ABSTRACT
The atrophy-hypertrophy complex (AHC) refers to the controlled restoration of liver parenchyma following hepatocyte loss. Different types of injury (e.g., toxins, ischemia/reperfusion, biliary obstruction, and resection) elicit the same hypertrophic response in the remnant liver. The AHC involves complex anatomical, histological, cellular, and molecular processes. The signals responsible for these processes are both intrinsic and extrinsic to the liver and involve both physical and molecular events. In patients in whom resection of large liver malignancies would result in an inadequate functional liver remnant, preoperative portal vein embolization may increase the remnant liver sufficiently to permit aggressive resections. Through continued basic science research, the cellular mechanisms of the AHC may be maximized to permit curative resections in patients with potentially prohibitive liver function.
Keywords: Portal vein embolization, atrophy-hypertrophy complex, liver regeneration
THE ATROPHY-HYPERTROPHY COMPLEX IN THE LIVER
The atrophy-hypertrophy complex (AHC) is the liver's regenerative response following hepatocyte loss and a dramatic demonstration of homeostasis in adult physiology. Although atrophy can be due to different types of injury (e.g., toxins, ischemia, biliary obstruction, and partial hepatectomy), the regenerative response is constant as long as a minimum amount of functional liver remnant exists.
The AHC may occur by liver disease that induces atrophy by impeding bile flow, portal venous inflow, or hepatic venous outflow to a portion of the liver. This atrophy, similar to parenchymal loss from resection, induces restorative hyperplasia through liver regeneration. The AHC is defined histologically by a decrease in the hepatocyte fraction and an increase in the composition of biliary components.1 The recognition that the AHC could be induced by portal vein embolization (PVE) to grow the potential remnant liver has allowed more aggressive liver resections while minimizing liver insufficiency. Further, the increasing use of PVE has driven research to define the mechanisms of the AHC.
In this article, the cellular and molecular mechanisms of the AHC are discussed. The roles of apoptosis and necrosis in hepatocellular atrophy and liver regeneration in compensatory hypertrophy are described, especially as they relate to PVE. Differences in these mechanisms in the healthy and diseased liver are also examined. Finally, strategies to potentiate these restorative mechanisms and their therapeutic potential are outlined.
THE AHC IN LIVER DISEASE
Biliary Obstruction
The AHC following biliary obstruction occurs when unilateral biliary obstruction induces atrophy of the ipsilateral liver and the contralateral liver undergoes hypertrophy. The AHC in this setting not only possesses the three characteristics of AHC just mentioned, but additional findings include (1) ductal inflammation and injury, (2) periductal venous injury, (3) ductal proliferation, (4) sinusoidal widening, and (5) a late progressive septal fibrosis and nodular changes.2,3 These findings and animal studies of bile duct ligation suggest that bile duct obstruction alone is insufficient to cause the AHC.4,5
Various causes of biliary obstruction are associated with the AHC. Some studies have found the AHC in association with 18% of hilar cholangiocarcinomas,6 15% of postcholecystectomy biliary strictures,7 15% of primary sclerosing cholangitides,8 hepatolithiasis in conditions such as recurrent pyogenic cholangitis,9 benign bile duct tumors (e.g., papillomas, cystadenomas, and granular cell tumors), and infestations of the biliary tree (e.g., Clonorchis sinensis and Ascaris lumbricoides).10 In other space-occupying diseases such as large hepatomas, metastases, and cystic lesions (e.g., simple and hydatid cysts),11,12 combined biliary and portal venous obstruction may induce the AHC.
Portal Vein Obstruction
The AHC following portal vein occlusion occurs when a disease process blocks inflow through a lobar or segmental portal vein, causing atrophy of the ischemic parenchyma and hypertrophy of the uninvolved liver. Portal vein obstruction is thought to be the most important factor in AHC associated with malignancy.13 PVE achieves the AHC through the same mechanisms and hence shares the histological characteristics and mechanisms that are discussed later.
Following early reports of the AHC in association with portal vein obstruction,14 various liver diseases have been implicated as underlying causes. In one series of 28 AHC cases from malignancy-induced portal vein occlusion, the tumor types were hilar cholangiocarcinoma in 21 (75%), hepatocellular carcinoma in 3 (10.7%), colon cancer metastases in 1 (3.6%), and pancreatic cancer in 1 (3.6%).5 Other causes of AHC from portal vein occlusion include hydatid cysts near the hilum,11,12 hepatolithiasis,11,15,16 and portal vein occlusion from hypercoagulable states resulting in cavernous transformation of the portal vein.17
Hepatic Vein Obstruction
The AHC may occur in hepatic vein obstruction. It is classically described in Budd-Chiari syndrome (BCS) in which the drainage from at least two of three hepatic veins is occluded, usually from post-thrombotic hepatic vein stenoses.18 Most cases of BCS are associated with hypercoagulable states such as primary myeloproliferative disorders, factor V Leiden mutations, anticardiolipin antibodies, and Behçet's disease. Compression of hepatic vein outflow from liver lesions/abscesses is the cause in only 5% of cases.19 The hepatic venous outflow obstruction in BCS causes increased sinusoidal pressure, sinusoidal portal hypertension, ischemic necrosis in a centrilobular distribution, and later fibrosis.18 Because the caudate lobe drains directly into the inferior vena cava, hypertrophy occurs here in 80% of patients with a chronic presentation.20
THE AHC IN PORTAL VEIN EMBOLIZATION: EARLY DISCOVERIES AND CURRENT USE
In 1920, Rous and Larimore first reported that ligation of a segmental portal vein in rabbits led to atrophy of that segment and hypertrophy of the remaining liver.21 Later clinical studies confirmed that portal vein occlusion by tumor or ligation induced ipsilateral atrophy and contralateral hypertrophy.14 In 1990, Makuuchi et al reported the use of preoperative PVE in patients undergoing extended right-sided hepatectomy for hilar cholangiocarcinoma.22 Many studies have since confirmed the utility of PVE to allow safe extended resections.
PORTAL VEIN EMBOLIZATION, LIVER ISCHEMIA, AND ATROPHY
Occlusion of the portal vein produces hepatic ischemia. Because the liver is a highly oxygen-dependent organ, impaired blood supply rapidly causes hepatic hypoxia, which subsequently progresses to anoxia, especially in pericentral regions of the liver lobule.23,24 Cardinal features of tissue ischemia are depletion of energy, loss of energy substrate, and acidosis.25 Contrary to conventional belief, cells often withstand extended ischemia and maintain their functionality and viability after prolonged periods of ischemia. One mechanism allowing hepatocyte survival during ischemia is acidosis that results from hydrolysis of high-energy phosphates, accumulation of lactate, and the release of protons from acidic organelles.26,27,28 Reperfusion of tissues recovers oxygen supply and normal physiological pH. However, paradoxically, reperfusion aggravates and precipitates cell and tissue death.25 The mechanisms underlying lethal ischemia/reperfusion (I/R) injury are multifactorial, including generation of reactive oxygen species (ROS), elevation of calcium, activation of injurious catabolic enzymes, and mitochondrial dysfunction.29 However, restoration of pH upon reperfusion is a major and independent event causing cell death25,27,28,30 because reoxygenation at acidic pH prevents I/R injury, whereas recovery to normal pH without reoxygenation does not block cell death after ischemia. In this paradoxical cell death after reperfusion, onset of the mitochondrial permeability transition (MPT), a phenomenon by which the mitochondria lose the integrity of mitochondrial inner membranes, is a major causative event in both necrosis and apoptosis after I/R.25,27,31
Mitochondrial Permeability Transition
The MPT, first characterized by Hunter et al in the mid-1970s,32 is a pathological phenomenon initiated by the opening of high conductance pores in the mitochondrial inner membrane.25 Under normal conditions, the mitochondrial membrane is virtually impermeable to all solutes except for those having specific transporters. However, when cells are exposed to toxic insults such as I/R, oxidative stress, and hepatocellular toxins, permeability transition pores in the mitochondria open. As a consequence, all solutes with a molecular mass of up to 1500 Da can nonselectively diffuse into the mitochondria.33 Onset of the MPT causes mitochondrial depolarization, uncoupling of oxidative phosphorylation, and large amplitude swelling leading to adenosine triphosphate (ATP) depletion and cell death.
Many agents are known to promote or prevent onset of the MPT.34 Calcium loading, inorganic phosphate, alkaline pH, ROS, and nitrogen radicals promote the MPT, whereas cyclosporin A, magnesium, acidic pH, and phospholipase inhibitors all prevent opening of permeability transition pores. Inhibition of the MPT by cyclosporin A or its derivatives prevents I/R injury to hepatocytes,27 myocytes,35 and other cells.36,37 The MPT can be directly assessed in live cells using confocal microscopy with calcein, a green fluorescing fluorophore.27,35,38
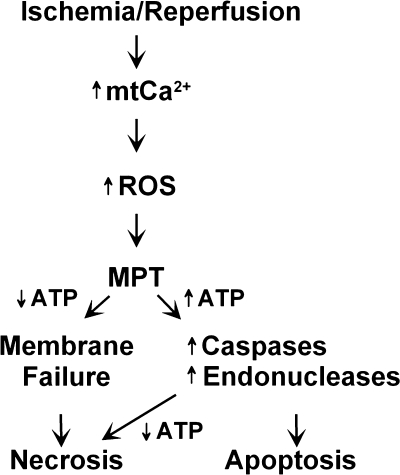
Hepatocellular Necrosis after Ischemia/Reperfusion (Fig. 1)
Figure 1.
Events leading to hepatocyte death following ischemia/reperfusion. ATP, adenosine triphosphate; MPT, mitochondrial permeability transition; mtCa2+, mitochondrial calcium; ROS, reactive oxygen species.
In hepatocytes, the formation of plasma membrane blebs is an initial indication of ischemic injury. This protrusion structure in the plasma membranes results from cytoskeletal alteration due to ATP depletion. Although the projection of surface blebs into the sinusoidal lumen can impair microcirculation, bleb formation is often reversible. However, rupture of blebs causes irreversible cell injury.39,40 After reperfusion and prior to cell death, hepatocytes develop a metastable state characterized by mitochondrial permeabilization, loss of lysosomal membrane integrity, coalescence and growth of surface blebs, and cell swelling. After rupture of the plasma membranes, cells release cytosolic enzymes and cofactors that are indispensable for cell survival. Furthermore, loss of permeability barrier in the plasma membranes causes disruption of ion homeostasis and electrical gradient. Necrosis can be assessed by cellular uptake of trypan blue or propidium iodide, which are normally excluded by healthy cells.
Hepatocellular Apoptosis after Ischemia/Reperfusion
Reperfusion of ischemic livers can cause apoptosis, characterized by cell shrinkage, caspase activation, chromatin condensation, and nuclear fragmentation.41,42 In contrast to necrosis, apoptosis develops with inflammation, scarring, and release of intracellular contents. Moreover, ATP (or deoxyadenosine triphosphate [dATP]) is required for execution of apoptotic cell death.43,44,45
As in necrosis, the mitochondria play an essential role in the development of apoptosis. A variety of proapoptotic proteins are localized in the mitochondria, including cytochrome c, apoptosis-inducing factor, and Smac-Diablo.46 Release of these proteins to the cytosol initiates apoptosis, which is tightly regulated under normal circumstances. However, pathological conditions, such as I/R or overproduction of tumor necrosis factor alpha (TNFα) and Fas ligand, induce apoptosis by either a mitochondrial or nonmitochondrial pathway.47,48 In the mitochondrial pathway, release of cytochrome c from the mitochondrial intermembrane space to the cytosol forms a complex with apoptosis protease activating factor-1 (APAF-1) and ATP (or dATP), leading to activation of caspases 9 and 3.49 The mechanisms of release of proapoptotic proteins remain controversial. One proposed mechanism is the formation of specific mitochondrial channels with the Bcl-2 family.50 Another mechanism is that the MPT induces mitochondrial swelling, rupture of mitochondrial outer membranes, and release of cytochrome c.51
Necrosis or Apoptosis after Ischemia/Reperfusion?
Although necrosis is the predominant cell death pathway after I/R, apoptotic cell death often coexists with necrosis.52,53 Thus this question arises: “How can I/R cause two different types of cell death?” The extreme conclusions that I/R induces all necrosis or all apoptosis may be missing the important fact that both types of cell death can occur simultaneously. Indeed, pathways to necrosis and apoptosis can be shared, and onset of the MPT is a common mechanism initiating both necrosis and apoptosis after I/R and toxic stress.31,54 If the MPT is widespread and glycolytic energy substrate is unavailable, cells become profoundly depleted of ATP. Because ATP is an essential player in the initiation of apoptosis, ATP depletion leads to failure of the plasma membrane integrity barrier and apoptosis. However, if the MPT is limited to a small population of the mitochondria and cells maintain 15 to 20% of normal ATP, necrosis does not occur. Instead, cells develop ATP-dependent apoptosis. After ATP-consuming apoptosis continues, cells become depleted of ATP, which generates a pattern of secondary necrosis that is often observed in pathological conditions. Thus necrosis and apoptosis can be switched, and these apparent independent types of cell death are not distinct entities.
PORTAL VEIN EMBOLIZATION AND LIVER HYPERTROPHY
Anatomical and Histological Changes in the Hypertrophic Response
The liver undergoes both anatomical and histological changes from the AHC. The extent to which these changes occur vary following PVE/occlusion versus partial hepatectomy. Anatomically, the liver rotates about the hilar axis and toward the atrophic side. This rotation can change the relative locations of the bile duct (posterior), hepatic artery (anterolateral), and portal vein (anteromedial) within the hepatoduodenal ligament.55
The rate of cell proliferation is not uniform throughout the lobule. Higher rates of cellular proliferation occur in the periportal regions (zone 1) with a gradual decrease toward the central veins (zone 3),56 and they may reflect the relative concentration of portal venous growth factors from across the liver lobule.4,56 The concept of hepatotrophic factors carried in portal venous blood is further corroborated by the occurrence of atrophy that occurs when portal blood flow is absent4 and circadian variations in DNA synthesis related to postprandial increases in portal venous blood flow.57,58
Liver Regeneration: Compensatory Hypertrophy and Hyperplasia in Response to Injury
Although only 0.0012 to 0.01% of hepatocytes in the uninjured adult liver replicate at any given time, this percentage can increase such that the volume from a two-thirds hepatectomy can be restored within weeks.56,59 Although loosely termed hypertrophy, the restorative process involves mainly hyperplasia or an increase in cell number. In addition, hypertrophy, or increased cell size, also occurs during regeneration.60,61,62
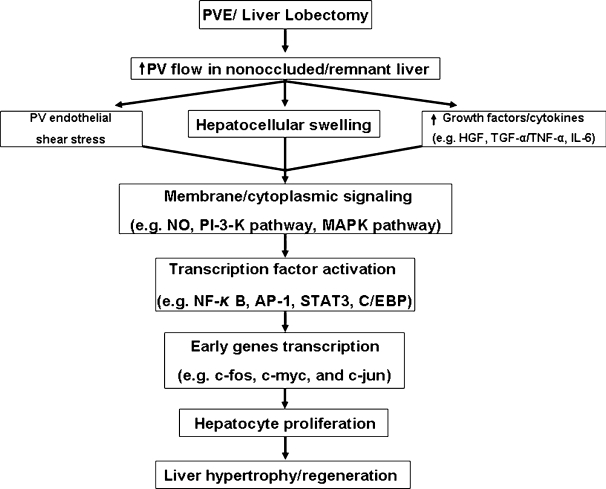
PROLIFERATIVE SIGNALING IN THE LIVER FOLLOWING PVE (FIG. 2)
Figure 2.
Changes promoting hypertrophy/regeneration in the nonoccluded/remnant liver following portal vein embolization (PVE)/liver lobectomy. HGF, hepatocyte growth factor; IL-6, interleukin-6; NO, nitric oxide; TGFα, transforming growth factor alpha; TNFα, tumor necrosis factor alpha.
The volume enlargement of the nonembolized liver lobe comes from both hepatocyte proliferation and hepatocyte hypertrophy.63 Although little is known about the connection between hepatocyte hypertrophy and hepatocyte proliferation after PVE, the initiation factors that cause hepatocyte proliferation have been well investigated using hepatectomized animal models. The proliferation of the nonoccluded liver appears to be independent of the loss of liver mass because compensatory hyperplasia is initiated before atrophy of the occluded liver ensues.64
Hemodynamic changes within the portal vein may be one signal that initiates liver regeneration. Increased portal vein pressure (shear stress) causes endothelial cells and/or hepatocytes to produce nitric oxide (NO) within 4 to 6 hours after partial hepatectomy. Inhibition studies have shown that NO promotes DNA synthesis in hepatocytes.65 In addition, inducible nitric oxide synthase (iNOS) knockout mice showed impaired liver regeneration and hepatocyte apoptosis.66 Although in vitro studies have shown that NO mediates the inactivation of methionine adenosyltransferase (MAT I/III) and consequently extracellular signal-regulated kinase (ERK 1 and 2) activation,67 these findings need to be confirmed in vivo.
Hepatocyte swelling caused by increasing portal venous flow in the nonoccluded liver lobe may be another mechanism by which liver regeneration following hepatectomy or PVE is initiated. Hepatocyte swelling can activate intracellular signaling pathway molecules such as mitogen-activated protein kinase (MAPKs), jun N-terminal kinases (JNK), and ERK.60
Systemic circulation of growth factors may also be important in hepatocyte proliferation in the nonoccluded liver lobe as well as liver regeneration after hepatectomy. In the regenerating liver, several growth factors and cytokines are important stimuli for hepatocyte replication and modulate subsequent transcription factors during liver regeneration. In contrast to liver regeneration after hepatectomy, little is known about the contribution of growth factors, cytokines, or transcription factors after PVE.
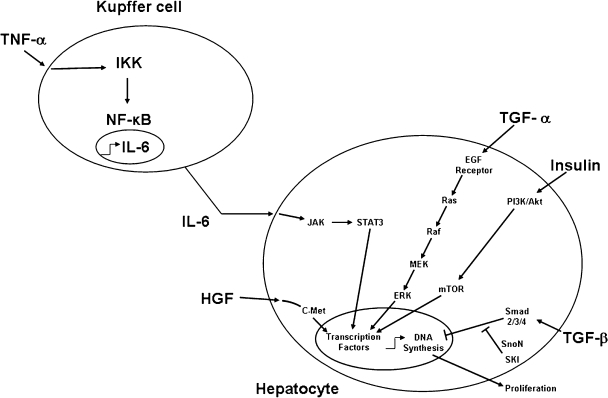
Growth Factors (Fig. 3)
Figure 3.
Signaling pathways that modulate the atrophy-hypertrophy complex. HGF, hepatocyte growth factor; IL-6, interleukin-6; TGFα, transforming growth factor alpha; TGFβ, transforming growth factor beta; TNFα, tumor necrosis factor alpha.
Hepatocyte growth factor (HGF) is a potent mitogen that binds the HGF receptor, c-met, and can induce hepatocyte DNA synthesis both in vitro and in vivo. HGF is produced by nonparenchymal cells in the liver and acts as a paracrine factor on hepatocytes. Uemura et al analyzed the expression of HGF mRNA in animals and showed an increase in the nonligated lobes between 6 and 24 hours, followed by a significant increase in DNA synthesis.68 In contrast, no increase of DNA synthesis was observed in the ligated lobe, although there was a slight elevation in HGF mRNA expression. Elevated serum levels of HGF may be also important because Kaido et al reported that the liver lobes of rats with continuous HGF infusion after portal vein ligation showed increased liver weight and DNA synthesis compared with that of untreated rats.69
Transforming growth factor alpha (TGFα), an autocrine factor produced by hepatocytes, binds the EGF receptor (EGFR) to stimulate hepatocyte replication in vitro and is important for liver regeneration after partial hepatectomy. TGFα expression in hepatocytes in both the embolized and nonembolized parts of the human liver was found to be increased.70
The HGF receptor c-met and EGFR are members of the receptor tyrosine kinase family and induce activation of intracellular signaling pathways. Two of these pathways, the phosphoinositide-3 kinase (PI3K)-Akt–mTOR and the Ras-Raf-MEK cascades, activate several transcription factors such as CCAAT/enhancer binding protein (C/EBP) and c-jun to induce hepatocyte proliferation during liver regeneration.
Activin is a growth and differentiation factor of the transforming growth factor beta (TGFβ) family that transduces signals to Smads (a class of signaling effectors described initially from Caenorhabditis elegans Sma and Drosophila Mad). Activin A, a dimeric protein of two βA subunits, has been most extensively examined and is expressed primarily in hepatocytes.71 Activin A is a negative regulator that promotes the termination of liver regeneration. Animal studies show that the pattern of βA mRNA expression of the ligated and nonligated lobes are similar; the levels increase initially at 12 hours after portal vein ligation (PVL), return to baseline, and then increase maximally at 120 hours.72
Cytokines (Fig. 3)
TNFα is a critical cytokine for the priming of hepatocyte replication. TNFα is produced primarily by Kupffer cells during liver regeneration and activates nuclear factor-kappa B (NF-κB) in nonparenchymal cells in an autocrine and paracrine fashion with resultant interleukin-6 (IL-6) production. Liver regeneration was shown to be inhibited by anti-TNFα antibody73 and in TNFα receptor type I knockout mice.74 TNFα may also play an important role even in the PVL model. Yokoyama et al measured the TNFα mRNA expression in the nonligated lobe by cDNA expression array and showed a fourfold increase over controls.75 In contrast, some investigators have questioned the importance of TNFα in liver regeneration. For example, Hayashi et al observed normal liver regeneration after partial hepatectomy in TNFα knockout mice.76
IL-6 is an important activator of signal transducers and activators of transcription (STAT3) and is released by hepatic stellate and Kupffer cells upon stimulation by TNFα. Activated STAT3 enters the nucleus and induces the transcription of specific immediate-early genes essential for liver regeneration. IL-6 mRNA was significantly induced in both ligated and nonligated liver lobes in the first hour after portal vein ligation, but no significant difference was observed between them.77 Others also observed similar results of IL-6 expression.75 Kobayashi et al reported that the serum IL-6 rose and reached maximum at 6 hours after PVL and then decreased. An in situ hybridization study showed that IL-6 mRNA in the nonligated lobe was predominantly expressed in sinusoidal endothelial cells around the periportal area (zone 1) 3 hours after PVL.78
Transcription Factors (Fig. 3)
Stärkel et al reported changes in expression of the transcription factors NF-κB, STAT3, c-fos, c-myc, and c-jun after portal branch ligation.77 The increased mRNA expressions of c-fos, c-myc, and c-jun during the first 2 hours were observed in both the ligated and nonligated lobe. However, the authors cautioned that such findings should be interpreted with caution because STAT3 levels also increased with sham operations. Recently, a nonstress-PVL rat model that minimizes the effects of surgical stress was established78 and has been used to examine the regenerative signals after PVL.75 The patterns of NF-κB p65, phosphorylated STAT3, c-fos, and c-jun levels differed. Nonligated lobes demonstrated biphasic activation of these transcription factors with peaks at 1 and 3 to 6 hours after PVL. However, ligated lobes showed a lower level of activation that reached maximum concentrations 2 hours after PVL.
Growth Arrest following the Hypertrophic Response (Fig. 3)
TGFβ plays an important growth regulatory role in epithelial cells. TGFβ binds to the type II TGFβ serine/threonine kinase receptor. Then, activated type II receptor forms a dimer with the type I receptor.79 These events activate the Smad pathway with resultant growth arrest and/or apoptosis. Through adaptors, Smads 2 and 3 are recruited to the TGFβ receptor complex and phosphorylated by the type I receptor. This releases Smad 2/3 from the transmembrane complex and allows formation of a heterotrimeric complex with the common mediator, Smad 4.80 The Smad complex then translocates to the nucleus where it activates TGFβ-responsive genes through cooperative interactions with DNA and other DNA-binding proteins.
Smad activity is modulated by adaptors such as Smad anchor for receptor activation (SARA), filamin, embryonic liver Fodrin (ELF), as well as functional interactions with multiple other signal transduction pathways.81,82,83 Other intracellular regulators of Smad function include microtubules that serve as cytoplasmic sequesters, controlling Smad 2 association and phosphorylation by the TGFβ receptor.84
TGFβ signaling is modulated by several different mechanisms. Smad 7, a target gene of TGFβ, acts at the level of the membrane receptor to inhibit Smad 2 and Smad 3 activation. Smad ubiquitin regulatory factor 1 (Smurf1) and Smurf2 associate with the nuclear Smad7 after stimulation by TGFβ. The Smurfs regulate ubiquitination of ELF, which can become displaced from the signaling pathway and degraded by proteasomes.
In the nucleus, gene transactivation can be blocked by binding of the Smad complex to nuclear corepressors such as Ski, SnoN, or TGIF. Additionally, p53 modulates TGFβ signaling through independent binding of the Smads.
Interestingly, even though TGFβ mRNA is increased in the regenerating liver after partial hepatectomy, the proliferating hepatocytes are resistant to its growth inhibitory effects. A 2002 study provided indirect evidence of active TGFβ signaling by observing an increase in Smad 2 phosphorylation during the first 5 days after hepatectomy.85 However, concomitant increases in protein levels of SnoN (2 to 48 hours) and Ski (24 to 72 hours) and increased activity of TGFβ repressors also occurred. A complex was formed between SnoN, Ski, phosphorylated-Smad 2, Smad 3, and Smad 4 during the 5 days following partial hepatectomy, thus explaining the resistance to TGFβ growth arrest. TGFβ signaling causes early ubiquitination and proteasome-mediated degradation of both Ski and SnoN to allow Smad-induced gene activation. The upregulation of SnoN mRNA can serve as negative feedback to return TGFβ signaling to basal levels.86,87 Further studies are needed to clarify what controls the upregulation of Ski and SnoN in the regenerating liver and whether their degradation is blocked to promote hepatocyte proliferation.
Inhibition of growth may also involve the cyclin-dependent kinase (CDK)-inhibitory proteins (CDKIs). The p21-Cdk interacting protein 1 (p21-Cip1) has bimodal upregulation as an immediate-early gene within 30 minutes of partial hepatectomy (PH) and also from 48 to 72 hours after PH. CDKIs bind cyclin/CDK complexes and p21 to regulate CDK1, CDK2, CDK4, and CDK6. The early expression of p21-Cip1 may synchronize entry into the G1 phase. The latter expression of p21-Cip1 may limit growth. Importantly, p21-Cip1 expression is also modulated by the growth inhibitory cytokines, TGFβ, and activin.88
Hypertrophy in the Injured Liver
Patients with compromised liver function may benefit from preoperative PVE to increase the size of the future liver remnant before extended liver resections. In one prospective clinical trial assessing the efficacy of PVE, patients with or without chronic liver disease in whom an elective right hepatectomy was indicated were assigned to have surgery either with or without PVE. The postoperative courses of those with normal livers were similar in embolized and nonembolized patients. In contrast, preoperative PVE in patients with chronic liver disease significantly decreased the incidence of postoperative complications. The authors concluded that preoperative PVE was beneficial only in patients with chronic liver disease.89
Investigators have shown that the injured liver regenerates less efficiently following both PVE and hepatectomy. The regeneration rate of the nonembolized lobes in cirrhosis (9.3 cm3/day) was slower than that of the normal liver (11.8 cm3/day).90 Despite theses difference, others have found only an insignificant difference in the final volume of the nonembolized lobe between cirrhotic versus normal livers,89 between livers with cirrhosis versus those with mild or moderate fibrosis,91 and between normal and injured livers due to viral hepatitis.92
In addition to the histological changes in regeneration, the changes in metabolic function are also important. Hypertrophy of the nonembolized lobe was impaired in patients with high serum bilirubin at PVE.93 Others have shown that liver regeneration in patients with hilar cholangiocarcinoma who underwent biliary decompression before PVE was not impaired with a growth rate of 12 cm3/day.94 Other clinical factors found to impact liver hypertrophy negatively include diabetes mellitus93,94 and male gender.93 The mechanisms underlying these associations remain unclear.
Some have shown that both diseased and normal livers are capable of hypertrophy following PVL. In a rat model, Lee et al induced cirrhosis with carbon tetrachloride and investigated the regenerative response following PVL.90 Both cirrhotic and noncirrhotic hepatocytes had comparable elevations in mitotic index at 3 days, and although DNA synthesis was increased in both, it was delayed in the cirrhotic livers.
Mizuno et al studied whether cholestasis could affect the hypertrophy of the nonligated lobe after PVL. The common bile duct was ligated 5 days before PVL in a rat model, and then DNA polymerase α expression, a marker of hepatocyte replication, was measured. These studies showed that the induction of liver hypertrophy in the cholestatic rats was similar to that of the noncholestatic rats following PVL.95
The Roles of Nonhepatocytes during Liver Regeneration
After PH, mature hepatocytes proliferate maximally at 24 hours followed by the replication of biliary ductal cells, Kupffer cells, stellate cells, and, lastly, endothelial cells.96 One important component of the regenerative process is extracellular matrix (ECM). Urokinase-like plasminogen activator (uPA) not only converts plasminogen to plasmin but also helps in the remodeling of the ECM to allow cell division, activation of extracellular pro-metalloproteinases, and release of the bound single-chain form of HGF from the ECM.97
The uPA also initiates the degradation of the ECM through activation of the matrix metalloproteinase (MMP) cascade.98,99 Within 5 minutes of PH, increased uPA activity correlates with conversion of inactive pro-MMP-2 and pro-MMP-9 to their active forms,100 and it initiates disruption of the ECM.101 This uPA-dependent degradation of the ECM causes release of bound HGF with a subsequent increase in serum HGF concentration.102,103 In uPA − / − mice treated with monoclonal anti-Fas antibody (a stimulator of apoptosis), serum HGF concentrations increased later than in controls, but delayed HGF release was reversed upon transfection with the uPA gene.104 Collectively, these studies suggest that uPA is an important initiator of free HGF and that ECM remodeling is needed in the early phase of liver regeneration.
“Oval cells,” a normally dormant hepatic stem cell population, may also contribute to liver regeneration. These small epithelial cells possess oval nuclei, scant cytoplasm, and reside in the bile ductules and canals of Hering. These cells can differentiate into cholangiocytes or hepatocytes. Hepatocyte differentiation leads to the formation of intermediate hepatocyte-like cells, which are defined as polygonal cells with a size between that of oval cells and hepatocytes. The current view is that these cells possess the potential to regenerate the hepatic mass when mature hepatocytes are unable to do so.105,106 Some have demonstrated that repopulation of the liver occurs following drug-induced injury that normally precludes mature hepatocyte replication, suggesting regeneration through the stem/oval cell compartment.107,108,109
In addition, bone marrow or hematopoietic derived stem cells enter the liver through the portal vasculature. During and after episodes of severe liver injury, a large proportion of mature hepatocytes and cholangiocytes are derived from hematopoietic stem cells.110 Even lethally damaged livers regenerate after transplantation of bone marrow–derived stem cells,111 although this process occurs through stem cells that fuse with hepatocytes rather than through pure stem cell repopulation of the liver.112,113 Further studies are needed to define the potential uses of stem cell transplantation as a therapy for end-stage liver disease.
STRATEGIES TO IMPROVE THE HYPERTROPHIC RESPONSE
Soon after the benefit of preoperative PVE was realized, potential strategies to maximize the hypertrophic response were evaluated. Bone morphogenic protein-1 (BMP-7), a protein involved in liver organogenesis, enhanced liver regeneration following partial hepatectomy in an animal model.114 Prostaglandin E(1) in lipid microspheres (Lipo PGE[1]) significantly increases DNA synthesis and survival following 90% hepatectomy in rats.115 Hepatopoietin is a hepatotrophic growth factor that stimulates proliferation in cultured hepatocytes and hepatoma cells and liver regeneration in animal studies.116 Proteases may help control the initiation and termination of liver regeneration by releasing growth factors anchored to the extracellular matrix.117 Ischemic preconditioning has beneficial effects on liver regeneration in animal models by upregulating growth-promoting factors, suppressing growth-inhibitory factors, and preserving energy levels for regeneration.118,119 Finally, in one prospective study of patients undergoing preoperative PVE for the resection of liver malignancies, 6 of 13 patients received CD133 + bone marrow stem cells directly to future remnant liver segments. Those patients receiving preoperative stem cells showed significantly increased remnant liver growth as compared with controls.120 Others have found that transplanted bone marrow cells can generate hepatocytes and help in liver repair and regeneration.121 Although preliminary, these strategies to promote liver regeneration may someday be used in conjunction with preoperative PVE to maximize the growth of the future liver remnant.
CONCLUSIONS
The AHC is a regulated compensatory response to liver injury that reestablishes adequate liver function for survival. The AHC involves complex anatomical, histological, cellular, and molecular processes that result in partial liver loss and regeneration. The signals responsible for these events are broad. They are both intrinsic and extrinsic to the liver and involve both physical forces and biochemical interactions. Differences in these mechanisms in the healthy and diseased liver may be used to maximize the liver's ability to heal. When used in conjunction with portal vein embolization, these mechanisms may someday be exploited to permit curative resections in patients with marginal liver function.
ACKNOWLEDGMENTS
We would like to thank Jason E. Cline for his assistance in formatting this article.
REFERENCES
- Bellentani S, Hardison W G, Manenti F. Mechanisms of liver adaptation to prolonged selective biliary obstruction (SBO) in the rat. J Hepatol. 1985;1:525–535. doi: 10.1016/s0168-8278(85)80750-9. [DOI] [PubMed] [Google Scholar]
- Schaffner F, Bacchin P G, Hutterer F, et al. Mechanism of cholestasis: 4. Structural and biochemical changes in the liver and serum in rats after bile duct ligation. Gastroenterology. 1971;60:888–897. [PubMed] [Google Scholar]
- Gall J A, Bhathal P S. Origin and involution of hyperplastic bile ductules following total biliary obstruction. Liver. 1990;10:106–115. doi: 10.1111/j.1600-0676.1990.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Schweizer W, Duda P, Tanner S, et al. Experimental atrophy/hypertrophy complex (AHC) of the liver: portal vein, but not bile duct obstruction, is the main driving force for the development of AHC in the rat. J Hepatol. 1995;23:71–78. doi: 10.1016/0168-8278(95)80313-0. [DOI] [PubMed] [Google Scholar]
- Hann L E, Getrajdman G I, Brown K T, et al. Hepatic lobar atrophy: association with ipsilateral portal vein obstruction. AJR Am J Roentgenol. 1996;167:1017–1021. doi: 10.2214/ajr.167.4.8819404. [DOI] [PubMed] [Google Scholar]
- Hadjis N S, Adam A, Gibson R, Blenkharn J I, Benjamin I S, Blumgart L H. Nonoperative approach to hilar cancer determined by the atrophy-hypertrophy complex. Am J Surg. 1989;157:395–399. doi: 10.1016/0002-9610(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Hadjis N S, Blumgart L H. Role of liver atrophy, hepatic resection and hepatocyte hyperplasia in the development of portal hypertension in biliary disease. Gut. 1987;28:1022–1028. doi: 10.1136/gut.28.8.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjis N S, Adam A, Blenkharn I, Hatzis G, Benjamin I S, Blumgart L H. Primary sclerosing cholangitis associated with liver atrophy. Am J Surg. 1989;158:43–47. doi: 10.1016/0002-9610(89)90314-0. [DOI] [PubMed] [Google Scholar]
- Jeyarajah D R. Recurrent pyogenic cholangitis. Curr Treat Options Gastroenterol. 2004;7:91–98. doi: 10.1007/s11938-004-0029-x. [DOI] [PubMed] [Google Scholar]
- Rana S S, Bhasin D K, Nanda M, Singh K. Parasitic infestations of the biliary tract. Curr Gastroenterol Rep. 2007;9:156–164. doi: 10.1007/s11894-007-0011-6. [DOI] [PubMed] [Google Scholar]
- Rozanes I, Acunas B, Celik L, Minareci O, Gokmen E. CT in lobar atrophy of the liver caused by alveolar echinococcosis. J Comput Assist Tomogr. 1992;16:216–218. doi: 10.1097/00004728-199203000-00008. [DOI] [PubMed] [Google Scholar]
- Karabulut K, Ozden I, Poyanli A, et al. Hepatic atrophy-hypertrophy complex due to Echinococcus granulosus. J Gastrointest Surg. 2006;10:407–412. doi: 10.1016/j.gassur.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Lorigan J G, Charnsangavej C, Carrasco C H, Richli W R, Wallace S. Atrophy with compensatory hypertrophy of the liver in hepatic neoplasms: radiographic findings. AJR Am J Roentgenol. 1988;150:1291–1295. doi: 10.2214/ajr.150.6.1291. [DOI] [PubMed] [Google Scholar]
- Bax H R, Mansens B J, Schalm L. Atrophy of the liver after occlusion of the bile ducts or portal vein and compensatory hypertrophy of the unoccluded portion and its clinical importance. Gastroenterology. 1956;31:131–155. [PubMed] [Google Scholar]
- Ishida H, Naganuma H, Konno K, et al. Lobar atrophy of the liver. Abdom Imaging. 1998;23:150–153. doi: 10.1007/s002619900309. [DOI] [PubMed] [Google Scholar]
- Kusano S, Okada Y, Endo T, Yokoyama H, Ohmiya H, Atari H. Oriental cholangiohepatitis: correlation between portal vein occlusion and hepatic atrophy. AJR Am J Roentgenol. 1992;158:1011–1014. doi: 10.2214/ajr.158.5.1566657. [DOI] [PubMed] [Google Scholar]
- Vilgrain V, Condat B, Bureau C, et al. Atrophy-hypertrophy complex in patients with cavernous transformation of the portal vein: CT evaluation. Radiology. 2006;241:149–155. doi: 10.1148/radiol.2411051102. [DOI] [PubMed] [Google Scholar]
- Valla D C. The diagnosis and management of the Budd-Chiari syndrome: consensus and controversies. Hepatology. 2003;38:793–803. doi: 10.1053/jhep.2003.50415. [DOI] [PubMed] [Google Scholar]
- Denninger M H, Chait Y, Casadevall N, et al. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology. 2000;31:587–591. doi: 10.1002/hep.510310307. [DOI] [PubMed] [Google Scholar]
- Matthieu D, Kracht M, Zafrani E, Dhumeaux D, Vasile N. In: Ferrucci J, Matthieu D, editor. Advances in Hepatobiliary Radiology. St. Louis: CV Mosby; 1990. Budd-Chiari syndrome. pp. 3–28.
- Rous P, Larimore L D. Relation of the portal blood flow to liver maintenance: a demonstration of liver atrophy conditional on compensation. J Exp Med. 1920;31:609–632. doi: 10.1084/jem.31.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makuuchi M, Thai B L, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- Lemasters J J, Ji S, Thurman R G. Centrilobular injury following hypoxia in isolated, perfused rat liver. Science. 1981;213(4508):661–663. doi: 10.1126/science.7256265. [DOI] [PubMed] [Google Scholar]
- Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31(2):255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- Kim J-S, He L, Lemasters J J. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun. 2003;304(3):463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- Gores G J, Nieminen A L, Wray B E, Herman B, Lemasters J J. Intracellular pH during “chemical hypoxia” in cultured rat hepatocytes: protection by intracellular acidosis against the onset of cell death. J Clin Invest. 1989;83(2):386–396. doi: 10.1172/JCI113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian T, Nieminen A L, Herman B, Lemasters J J. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am J Physiol. 1997;273:C1783–C1792. doi: 10.1152/ajpcell.1997.273.6.C1783. [DOI] [PubMed] [Google Scholar]
- Currin R T, Gores G J, Thurman R G, Lemasters J J. Protection by acidotic pH against anoxic cell killing in perfused rat liver: evidence for a pH paradox. FASEB J. 1991;5(2):207–210. doi: 10.1096/fasebj.5.2.2004664. [DOI] [PubMed] [Google Scholar]
- McCord J M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Kim J S, He L, Qian T, Lemasters J J. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med. 2003;3(6):527–535. doi: 10.2174/1566524033479564. [DOI] [PubMed] [Google Scholar]
- Kim J-S, Qian T, Lemasters J J. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology. 2003;124:494–503. doi: 10.1053/gast.2003.50059. [DOI] [PubMed] [Google Scholar]
- Hunter D R, Haworth R A, Southard J H. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976;251(16):5069–5077. [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79(4):1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Gunter T E, Pfeiffer D R. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Kim J-S, Jin Y, Lemasters J J. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia/reperfusion. Am J Physiol Heart Circ Physiol. 2006;290(5):H2024–H2034. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- Zazueta C, Franco M, Correa F, et al. Hypothyroidism provides resistance to kidney mitochondria against the injury induced by renal ischemia-reperfusion. Life Sci. 2007;80(14):1252–1258. doi: 10.1016/j.lfs.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Li P A, Uchino H, Elmer E, Siesjo B K. Amelioration by cyclosporin A of brain damage following 5 or 10 min of ischemia in rats subjected to preischemic hyperglycemia. Brain Res. 1997;753(1):133–140. doi: 10.1016/s0006-8993(97)00005-x. [DOI] [PubMed] [Google Scholar]
- Kim J-S, Ohshima S, Pediaditakis P, Lemasters J J. Nitric oxide protects rat hepatocytes against reperfusion injury mediated by the mitochondrial permeability transition. Hepatology. 2004;39:1533–1543. doi: 10.1002/hep.20197. [DOI] [PubMed] [Google Scholar]
- Herman B, Nieminen A L, Gores G J, Lemasters J J. Irreversible injury in anoxic hepatocytes precipitated by an abrupt increase in plasma membrane permeability. FASEB J. 1988;2(2):146–151. doi: 10.1096/fasebj.2.2.3342967. [DOI] [PubMed] [Google Scholar]
- Nieminen A L, Gores G J, Wray B E, Tanaka Y, Herman B, Lemasters J J. Calcium dependence of bleb formation and cell death in hepatocytes. Cell Calcium. 1988;9(5-6):237–246. doi: 10.1016/0143-4160(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Gao W, Bentley R C, Madden J F, Clavien P A. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology. 1998;27(6):1652–1660. doi: 10.1002/hep.510270626. [DOI] [PubMed] [Google Scholar]
- Natori S, Selzner M, Valentino K L, et al. Apoptosis of sinusoidal endothelial cells occurs during liver preservation injury by a caspase-dependent mechanism. Transplantation. 1999;68(1):89–96. doi: 10.1097/00007890-199907150-00018. [DOI] [PubMed] [Google Scholar]
- Richter C, Schweizer M, Cossarizza A, Franceschi C. Control of apoptosis by the cellular ATP level. FEBS Lett. 1996;378(2):107–110. doi: 10.1016/0014-5793(95)01431-4. [DOI] [PubMed] [Google Scholar]
- Leist M, Single B, Castoldi A F, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185(8):1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57(10):1835–1840. [PubMed] [Google Scholar]
- Galluzzi L, Larochette N, Zamzami N, Kroemer G. Mitochondria as therapeutic targets for cancer chemotherapy. Oncogene. 2006;25(34):4812–4830. doi: 10.1038/sj.onc.1209598. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17(6):1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano E, Bradham C A, Stark A, Iimuro Y, Lemasters J J, Brenner D A. The mitochondrial permeability transition augments Fas-induced apoptosis in mouse hepatocytes. J Biol Chem. 2000;275(16):11814–11823. doi: 10.1074/jbc.275.16.11814. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15(22):2922–2933. [PubMed] [Google Scholar]
- Wei M C, Zong W X, Cheng E H, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzami N, Susin S A, Marchetti P, et al. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183(4):1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral J S, Bucci T J, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33(2):397–405. doi: 10.1053/jhep.2001.22002. [DOI] [PubMed] [Google Scholar]
- Kohli V, Madden J F, Bentley R C, Clavien P A. Calpain mediates ischemic injury of the liver through modulation of apoptosis and necrosis. Gastroenterology. 1999;116(1):168–178. doi: 10.1016/s0016-5085(99)70241-6. [DOI] [PubMed] [Google Scholar]
- Qian T, Herman B, Lemasters J J. The mitochondrial permeability transition mediates both necrotic and apoptotic death of hepatocytes exposed to Br-A23187. Toxicol Appl Pharmacol. 1999;154(2):117–125. doi: 10.1006/taap.1998.8580. [DOI] [PubMed] [Google Scholar]
- Czerniak A, Soreide O, Gibson R N, et al. Liver atrophy complicating benign bile duct strictures: surgical and interventional radiologic approaches. Am J Surg. 1986;152:294–300. doi: 10.1016/0002-9610(86)90261-8. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G K, DeFrances M C. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Barbason H, Bouzahzah B, Herens C, et al. Circadian synchronization of liver regeneration in adult rats: the role played by adrenal hormones. Cell Tissue Kinet. 1989;22:451–460. doi: 10.1111/j.1365-2184.1989.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Souto M, Llanos J M. The circadian optimal time for hepatectomy in the study of liver regeneration. Chronobiol Int. 1985;2:169–175. doi: 10.3109/07420528509055556. [DOI] [PubMed] [Google Scholar]
- Koniaris L G, McKillop I H, Schwartz S I, Zimmers T A. Liver regeneration. J Am Coll Surg. 2003;197:634–659. doi: 10.1016/S1072-7515(03)00374-0. [DOI] [PubMed] [Google Scholar]
- Kim R D, Stein G S, Chari R S. Impact of cell swelling on proliferative signal transduction in the liver. J Cell Biochem. 2001;83:56–69. doi: 10.1002/jcb.1205. [DOI] [PubMed] [Google Scholar]
- Nagy P, Teramoto T, Factor V M, et al. Reconstitution of liver mass via cellular hypertrophy in the rat. Hepatology. 2001;33:339–345. doi: 10.1053/jhep.2001.21326. [DOI] [PubMed] [Google Scholar]
- Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Komori K, Nagino M, Nimura Y. Hepatocyte morphology and kinetics after portal vein embolization. Br J Surg. 2006;93(6):745–751. doi: 10.1002/bjs.5332. [DOI] [PubMed] [Google Scholar]
- Lambotte L, Li B, Leclercq I, Sempoux C, Saliez A, Horsmans Y. The compensatory hyperplasia (liver regeneration) following ligation of a portal branch is initiated before the atrophy of the deprived lobes. J Hepatol. 2000;32(6):940–945. doi: 10.1016/s0168-8278(00)80098-7. [DOI] [PubMed] [Google Scholar]
- Hortelano S, Dewez B, Genaro A M, Diaz-Guerra M J, Bosca L. Nitric oxide is released in regenerating liver after partial hepatectomy. Hepatology. 1995;21(3):776–786. [PubMed] [Google Scholar]
- Rai R M, Lee F Y, Rosen A, et al. Impaired liver regeneration in inducible nitric oxide synthase deficient mice. Proc Natl Acad Sci U S A. 1998;95(23):13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Trevijano E R, Martinez-Chantar M L, Latasa M U, Mato J M, Avila M A. NO sensitizes rat hepatocytes to proliferation by modifying S-adenosylmethionine levels. Gastroenterology. 2002;122(5):1355–1363. doi: 10.1053/gast.2002.33020. [DOI] [PubMed] [Google Scholar]
- Uemura T, Miyazaki M, Hirai R, et al. Different expression of positive and negative regulators of hepatocyte growth in growing and shrinking hepatic lobes after portal vein branch ligation in rats. Int J Mol Med. 2000;5(2):173–179. doi: 10.3892/ijmm.5.2.173. [DOI] [PubMed] [Google Scholar]
- Kaido T, Yoshikawa A, Seto S, et al. Hepatocyte growth factor supply accelerates compensatory hypertrophy caused by portal branch ligation in normal and jaundiced rats. J Surg Res. 1999;85(1):115–119. doi: 10.1006/jsre.1999.5639. [DOI] [PubMed] [Google Scholar]
- Kusaka K, Imamura H, Tomiya T, Takayama T, Makuuchi M. Expression of transforming growth factor-alpha and -beta in hepatic lobes after hemihepatic portal vein embolization. Dig Dis Sci. 2006;51(8):1404–1412. doi: 10.1007/s10620-006-9105-5. [DOI] [PubMed] [Google Scholar]
- Vejda S, Cranfield M, Peter B, et al. Expression and dimerization of the rat activin subunits betaC and betaE: evidence for the formation of novel activin dimers. J Mol Endocrinol. 2002;28(2):137–148. doi: 10.1677/jme.0.0280137. [DOI] [PubMed] [Google Scholar]
- Takamura K, Tsuchida K, Miyake H, Tashiro S, Sugino H. Activin and activin receptor expression changes in liver regeneration in rat. J Surg Res. 2005;126(1):3–11. doi: 10.1016/j.jss.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Akerman P, Cote P, Yang S Q, et al. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263(4):G579–G585. doi: 10.1152/ajpgi.1992.263.4.G579. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Kirillova I, Peschon J J, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci U S A. 1997;94(4):1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S, Yokoyama Y, Kawai T, et al. Biphasic activation of liver regeneration-associated signals in an early stage after portal vein branch ligation. Biochem Biophys Res Commun. 2006;349(2):732–739. doi: 10.1016/j.bbrc.2006.08.083. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Nagaki M, Imose M, et al. Normal liver regeneration and liver cell apoptosis after partial hepatectomy in tumor necrosis factor-alpha-deficient mice. Liver Int. 2005;25(1):162–170. doi: 10.1111/j.1478-3231.2005.01029.x. [DOI] [PubMed] [Google Scholar]
- Stärkel P, Horsmans Y, Sempoux C, et al. After portal branch ligation in rat, nuclear factor kappaB, interleukin-6, signal transducers and activators of transcription 3, c-fos, c-myc, and c-jun are similarly induced in the ligated and nonligated lobes. Hepatology. 1999;29(5):1463–1470. doi: 10.1002/hep.510290503. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Nagino M, Yokoyama Y, Nimura Y, Sokabe M. Evaluation of hepatic interleukin-6 secretion following portal vein ligation using a minimal surgical stress model. J Surg Res. 2006;135(1):27–33. doi: 10.1016/j.jss.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Wrana J L, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370(6488):341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Abdollah S, Macias-Silva M, Tsukazaki T, et al. TbetaRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J Biol Chem. 1997;272(44):27678–27685. doi: 10.1074/jbc.272.44.27678. [DOI] [PubMed] [Google Scholar]
- Wu G, Chen Y G, Ozdamar B, et al. Structural basis of Smad2 recognition by the Smad anchor for receptor activation. Science. 2000;287(5450):92–97. doi: 10.1126/science.287.5450.92. [DOI] [PubMed] [Google Scholar]
- Hocevar B A, Smine A, Xu X X, Howe P H. The adaptor molecule Disabled-2 links the transforming growth factor beta receptors to the Smad pathway. EMBO J. 2001;20(11):2789–2801. doi: 10.1093/emboj/20.11.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra L, Marshall B. Adaptor proteins and ubiquinators in TGF-beta signaling. Cytokine Growth Factor Rev. 2006;17(1–2):75–87. doi: 10.1016/j.cytogfr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Dong C, Li Z, Alvarez R, Jr, Feng X H, Goldschmidt-Clermont P J. Microtubule binding to Smads may regulate TGF beta activity. Mol Cell. 2000;5(1):27–34. doi: 10.1016/s1097-2765(00)80400-1. [DOI] [PubMed] [Google Scholar]
- Macias-Silva M, Li W, Leu J I, Crissey M A, Taub R. Up-regulated transcriptional repressors SnoN and Ski bind Smad proteins to antagonize transforming growth factor-beta signals during liver regeneration. J Biol Chem. 2002;277(32):28483–28490. doi: 10.1074/jbc.M202403200. [DOI] [PubMed] [Google Scholar]
- Stroschein S L, Wang W, Zhou S, Zhou Q, Luo K. Negative feedback regulation of TGF-beta signaling by the SnoN oncoprotein. Science. 1999;286(5440):771–774. doi: 10.1126/science.286.5440.771. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu X, Ng-Eaton E, Lodish H F, Weinberg R A. SnoN and Ski protooncoproteins are rapidly degraded in response to transforming growth factor beta signaling. Proc Natl Acad Sci U S A. 1999;96(22):12442–12447. doi: 10.1073/pnas.96.22.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht J H, Meyer A H, Hu M Y. Regulation of cyclin-dependent kinase inhibitor p21(WAF1/Cip1/Sdi1) gene expression in hepatic regeneration. Hepatology. 1997;25(3):557–563. doi: 10.1002/hep.510250311. [DOI] [PubMed] [Google Scholar]
- Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237(2):208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K C, Kinoshita H, Hirohashi K, Kubo S, Iwasa R. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg. 1993;17(1):109–115. doi: 10.1007/BF01655721. [DOI] [PubMed] [Google Scholar]
- Azoulay D, Castaing D, Krissat J, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232(5):665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi H, Ishimura K, Okano K, et al. Application of preoperative portal vein embolization before major hepatic resection in patients with normal or abnormal liver parenchyma. Surgery. 2002;131(1):26–33. doi: 10.1067/msy.2002.118259. [DOI] [PubMed] [Google Scholar]
- Imamura H, Shimada R, Kubota M, et al. Preoperative portal vein embolization: an audit of 84 patients. Hepatology. 1999;29(4):1099–1105. doi: 10.1002/hep.510290415. [DOI] [PubMed] [Google Scholar]
- Nagino M, Nimura Y, Kamiya J, et al. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology. 1995;21(2):434–439. [PubMed] [Google Scholar]
- Mizuno S, Nimura Y, Suzuki H, Yoshida S. Portal vein branch occlusion induces cell proliferation of cholestatic rat liver. J Surg Res. 1996;60(1):249–257. doi: 10.1006/jsre.1996.0039. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G K. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangnall D, Smith K, Bird N C, Majeed A W. Early increases in plasminogen activator activity following partial hepatectomy in humans. Comp Hepatol. 2004;3(1):11. doi: 10.1186/1476-5926-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi F. Urokinase and urokinase receptor: a paracrine/autocrine system regulating cell migration and invasiveness. Bioessays. 1993;15(2):105–111. doi: 10.1002/bies.950150206. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W G. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol. 1996;148(5):1345–1350. [PMC free article] [PubMed] [Google Scholar]
- Kim T H, Mars W M, Stolz D B, Michalopoulos G K. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000;31(1):75–82. doi: 10.1002/hep.510310114. [DOI] [PubMed] [Google Scholar]
- Mars W M, Liu M L, Kitson R P, Goldfarb R H, Gabauer M K, Michalopoulos G K. Immediate early detection of urokinase receptor after partial hepatectomy and its implications for initiation of liver regeneration. Hepatology. 1995;21(6):1695–1701. [PubMed] [Google Scholar]
- Liu M L, Mars W M, Zarnegar R, Michalopoulos G K. Uptake and distribution of hepatocyte growth factor in normal and regenerating adult rat liver. Am J Pathol. 1994;144(1):129–140. [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Vigna E, Bardelli A, Follenzi A, Galimi F, Comoglio P M. Biological activation of pro-HGF (hepatocyte growth factor) by urokinase is controlled by a stoichiometric reaction. J Biol Chem. 1995;270(2):603–611. doi: 10.1074/jbc.270.2.603. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Hara A, Okuno M, et al. Mechanism of retarded liver regeneration in plasminogen activator-deficient mice: impaired activation of hepatocyte growth factor after Fas-mediated massive hepatic apoptosis. Hepatology. 2001;33(3):569–576. doi: 10.1053/jhep.2001.22650. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson S S, Evarts R P, Bisgaard H C, Fujio K, Hu Z. Hepatic stem cell compartment: activation and lineage commitment. Proc Soc Exp Biol Med. 1993;204(3):253–260. doi: 10.3181/00379727-204-43661. [DOI] [PubMed] [Google Scholar]
- Oh S H, Witek R P, Bae S H, et al. Bone marrow-derived hepatic oval cells differentiate into hepatocytes in 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Gastroenterology. 2007;132(3):1077–1087. doi: 10.1053/j.gastro.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Gordon G J, Coleman W B, Grisham J W. Temporal analysis of hepatocyte differentiation by small hepatocyte-like progenitor cells during liver regeneration in retrorsine-exposed rats. Am J Pathol. 2000;157(3):771–786. doi: 10.1016/S0002-9440(10)64591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon G J, Coleman W B, Hixson D C, Grisham J W. Liver regeneration in rats with retrorsine-induced hepatocellular injury proceeds through a novel cellular response. Am J Pathol. 2000;156(2):607–619. doi: 10.1016/S0002-9440(10)64765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio K, Evarts R P, Hu Z, Marsden E R, Thorgeirsson S S. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab Invest. 1994;70(4):511–516. [PubMed] [Google Scholar]
- Roskams T A, Libbrecht L, Desmet V J. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23(4):385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6(11):1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422(6934):897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos G, Wang P R, Russell D W. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422(6934):901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- Sugimoto H, Yang C, LeBleu V S, et al. BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J. 2007;21:256–264. doi: 10.1096/fj.06-6837com. [DOI] [PubMed] [Google Scholar]
- Ando K, Miyazaki M, Shimizu H, Okuno A, Nakajima N. Beneficial effects of prostaglandin E(1) incorporated in lipid microspheres on liver injury and regeneration after 90% partial hepatectomy in rats. Eur Surg Res. 2000;32:155–161. doi: 10.1159/000008757. [DOI] [PubMed] [Google Scholar]
- Gatzidou E, Kouraklis G, Theocharis S. Insights on augmenter of liver regeneration cloning and function. World J Gastroenterol. 2006;12:4951–4958. doi: 10.3748/wjg.v12.i31.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed F F, Khokha R. Thinking outside the cell: proteases regulate hepatocyte division. Trends Cell Biol. 2005;15:555–563. doi: 10.1016/j.tcb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Gomez D, Homer-Vanniasinkam S, Graham A M, Prasad K R. Role of ischaemic preconditioning in liver regeneration following major liver resection and transplantation. World J Gastroenterol. 2007;13:657–670. doi: 10.3748/wjg.v13.i5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- Fürst G, Schulte am Esch J, Poll L W, et al. Portal vein embolization and autologous CD133 + bone marrow stem cells for liver regeneration: initial experience. Radiology. 2007;243:171–179. doi: 10.1148/radiol.2431060625. [DOI] [PubMed] [Google Scholar]
- Dorrell C, Grompe M. Liver repair by intra- and extrahepatic progenitors. Stem Cell Rev. 2005;1:61–64. doi: 10.1385/SCR:1:1:061. [DOI] [PubMed] [Google Scholar]