ABSTRACT
Segmental arterial mediolysis (SAM) is a nonatherosclerotic, noninflammatory arteriopathy, which is characterized by dissecting aneurysms resulting from lysis of the outer media of the arterial wall. The most common presentation is abdominal pain and hemorrhage in the elderly. Computed tomography (CT) and angiography imaging findings overlap with various vasculitides and include segmental changes of aneurysm and stenosis. A key distinguishing feature is the presence of dissections, the principle morphologic expression of SAM. Differentiation and exclusion of an inflammatory arteritis is crucial in appropriate management, as immunosuppressants generally used for treatment of vasculitis may be ineffective or even worsen the vasculopathy. Although the disease can be self-limiting without treatment or with conservative medical therapy, the acute process carries a 50% mortality rate and may necessitate urgent surgical and/or endovascular therapy. Prompt recognition and diagnosis are therefore of utmost importance in appropriate management of this rare entity.
Keywords: Segmental arterial mediolysis, imaging findings
The first cases of a distinct arterial pathology involving the intraabdominal arteries were described by Slavin and Gonzales-Vitale in 1976 and termed segmental mediolytic arteritis.1 The same histologic appearance of arterial “medial necrosis” reported by Gruenwald in the coronary arteries of newborns in 1949 is now recognized to be the same pathologic process and considered to be the first report of this entity.2 Since those early reports, SAM has been further characterized as a nonatherosclerotic, nonhereditary vasculopathy with an absence of an inflammatory component. The last characteristic is in contradistinction to the other arteritides and has led to the current terminology of segmental arterial mediolysis.3,4,5,6,7
The underlying pathogenesis of SAM is unknown, although repeated vasoconstrictor stimuli have been associated with the histologic finding of medial lysis in vessel walls, the hallmark of SAM.4,8 Histologic findings of SAM also overlap with fibromuscular dysplasia (FMD), leading some authors to propose that SAM may represent a variant of FMD5,9,10 or a precursor of certain types of FMD.4,11 However, SAM exists as a distinct clinical entity that manifests most commonly in the late middle-age and elderly population as acute abdominal pain and/or hemorrhage resulting from arterial rupture or dissection from the weakened arterial wall.1,2,3,4,12 Because (1) angiography is usually not part of the routine workup of patients with abdominal pain, and (2) the disease can be acute and self-limiting such that an imaging workup may not ever be pursued, the incidence of SAM is probably underestimated.13,14 The more widespread use of computed tomography (CT) and CT angiography (CTA) in the evaluation of abdominal pathologies is likely to aid in increased recognition of this entity.
Although histologic confirmation is the gold standard, it is usually unavailable and the diagnosis of SAM is established by the presence of characteristic imaging findings and clinical and laboratory exclusion of other differentials.
PATHOPHYSIOLOGY
The observation that patients with SAM commonly have had a clinical history of hypoxia, shock, or other vasoconstrictor stimuli such as Raynaud's disease, migraine, recent surgery, stroke, hypertension, pulmonary hypertension, or exogenous pressor agents, has led to the belief that repeated vasoconstrictive responses in the splanchnic vascular bed may result in arterial mediolysis.4,8 This is further supported by the finding that arteries with chronic vasospasm have histologic similarities to SAM.1,3,4,7
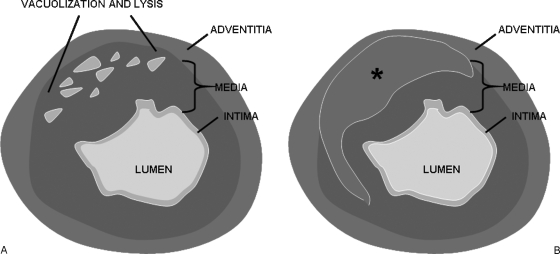
The four diagnostic lesions of SAM as outlined by Slavin et al, include mediolysis, separation, arterial gaps, and reparative fibrosis.1,3,4,11 Mediolysis refers to partial or total vacuolization and lysis of the outer arterial media (Fig. 1A). This leads to weakening of the outer layer of the media, formation of arterial gaps, patchy transmural loss of the external elastic lamina, and separation of the media from the adventitia. Ultimately, dissecting hematomas and aneurysms occur at the arterial gaps, which could result in sudden massive intraabdominal or retroperitoneal hemorrhage (Fig. 1B). Dissection or associated thrombi may also result in arterial luminal occlusion that is responsible for ischemia or infarction of endorgans such as the bowel or kidneys.3,5,6 The acute phase is then followed by a late or reparative phase in which there is exuberant granulation tissue that is eventually replaced by fibrosis and ultimately results in vessel remodeling and restoration of a smooth wall.3,15
Figure 1.
(A) Pathogenesis of segmental arterial mediolysis (SAM). Vacuolization and lysis of the outer arterial media leads to formation of arterial gaps and patchy transmural loss of the external elastic lamina. (B) Pathogenesis of SAM. Dissecting hematomas (asterisk “*”) and aneurysms occur at the arterial gaps with separation of the media from the adventitia.
CLINICAL PRESENTATION
SAM most commonly afflicts the splanchnic arteries of the late middle-age and elderly population. Pooled studies by Slavin et al, and Inada et al show the age distribution to range from the fourth to the ninth decades, with an average age of 60.5 years in Inada's study.11,12 No gender difference was noted.12 SAM may be clinically silent, or when symptomatic, usually manifests as abdominal apoplexy—a syndrome consisting of abdominal pain and distension, rapidly decreasing hematocrit, and hypovolemic shock. Associated hypotension, ischemic bowel, guaiac-positive stools, and vomiting may be present.11,12,16,17 Less commonly, patients may present with hematuria or acute flank pain if the renal artery is involved,12,18 or hemobilia secondary to erosion of an aneurysm into the biliary tract.12
SAM has been found less commonly in the coronary arteries of newborns exhibiting hypoxia or asphyxia associated with in utero or immediate postpartum fetal distress.2,3,4 SAM has also been reported in the cerebral arteries of young adults. Those patients present with intensive headache for 12 to 48 hours prior to a sudden cerebrovascular accident, or present with other symptoms including dizziness, episodes of hemicrania, and visual disturbances.7,17
IMAGING FINDINGS
This article will focus discussion of the imaging findings of SAM on those of abdominal visceral involvement, as it is the most common presentation of this rare entity. However, similar radiologic appearances are also seen in the nonsplanchnic arteries that are involved, such as the carotid artery,17 because the imaging findings reflect the histologic changes of the disease.
The imaging findings of SAM overlap extensively with those of the vasculitides, which occur much more commonly in the general population. Unfortunately, the radiologic appearance of SAM is sometimes indistinguishable from other small-to-medium-sized vasculitides such as polyarteritis nodosa and Wegener's granulomatosis.16 Luckily, most vasculitides can be diagnosed or excluded on the basis of associated clinical or laboratory data. Otherwise, imaging clues that help allow differentiation of SAM from other vasculitides include its distribution of involvement, its hallmark radiologic characteristics, and associated secondary findings.
SAM most commonly involves the large abdominal aortic branches, considered to be medium-sized vessels.8,19 A review of 23 cases by Slavin et al, revealed that it involved the artery itself and/or branches of the celiac artery in 60%, the superior mesenteric artery in 17%, the renal arteries in 14%, and the inferior mesenteric artery in 9%.4 A review of 27 cases by Inada et al, revealed the middle colic artery to be the most frequently involved (50%) followed by gastric and gastroepiploic arteries.11 Involvement of the iliac arteries has been reported by at least one author.15
There may be between a few to numerous lesions affecting an artery in a segmental, skip pattern.1 One or more arteries may be involved simultaneously or at different times.4,8 The involved segments may demonstrate circumferential involvement or involvement of only a portion of the arterial wall.4
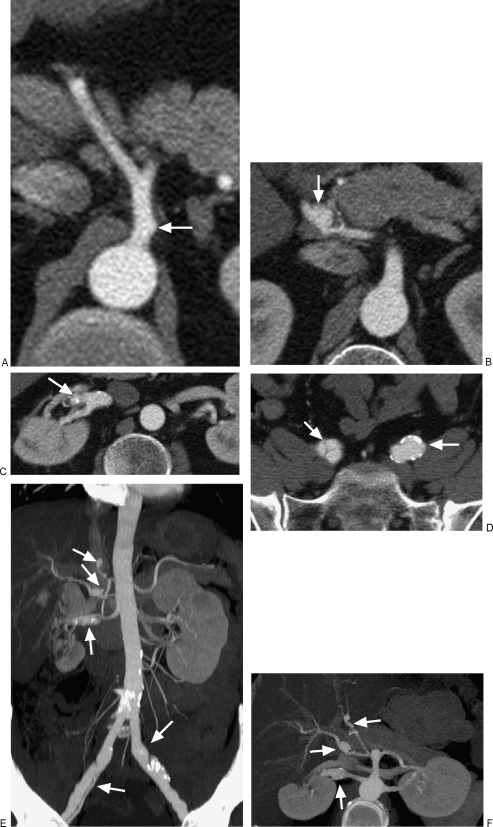
On CTA, magnetic resonance angiography (MRA), or catheter angiography, the classic appearance of alternating aneurysms and stenoses–“beading,” irregularity of the vascular wall, and occlusions is seen, often indistinguishable from vasculitis.13,16,20,21 However, the principle radiologic hallmark of SAM is the greater prevalence of dissecting aneurysms.6,12 A study by Inada et al found dissections in 78% of cases and pseudoaneurysms in the remainder of cases. 33% of cases had multiple aneurysms (Fig. 2).11 Dissection of the peripheral arteries that are unrelated to the aorta is rare, and its presence should suggest a diagnosis of SAM.6,11,12,18 Aneurysms may be fusiform or saccular10; focal areas of vascular dilation with regions of stenosis proximally may actually represent dissection or dissecting hematoma (Fig. 3).17,18 Affected arteries may become elongated and kinked. In contradistinction to mycotic aneurysms, there is no preferential involvement of a bifurcating site.1,3,14
Figure 2.
(A) Multiple simultaneous visceral arterial lesions in segmental arterial mediolysis (SAM). Axial contrast-enhanced computed tomography (CT) scan demonstrates focal pseudoaneurysm of the celiac artery (arrow). (B) Multiple simultaneous visceral arterial lesions in SAM. Axial contrast-enhanced CT scan demonstrates a dissecting aneurysm of the common hepatic artery (arrow). (C) Multiple simultaneous visceral arterial lesions in SAM. Axial contrast-enhanced CT scan demonstrates a fusiform dissecting aneurysm of the distal right renal artery (arrow). (D) Multiple simultaneous visceral arterial lesions in SAM. Axial contrast-enhanced CT scan demonstrates a right common iliac dissection and saccular aneurysm of the left common iliac artery (arrows). (E) Multiple simultaneous visceral arterial lesions in SAM. Coronal maximal intensity projection (MIP) image better demonstrates the multiplicity of dissecting aneurysms throughout the abdomen and pelvis (arrows). (F) Multiple simultaneous visceral arterial lesions in SAM. Axial MIP image better demonstrates the multiplicity of dissecting aneurysms throughout the abdomen (arrows).
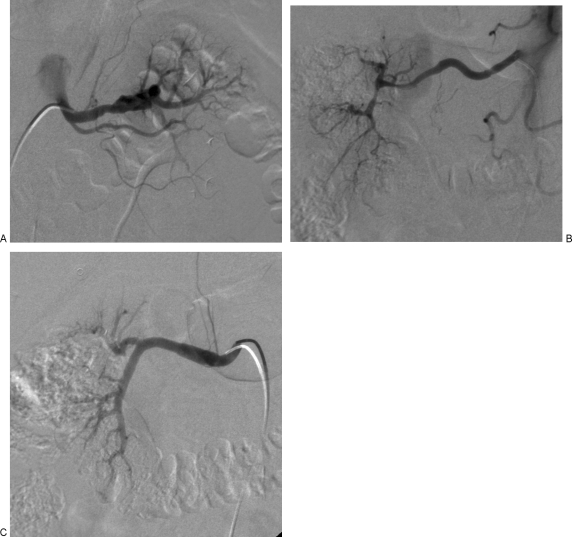
Figure 3.
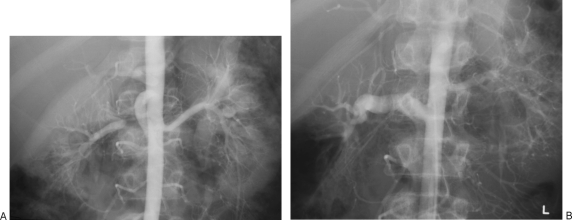
(A) Angiographic appearance of segmental arterial mediolysis (SAM). Catheter angiography demonstrates segmental alternating fusiform and saccular appearance of the distal left renal artery, representing dissecting aneurysms. (B) Angiographic appearance of SAM. Catheter angiography demonstrates segmental stenoses and irregularity of the distal right renal artery with resultant occlusion of the interlobar and interlobular arteries. (C) Angiographic appearance of SAM. Catherter angiography demonstrates fusiform enlargement of the right renal artery, representings a dissecting aneurysm.
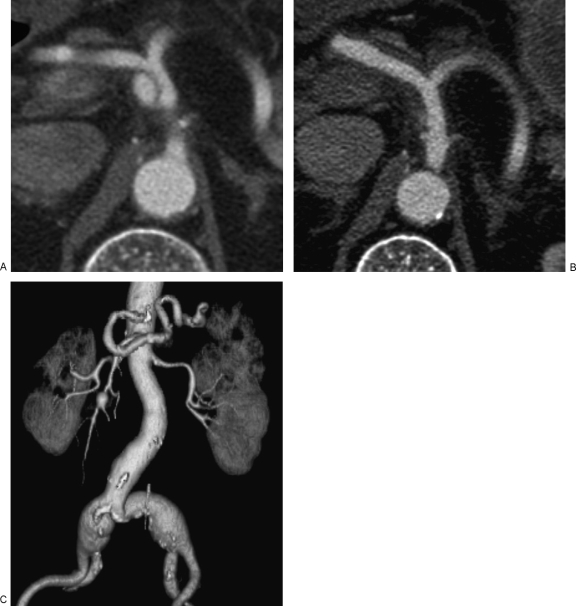
CT offers the additional advantages of detecting omental, mesenteric, or retroperitoneal hematomas or secondary findings of endorgan injury, such as bowel wall thickening, pneumatosis coli, pancreatitis, and renal or splenic infarction (Fig. 4).1,4,20,22,23,24 CT is also more sensitive for detecting arterial wall thickening associated with dissection (Fig. 5). Evaluation with a multidetector row CT with three-dimensional (3D) reconstruction has been advocated due to its high temporal and spatial resolution.24 A comparison of CTA and catheter angiography concluded that CTA was sufficient for a radiologic diagnosis of SAM.14
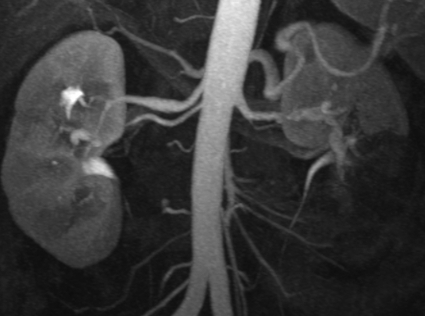
Figure 4.
Endorgan injury in segmental arterial mediolysis (SAM). Coronal maximal intensity projection image demonstrates a wedge-like area of nonenhancement of the left renal lower pole, consistent with renal infarction. Note the associated irregularity and dissecting aneurysms involving the distal bilateral renal arteries, better seen on corresponding angiographic exam (Fig. 3).
Figure 5.
(A) Dissecting hematomas in segmental arterial mediolysis (SAM). Axial contrast-enhanced computed tomography (CT) scan demonstrates a dissecting aneurysm involving the celiac artery. (B) Dissecting hematomas in SAM. Axial contrast-enhanced CT scan is more sensitive for depiction of diffuse arterial wall thickening in the common hepatic and splenic arteries representing dissecting hematomas. (C) Dissecting hematomas in SAM. Coronal three-dimensional reconstructed image in the same patient demonstrates additional saccular aneurysms in the splenic and superior mesenteric arteries as well as fusiform aneurysms involving the bilateral common iliac arteries.
Nevertheless, catheter angiography still plays a role in the diagnosis and management of SAM. Albeit rare, an associated arteriovenous fistula may be detected more easily by traditional angiography. Furthermore, catheter angiography allows for the possibility of endovascular treatment at the time of diagnosis.
DIFFERENTIAL DIAGNOSES
Many vasculopathies exhibit similar radiologic findings as SAM. However, most can be excluded on clinical grounds or by laboratory abnormalities. SAM may mimic small- to medium-sized vasculitides such as polyarteritis nodosa (PAN), Kawasaki disease, primary granulomatous central nervous system vasculitis, Wegener granulomatosis, Churg-Strauss syndrome, microscopic polyangiitis, Henoch-Schönlein syndrome, systemic lupus erythematosus, rheumatoid vasculitis, and Behçet syndrome.14,19,25 However, not only do those vasculitides demonstrate abnormal elevations of inflammatory and immune markers including C-reactive protein, erythrocyte sedimentation rate, antinuclear antibodies, antineutrophil cytoplasmic antibodies, rheumatoid factor, and presence of immune complexes, they often also demonstrate other laboratory abnormalities such as anemia, leukocytosis, thrombocytosis, reduced serum albumin concentration, and abnormal urinalysis results.10,25
Although multiple visceral aneurysms should raise concern for infectious etiologies such as mycotic aneurysms, the lesions in SAM are randomly distributed and do not demonstrate the predilection for vascular bifurcations seen with mycotic aneurysms8,14 or an associated systemic infection. Several degenerative and connective tissue vasculopathies such as cystic medial necrosis and cystic adventitial artery disease are also in the differential, but exhibit distinct clinical presentations. Cystic medial necrosis usually involves larger arteries and is seen in patients with Marfan's disease, and cystic adventitial artery disease involves the extremity arteries of younger adult men. Histologically, both entities contain mucopolysaccharides, which stain positive on ground substance stains such as alcian blue, a characteristic not exhibited by SAM.1,13
Fibromuscular dysplasia (FMD) is the one vasculopathy that is most difficult to differentiate from SAM. Because the histologic findings of SAM are so similar to FMD, the imaging findings of SAM involving the renal arteries are likewise very similar. However, despite the radiologic and histologic similarities, the two entities exhibit a different clinical profile in terms of age of onset, gender, distribution of affected arteries, and clinical symptoms. FMD afflicts young to middle-aged women, is rarely painful, and is usually asymptomatic or associated with symptoms of occlusive disease and rarely rupture. Furthermore, involvement of the renal and internal carotid arteries is more common than the visceral arteries, whereas the converse is true in SAM. In fact, FMD of the extrarenal visceral arteries is infrequent and only accounts for 9% of all FMD.5,11,14,18
NATURAL HISTORY AND TREATMENT
The acute phase of SAM carries a 50% mortality rate most commonly due to acute intraabdominal hemorrhage.13,20 With the exception of a case in which a patient was well without recurrence 9 years after surgical resection of a middle colic artery aneurysm,11 the long-term natural history of the disease has yet to be defined, as only medium range follow-up between a few months and 4 years is available. Case reports have indicated complete resolution of the vascular abnormalities ranging from 2 months21 to 4 years,14 partial resolution from 5 months16 and 9 months,14 stabilization of disease at 3 years,14 and development of new vascular abnormalities such as aneurysms or dissection from 9 months10 to 1.5 years8 following treatment of an initial lesion.
Given the “spatiotemporal character” of SAM evidenced by the diverse timeline of its progression, stabilization, and regression, and the potential for the development of new aneurysms in new vascular territories or the rupture of existing aneurysms, some authors advocate long-term imaging evaluation8 or even short-interval follow-up to monitor the rapid transformation of vascular morphology.10
Treatment of SAM has been tailored to each distinct clinical presentation. Commonly, laparotomy with urgent segmental resection of affected bowel, or ligation or excision of an aneurysm is performed.26 Surgical reconstruction with autologous grafts has also been reported (Fig. 6).17 Recently, successful transcatheter embolization with coils and N-butyl cyanoacrylate (glue utilized due to vascular tortuosity precluding microcatheter placement into the aneurysm sac) in four patients was reported by Shimohira et al, who argue for embolization of incidentally found SAM due to the high mortality associated with acute rupture.10 Another author reported treatment with coil embolization of hepatic aneurysms greater than 1 cm due to greater likelihood for pain, hemobilia, and rupture.16 Soulen et al reported balloon angioplasty of renal artery stenosis for renovascular hypertension, which was successful in decreasing the patient's hypertension. The patient was initially felt to have FMD, but at time of angiography was found to have multiple abnormalities involving the splenic artery, common hepatic artery, and both renal and iliac arteries, supporting a diagnosis of SAM. The authors emphasized that specific attention was directed to not dilating beyond the nominal diameter of the adventitia to avoid rupture of an already weakened artery.15 The role of endovascular exclusion or stent-grafts has yet to be determined; there are no reports to date (to my knowledge).
Figure 6.
(A) Surgical treatment of segmental arterial mediolysis (SAM). Catheter angiography reveals a segment of severe stenosis involving the proximal right renal artery. Clinical and radiologic findings were consistent with SAM. (B) Surgical treatment of SAM. Catheter angiography performed after autologous vein bypass grafting of the segment of stenosis demonstrates a widely patent right renal artery.
Medical therapy including control of hypertension may be indicated in patients with existing aneurysms and a lifetime risk of rupture.15 Patients may need to be evaluated for gestational factors and portal hypertension to assess their influence on the development of aneurysms and the risk of rupture.26 Inappropriate treatment with steroids or immunosuppressants should also be avoided as it not only ineffective, but may result in adverse outcomes.27
In the absence of a better understanding of the natural history of SAM and given the high mortality associated with the acute phase, some authors advocate early diagnosis, prompt resuscitation, and early intervention.10,20,26 In patients without initial bleeding complications or those with aneurysms of small size, a more conservative approach with CTA follow-up may be a considered.14
CONCLUSION
SAM is a nonarteriosclerotic, noninflammatory vasculopathy characterized most distinctively by the presence of dissecting aneurysms in the setting of alternating aneurysms and stenoses. Although histology is the gold standard, the diagnosis is established by the presence of imaging findings and the clinical and laboratory exclusion of other differentials. Vigilance for this entity in the evaluation of acute abdominal pain in the late middle-age and elderly population may allow prompt and accurate diagnosis and facilitate appropriate treatment and management. Although treatment has been historically surgical, increasing experience with endovascular therapy and a better understanding of the natural history of the disease may permit a greater role for minimally invasive therapy in the future. The need for short-interval or long-term imaging follow-up of the disease entity has yet to be confirmed, but may be beneficial.
ACKNOWLEDGMENTS
The author would like to thank Dr. Sanjeeva Kalva, Assistant Professor at Massachusetts General Hospital, for the invitation and idea to write on this topic as well as for providing case examples for inclusion in this article. The author also expresses gratitude to Ms. Carrie Walsh, Assistant Medical Librarian at the Kaiser Permanente Medical Center, for her help in the literature search.
REFERENCES
- Slavin R E, Gonzalez-Vitale J C. Segmental mediolytic arteritis: a clinical pathologic study. Lab Invest. 1976;35:23–29. [PubMed] [Google Scholar]
- Gruenwald P. Necrosis in the coronary arteries of newborn infants. Am Heart J. 1949;38(6):889–897. doi: 10.1016/0002-8703(49)90889-3. [DOI] [PubMed] [Google Scholar]
- Slavin R E, Cafferty L, Cartwright J., Jr Segmental mediolytic arteritis. A clinicopathologic and ultrastructural study of two cases. Am J Surg Pathol. 1989;13(7):558–568. [PubMed] [Google Scholar]
- Slavin R E, Saeki K, Bhagavan B, Maas A E. Segmental arterial mediolysis: a precursor to fibromuscular dysplasia? Mod Pathol. 1995;8(3):287–294. [PubMed] [Google Scholar]
- Armas O A, Donovan D C. Segmental mediolytic arteritis involving hepatic arteries. Arch Pathol Lab Med. 1992;116(5):531–534. [PubMed] [Google Scholar]
- Inayama Y, Kitamura H, Kitamura H, Tobe M, Kanisawa M. Segmental mediolytic arteritis. Clinicopathologic study and three-dimensional analysis. Acta Pathol Jpn. 1992;42(3):201–209. [PubMed] [Google Scholar]
- Leu H J. Cerebrovascular accidents resulting from segmental mediolytic arteriopathy of the cerebral arteries in young adults. Cardiovasc Surg. 1994;2(3):350–353. [PubMed] [Google Scholar]
- Hashimoto T, Deguchi J, Endo H, Miyata T. Successful treatment tailored to each splanchnic arterial lesion due to segmental arterial mediolysis (SAM): report of a case. J Vasc Surg. 2008;48(5):1338–1341. doi: 10.1016/j.jvs.2008.05.056. [DOI] [PubMed] [Google Scholar]
- Lie J T. Segmental mediolytic arteritis. Not an arteritis but a variant of arterial fibromuscular dysplasia. Arch Pathol Lab Med. 1992;116(3):238–241. [PubMed] [Google Scholar]
- Shimohira M, Ogino H, Sasaki S, et al. Transcatheter arterial embolization for segmental arterial mediolysis. J Endovasc Ther. 2008;15(4):493–497. doi: 10.1583/08-2384.1. [DOI] [PubMed] [Google Scholar]
- Inada K, Maeda M, Ikeda T. Segmental arterial mediolysis: unrecognized cases culled from cases of ruptured aneurysm of abdominal visceral arteries reported in the Japanese literature. Pathol Res Pract. 2007;203(11):771–778. doi: 10.1016/j.prp.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Slavin R E, Inada K. Segmental arterial mediolysis with accompanying venous angiopathy: a clinical pathologic review, report of 3 new cases, and comments on the role of endothelin-1 in its pathogenesis. Int J Surg Pathol. 2007;15(2):121–134. doi: 10.1177/1066896906297684. [DOI] [PubMed] [Google Scholar]
- Heritz D M, Butany J, Johnston K W, Sniderman K W. Intraabdominal hemorrhage as a result of segmental mediolytic arteritis of an omental artery: case report. J Vasc Surg. 1990;12:561–565. doi: 10.1067/mva.1990.24040. [DOI] [PubMed] [Google Scholar]
- Michael M, Widmer U, Wildermuth S, Barghorn A, Duewell S, Pfammatter T. Segmental arterial mediolysis: CTA findings at presentation and follow-up. AJR Am J Roentgenol. 2006;187(6):1463–1469. doi: 10.2214/AJR.05.0281. [DOI] [PubMed] [Google Scholar]
- Soulen M C, Cohen D L, Itkin M, Townsend R R, Roberts D A. Segmental arterial mediolysis: angioplasty of bilateral renal artery stenoses with 2-year imaging follow-up. J Vasc Interv Radiol. 2004;15(7):763–767. doi: 10.1097/01.rvi.0000133543.32123.dc. [DOI] [PubMed] [Google Scholar]
- Ryan J M, Suhocki P V, Smith T P. Coil embolization of segmental arterial mediolysis of the hepatic artery. J Vasc Interv Radiol. 2000;11(7):865–868. doi: 10.1016/s1051-0443(07)61802-8. [DOI] [PubMed] [Google Scholar]
- Obara H, Matsumoto K, Narimatsu Y, Sugiura H, Kitajima M, Kakefuda T. Reconstructive surgery for segmental arterial mediolysis involving both the internal carotid artery and visceral arteries. J Vasc Surg. 2006;43(3):623–626. doi: 10.1016/j.jvs.2005.11.033. [DOI] [PubMed] [Google Scholar]
- LaBerge J, Kerlan R L. SCVIR Annual Meeting film panel sessions: case 3. Segmental arterial mediolysis (SAM) resulting in spontaneous dissections of the middle colic and left renal arteries and occlusion of SMA. Society of the Cardiovascular & Interventional Radiology. J Vasc Interv Radiol. 1999;10:509–513. doi: 10.1016/s1051-0443(99)70074-6. [DOI] [PubMed] [Google Scholar]
- Molloy E S, Langford C A. Vasculitis mimics. Curr Opin Rheumatol. 2008;20(1):29–34. doi: 10.1097/BOR.0b013e3282f1dcf2. [DOI] [PubMed] [Google Scholar]
- Rengstorff D S, Baker E L, Wack J, Yee L F. Intra-abdominal hemorrhage caused by segmental arterial mediolysis of the inferior mesenteric artery: report of a case. Dis Colon Rectum. 2004;47:769–772. doi: 10.1007/s10350-003-0103-9. [DOI] [PubMed] [Google Scholar]
- Sakano T, Morita K, Imaki M, Ueno H. Segmental arterial mediolysis studied by repeated angiography. Br J Radiol. 1997;70(834):656–658. doi: 10.1259/bjr.70.834.9227264. [DOI] [PubMed] [Google Scholar]
- Wang J JL, Huang T W. Ischemic colitis caused by an isolated dissecting aneurysm of the left colic artery: a presumed case of segmental mediolytic arteriopathy. J Formos Med Assoc. 1994;93(8):715–720. [PubMed] [Google Scholar]
- Phillips C K, Lepor H. Spontaneous retroperitoneal hemorrhage caused by segmental arterial mediolysis. Rev Urol. 2006;8(1):36–40. [PMC free article] [PubMed] [Google Scholar]
- Frauenfelder T, Wildermuth S, Marincek B, Boehm T. Nontraumatic emergent abdominal vascular conditions: advantages of multi-detector row CT and three-dimensional imaging. Radiographics. 2004;24(2):481–496. doi: 10.1148/rg.242025714. [DOI] [PubMed] [Google Scholar]
- Ha H K, Lee S H, Rha S E, et al. Radiologic features of vasculitis involving the gastrointestinal tract. Radiographics. 2000;20:779–794. doi: 10.1148/radiographics.20.3.g00mc02779. [DOI] [PubMed] [Google Scholar]
- Abdelrazeq A S, Saleem T B, Nejim A, Leveson S H. Massive hemoperitoneum caused by rupture of an aneurysm of the marginal artery of Drummond. Cardiovasc Intervent Radiol. 2008;31(Suppl 2):S108–S110. doi: 10.1007/s00270-007-9117-3. [DOI] [PubMed] [Google Scholar]
- Lie J T. Systemic, cerebral and pulmonary segmental mediolytic arteriopathy: villainous masqueraders of vasculitis. Cardiovasc Pathol. 1996;5:305–314. doi: 10.1016/s1054-8807(96)00071-3. [DOI] [PubMed] [Google Scholar]