1 μm and 100 μm). After embolization with iodized oil, Kupffer cells were activated as long as the oil was retained in the liver and returned to their normal state as the oil was cleared from the liver.25
The efficiency of Kupffer cells' clearance of embolic agents was remarkable in that particulate agents circulating in the liver were usually cleared from the circulation in < 10 minutes. Coating of the particulates with amino acid helped the particles avoid capture by the Kupffer cells and prolonged their circulation in the liver.
The presence and activation of Kupffer cells have generally been considered a favorable prognostic sign of the host's defense response to tumors. Whether certain therapeutic and diagnostic agents such as those used in interventional radiology have an immunostimulative effect on Kupffer cells, achieving synergistic tumoricidal results, is worth further study.
CONCLUSION
As discussed here, the various vascular communications play important roles in the balance of blood perfusion to the liver parenchyma and in controlling the blood supply to hepatic tumors and the anticipated remnant liver (in cases of PVE). A thorough comprehension of the structural and functional characteristics of the hepatic microcirculation may help improve the design, planning, and practice of imaging-guided treatment for hepatic tumors and for PVE.
REFERENCES
- Grisham J W, Nopanitaya W. In: Lautt WW, editor. Hepatic Circulation in Health and Disease. New York: Raven Press; 1981. Scanning electron microscopy of casts of hepatic microvessels: review of methods and results. pp. 87–109.
- McCuskey R S. A dynamic and static study of hepatic arterioles and hepatic sinusoids. Am J Anat. 1966;119:455–478. doi: 10.1002/aja.1001190307. [DOI] [PubMed] [Google Scholar]
- Oda M, Yokomori H, Han J Y. Regulatory mechanisms of hepatic microcirculation. Clin Hemorheol Microcirc. 2003;29:167–182. [PubMed] [Google Scholar]
- Kan Z, Wallace S. Sinusoidal embolization: impact of iodized oil on hepatic microcirculation. J Vasc Interv Radiol. 1994;5:881–886. doi: 10.1016/s1051-0443(94)71629-8. [DOI] [PubMed] [Google Scholar]
- Young B, Lowe J S, Stevens A, Heath J W. Wheater's Functional Histology: A Text and Colour Atlas. 4th ed. Oxford, UK: Churchill Livingstone; 2006. pp. 288–301.
- Rappaport A M. In: Lautt WW, editor. Hepatic Circulation in Health and Disease. New York: Raven Press; 1981. The acinus: microvascular unit of the liver. pp. 175–192.
- Segall H N. An experimental anatomical investigation of the blood and bile canals of the liver. Surg Gynecol Obstet. 1923;37:152–164. [Google Scholar]
- Kan Z, Ivancev K, Lunderquist A, et al. In vivo microscopy of hepatic tumors in animal models: a dynamic investigation of blood supply to hepatic metastases. Radiology. 1993;187:621–626. doi: 10.1148/radiology.187.3.8497606. [DOI] [PubMed] [Google Scholar]
- Gonda T, Ishida H, Yoshinaga K, Sugihara K. Microvasculature of small liver metastases in rats. J Surg Res. 2000;94:43–48. doi: 10.1006/jsre.2000.5978. [DOI] [PubMed] [Google Scholar]
- Kan Z, Ivancev K, Lunderquist A, et al. In vivo microscopy of hepatic metastases: dynamic observation of tumor invasion and interaction with Kupffer cells. Hepatology. 1995;21:487–494. [PubMed] [Google Scholar]
- Kan Z, Liu T J. Video microscopy of tumor metastasis: using the green fluorescent protein (GFP) gene as a cancer-cell-labeling system. Clin Exp Metastasis. 1999;17:49–55. doi: 10.1023/a:1026478105365. [DOI] [PubMed] [Google Scholar]
- Nakakuma K, Tashiro K, Hiraoka T, Ogata K, Ootsuka K. Hepatocellular carcinoma and metastatic cancer detected by iodized oil. Radiology. 1985;154:15–17. doi: 10.1148/radiology.154.1.2981111. [DOI] [PubMed] [Google Scholar]
- Kan Z, Ivancev K, Hagerstrand I, Chuang V, Lunderquist A. In vivo microscopy of the liver after injection of Lipiodol into the hepatic artery and portal vein in the rat. Acta Radiol. 1989;30:419–425. [PubMed] [Google Scholar]
- Kan Z, Sato M, Ivancev K, et al. Distribution and effect of poppyseed oil in the liver after hepatic artery embolization: experimental study in several animal species. Radiology. 1993;186:861–866. doi: 10.1148/radiology.186.3.8381552. [DOI] [PubMed] [Google Scholar]
- Kan Z, Wright K C, Wallace S. Ethiodol emulsions in hepatic microcirculation: in vivo microscopic observation in rat liver. Acad Radiol. 1997;4:275–282. doi: 10.1016/s1076-6332(97)80029-3. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Kan Z, Chen C, et al. Efficacy and safety of preoperative lobar or segmental ablation via transarterial administration of Ethiodol and ethanol mixture for treatment of hepatocellular carcinoma: a clinical study. World J Surg. 2000;24:844–850. doi: 10.1007/s002680010135. [DOI] [PubMed] [Google Scholar]
- Madoff D C, Gupta S, Pillsbury E P, et al. Transarterial versus transhepatic portal vein embolization to induce selective hepatic hypertrophy: a comparative study in swine. J Vasc Interv Radiol. 2007;18:79–83. doi: 10.1016/j.jvir.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Majors J E. Retroviral vectors strategies and applications. Semin Virol. 1992;3:285–295. [Google Scholar]
- Shibayama Y, Hashimoto K, Nakata K. Hepatic haemodynamics and microvascular architecture after portal venular embolization in the rat. J Hepatol. 1992;14:94–98. doi: 10.1016/0168-8278(92)90136-d. [DOI] [PubMed] [Google Scholar]
- Liu Y, Matsui O. Collaterals through hepatic sinusoids after embolization of terminal portal venules: an in vivo study on mice. Hepatol Res. 2005;31:36–42. doi: 10.1016/j.hepres.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Yamakado K, Takeda K, Nishide Y, et al. Portal vein embolization with steel coils and absolute ethanol: a comparative experimental study with canine liver. Hepatology. 1995;22:1812–1818. [PubMed] [Google Scholar]
- Madoff D C, Abdalla E K, Gupta S, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215–225. doi: 10.1097/01.RVI.0000147067.79223.85. [DOI] [PubMed] [Google Scholar]
- McCuskey R S, McCuskey P. Fine structure and function of Kupffer cells. J Electron Microsc Tech. 1990;14:237–246. doi: 10.1002/jemt.1060140305. [DOI] [PubMed] [Google Scholar]
- Enomoto N, Ikejima K, Bradford B U, et al. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15(suppl):D20–D25. doi: 10.1046/j.1440-1746.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- Kan Z, McCuskey P A, Wright K C, Wallace S. Role of Kupffer cells in iodized oil embolization. Invest Radiol. 1994;29:990–993. doi: 10.1097/00004424-199411000-00007. [DOI] [PubMed] [Google Scholar]