ABSTRACT
Visceral artery aneurysms (VAAs) and visceral artery pseudoaneurysms (VAPAs) frequently present as life-threatening emergencies. VAAs are now being diagnosed with increasing frequency, related to routine use of magnetic resonance imaging (MRI), computed tomography (CT), and ultrasound. Both surgery as well as endovascular techniques are well established in their management. Endovascular management includes transarterial deployment of coils, N-butyl cyanoacrylate, or stent grafts. Direct percutaneous embolization of visceral aneurysms and pseudoaneurysms may also be performed. Special attention to aneurysmal etiology—congenital, atherosclerotic, infectious, and inflammatory is outlined. Advances in endovascular management with various aneurysmal isolation techniques are discussed. It is concluded that percutaneous endovascular management, now offers a safe and effective alternative to conventional surgery with lower procedural morbidity and mortality and high technical success rates.
Keywords: Visceral artery aneurysms, endovascular, surgery, aneurysmal rupture
Visceral artery aneurysms (VAAs) are rare with a reported incidence of 0.01 to 0.2% on routine autopsies.1 However, VAAs are clinically important and potentially lethal; 22% of all visceral artery aneurysms present as clinical emergencies; 8.5% result in death.1 VAAs include both true aneurysms, limited by all three layers of the arterial wall, which undergo progressive dilation and wall thinning, and pseudoaneurysms (VAPAs), wherein there is a tear of the vessel wall and a periarterial hematoma.2 The diagnosis and incidence of VAAs and VAPAs have been steadily increasing due to the increased use of cross-sectional imaging for various intraabdominal pathologies, and increased instrumentation of the biliary tract, and placement of intravascular catheters for regional cancer therapy.1 In contrast to splenic artery aneurysms, which are historically the most common VAAs, hepatic artery aneurysms (HAAs), are the most frequently reported VAAs during the past two decades, owing to the growing use of percutaneous biliary procedures, liver transplantation, and nonoperative management of blunt trauma to the abdomen.1
Most VAAs are secondary to vessel wall degeneration, and demonstrate deficiency of the arterial media with loss and/or fragmentation of the elastic fibers and reduced smooth muscle. Arteriosclerosis, congenital syndromes, fibromuscular dysplasia, gestational alterations, and collagen disorders are other possible causes of VAAs. Pseudoaneurysms can develop as a result of blunt or penetrating trauma, inflammation, infection, vasculitis, and iatrogenic trauma secondary to surgical, endoscopic, and radiologic procedures.2
Typically, surgery or endovascular management is considered for VAAs when they are larger than 2 cm in diameter, demonstrate rapid growth, and when patients present with symptoms attributable to the aneurysm. In addition, therapy is often advocated for VAAs in women of childbearing age, pregnant women, and liver transplant recipients irrespective of their size and presence of symptoms.3 However, considering the natural history of the VAAs and the risk of rupture, there is a general agreement in the literature to treat these lesions even when they are asymptomatic.4
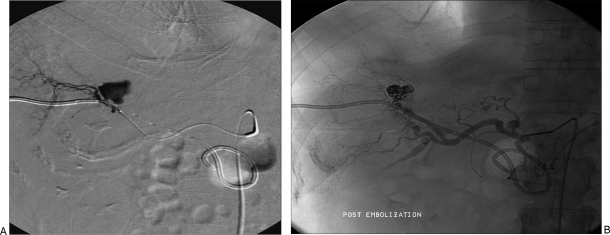
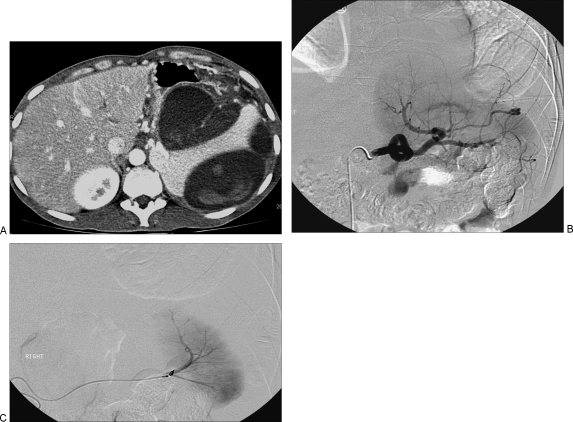
VAPAs are distinguished from the true aneurysms by clinical and imaging criteria. Clinically, the patients with VAPAs, typically present with an antecedent history of arterial trauma, intraabdominal or, retroperitoneal inflammation, malignancy, or biliary tract manipulation. Imaging demonstrates focal arterial disruption in the setting of an otherwise normal artery. Presence of perivascular inflammation in the setting of an irregular aneurysmal wall also suggests a pseudoaneurysm (Fig. 1).
Figure 1.
(A) Hepatic artery pseudoaneurysm, secondary to placement of an internal–external biliary drainage catheter. (B) Successful coil embolization was performed.
Aneurysmal morphology favorable for endovascular therapy include saccular aneurysms with a narrow neck, aneurysms with adequate collateral flow, and aneurysms of vessels that are not the only source of blood supply to that organ.4 Patients are considered candidates for endovascular treatment if inflow and outflow vessels to and from the aneurysm can be accessed and occluded by a catheter-based system and if end organ perfusion can be preserved by collateral flow or stent graft therapy.3 Mortality rates after elective treatment of VAAs is estimated to be 5%.
Management depends in part on the location of the aneurysm, with an endovascular approach being most appropriate for aneurysms involving the parenchymal branches of the hepatic, splenic, renal, or pancreaticoduodenal arteries.2 Pseudoaneurysms should be treated with proximal and distal coil embolization, whereas true aneurysms are treated with coil packing, preserving the native arterial circulation. Arterial patency may be preserved in saccular aneurysms in which catheterization of the neck allows embolization to be limited to the sac. This may be performed using coils, glue, thrombin, or by placement of a stent graft. Fusiform aneurysms involving bifurcations, require endovascular exclusion with placement of coils in the efferent and afferent arteries, to obtain complete occlusion. In these cases, perfusion of the end organ can be at least partly maintained by collateral flow.
Surgical and endovascular treatment of VAAs share the common goal of preventing aneurysm expansion and rupture. This goal is best accomplished by excluding the aneurysm from the arterial circulation and pressure. In the presence of adequate collateral flow, surgical management consists of ligation of the parent artery proximal and distal to the aneurysm. This approach is appropriate for splenic artery aneurysms (SAAs) with collateral flow from the short gastric and gastroepiploic arteries to the distal splenic artery and spleen. The celiac trunk, proximal superior mesenteric artery (SMA), and common hepatic artery (CHA) may also be ligated, with collateral flow provided by the pancreaticoduodenal and gastroduodenal arteries. For proper hepatic artery (PHA) and main renal artery (MRA) that lack collateral flow, and for celiac artery and SMA with inadequate collateral flow, ligation must be accompanied by arterial bypass surgery.2
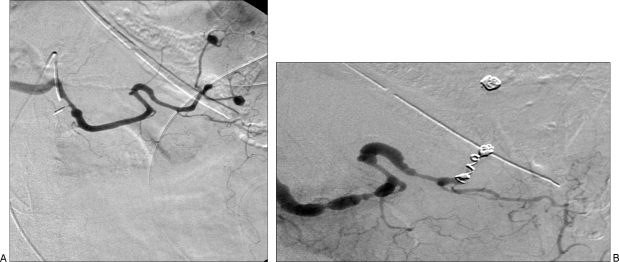
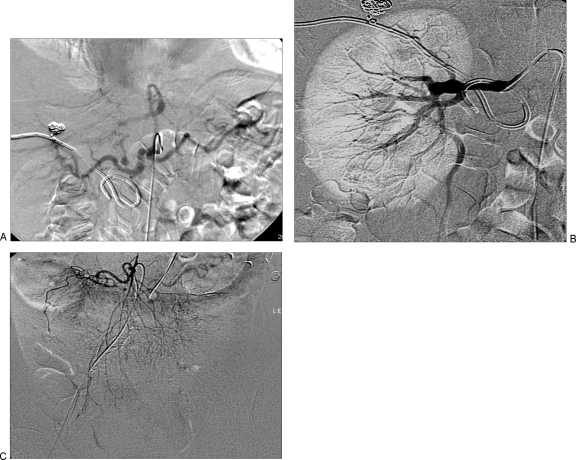
Aneurysm isolation can be accomplished in several ways. For aneurysms involving large arteries (e.g., the splenic artery), the aneurysm can be “trapped” between coils placed in the parent artery distal and then proximal to the aneurysm, thereby eliminating both antegrade flow and the potential for retrograde flow to the aneurysm. For aneurysms involving smaller arteries, the distal parent artery or its branches can be occluded with large particles, followed by coil placement in the larger proximal parent artery, again trapping the aneurysm and isolating it from the circulation (Fig. 2).5 Care must be taken to ensure that any collateral vessels that might support continued perfusion of the aneurysm are occluded with either coils or particles.2
Figure 2.
(A, B) Multiple aneurysms of the splenic artery treated with coil embolization of the proximal main splenic artery.
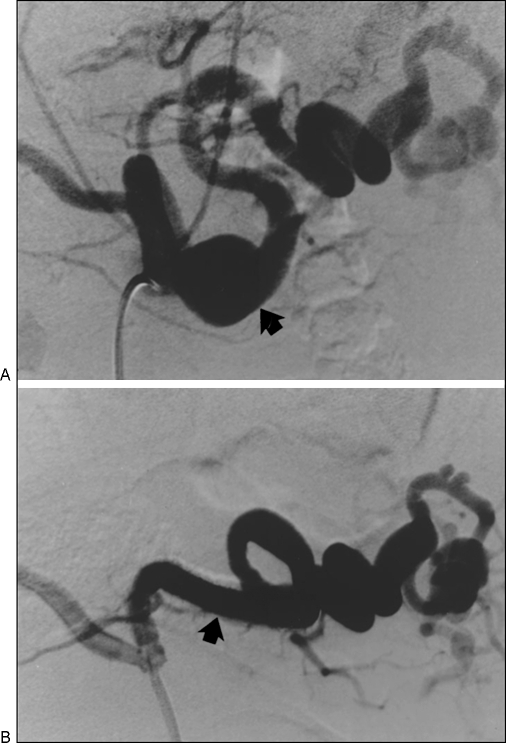
Covered stents provide another means of excluding VAAs from the circulation. These stents are reserved for major branches of the visceral or splanchnic arteries for which preservation of arterial perfusion is required.6 Limitations of covered stents include delivery systems, the size and rigidity of which preclude stent placement in distal tortuous branches. Covered stents are usually reserved for arteries 6 mm or larger in diameter because of the risk of thrombosis when placed in smaller vessels (Fig. 3).2
Figure 3.
(A) Broad-necked aneurysm (arrow) at the junction of the proximal and mid third of the splenic artery. (B) Treated with placement of a covered stent (arrow).
For saccular aneurysms, coil occlusion (as applied to berry aneurysms, for example) provides another option. If the aneurysm has a narrow neck, the coils are constrained within the aneurysm sac by the narrow neck. For wider neck aneurysms, stent-assisted coil placement is appropriate. A stent is first placed across the neck of the aneurysm, followed by the insertion of coils into the aneurysm sac through the interstices of the stent, which in turn constrain the coils within the sac. Occasionally, glue or thrombin is injected into the coil-filled sac to hasten thrombosis.2
Thrombin injection, as used in femoral artery pseudoaneurysms has been adopted for treatment of VAAs after a failed endovascular approach or as an alternative to endovascular treatment. Under computed tomography (CT) or (preferably) ultrasound (US) guidance, a 20- or 22-gauge needle is introduced into the pseudoaneurysm and thrombin is injected until there is cessation of flow. This approach is most useful for small peripheral traumatic pseudoaneurysms of the liver, spleen, or kidney and has reportedly been used to treat pancreaticoduodenal aneurysms after endovascular treatment has failed.2 Although complete exclusion of the aneurysm from the arterial circulation guarantees successful treatment, coil occlusion or thrombin-induced thrombosis does not necessarily help achieve this goal, with aneurysm expansion or recurrence remaining a possibility.2
Technical success of percutaneous transcatheter coil embolization techniques has been acceptable according to published series, the results varying from 67 to 92%.4 However, disappointment has been expressed concerning the prevention of pressure transmission to the aneurysmal wall that may be present even in successfully embolized aneurysms. Therefore, case reports of rupture after successful embolization and reperfusion at follow-up are encountered. Intraprocedural aneurysm rupture has also been reported. Another complication of transcatheter embolization is arterial thrombosis/ embolization that may result in organ infarction and subsequent infection.4 Also, because ablative endoluminal therapy requires highly radiodense materials, such as coils and glue, postoperative surveillance for aneurysm reperfusion and parent vessel patency are suboptimal.7
INFECTED ANEURYSMS
Infectious or inflammatory etiology is suspected when a soft tissue mass with stranding or gas adjacent to the aneurysm is noted on imaging. Infected aneurysms merit special consideration due to their proclivity to rapid expansion and rupture.8 Vascular trauma with inoculation of bacteria into the arterial wall is the most common etiology.9 Staphylococcus aureus is the most commonly cultured microorganism followed by various species of Streptococcus.8 In a few cases, multiple organisms may be cultured and in up to 25% of cases, no causative organism is found. Septic emboli secondary to bacterial endocarditis are a well-known cause of infected aneurysms, and may affect multiple sites (Fig. 4).9 Therefore, cross-sectional imaging of the head and the abdomen is required preoperatively. Underlying substance abuse is usually the cause of endocarditis in the absence of immunosuppression or poor dentition.8 Along with antibacterial therapy, resection of the infected aneurysm is usually required. In cases of infected splenic artery aneurysms, surgical excision of the aneurysms well as concomitant splenectomy is advocated. In addition, bacterial endocarditis may produce infarcts or abscesses in the spleen, leading to its spontaneous rupture.9 The role of endografts here is yet to be defined because of the potential for subsequent infection of the endograft.
Figure 4.
(A) A computed tomography (CT) scan of the abdomen demonstrating multiple foci of septic emboli, in a patient with bacterial endocarditis. (B) On angiography, note is made of an infectious pseudoaneurysm from the third-order branches of the right hepatic artery. (C) This was treated with transcatheter coil embolization.
INFLAMMATORY ANEURYSMS
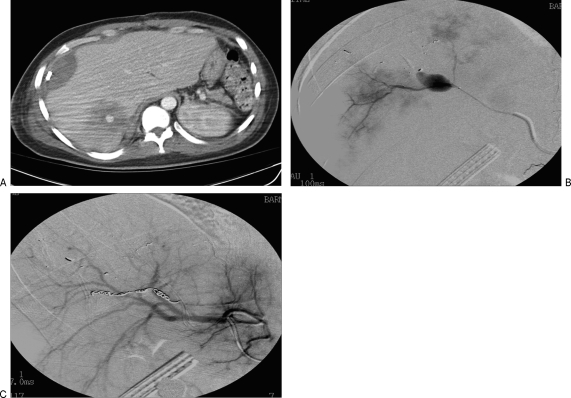
Inflammatory aneurysms are rare but a serious complication of pancreatitis, having an incidence of 15% of all VAAs. Direct bleeding into the gastrointestinal tract, an associated pseudocyst, pancreatic duct, or retroperitoneum may occur. Upon rupture, a mortality rate of 37% is reported. Severity of pancreatitis does not correlate with the occurrence of major hemorrhage, emphasizing the unpredictable nature of this complication. The pathogenesis involves uncontrolled severe inflammation causing necrosis and autodigestion of the pancreatic or peripancreatic artery including splenic, hepatic, gastroduodenal, and pancreaticoduodenal arteries.10 If an associated pseudocyst does not develop a thick wall, the pseudoaneurysm can rupture into the retroperitoneum or freely into the peritoneal cavity. Diagnosis is usually made on cross-sectional imaging, where the extent of pancreatic disease, hemorrhage, and presence of a pseudocyst can be ascertained (Fig. 5).
Figure 5.
(A) A computed tomography (CT) scan of the abdomen demonstrates a large pancreatic pseudocyst with active extravasation from a splenic artery pseudoaneurysm into the pseudocyst. (B) At angiography, a pseudoaneurysm from the proximal splenic artery was noted. (C) This was treated by coil embolization.
Selective visceral artery angiography as well as transcatheter arterial embolization is emerging as the treatment of choice in localizing the source of bleeding and controlling hemorrhage in patients with bleeding from duodenal ulceration, visceral pseudoaneurysm, and postoperative hemorrhage. Empiric embolization is defined as therapeutic embolization of a vessel in which no active bleeding was identified on angiography, but the artery itself is attenuated or irregular and thought to be the likely site of bleeding in a case of clinically acute hemorrhage.10 The use of microcatheters facilitates highly selective catheterization of small and tortuous vessels for embolization in the peripancreatic territory. The embolic material is usually deployed upstream as well as downstream from the pseudoaneurysm, which is relatively safe in the case of pseudoaneurysms of the gastroduodenal artery. Aneurysms of the pancreaticoduodenal arteries are located deep within the pancreatic parenchyma and their detection at surgery can be unsuccessful even in an elective setting. Recurrent bleeding from new sites after successful embolization is known to occur in patients with necrotizing pancreatitis as ongoing inflammation can cause simultaneous enzymatic destruction of multiple vessels. These intense inflammatory changes make surgical control very challenging; such surgical options include ligation of the aneurysm, drainage of the pseudocyst, Roux en Y cystojejunostomy, or distal pancreatectomy.10 Despite successful treatment of the bleeding site in patients with acute severe pancreatitis, multiple organ dysfunction accounts for mortality in up to 90% of pancreatitis-related deaths.10
CONGENITAL ANEURYSMS
Arterial vasculopathy in von Recklinghausen's disease is well known, but infrequent. Arterial stenosis and dilatations in children with renal localizations predominate, with renovascular hypertension being the most common presentation. Less commonly, aneurysmal involvement of the intracranial arteries, supra-aortic trunks, thoracic aorta, pulmonary and visceral vessels occur. The pathogenesis involves fibrodysplastic changes in the media with segmental fragmentation of the vessel wall caused by atrophy of the muscularis and formation of saccular aneurysms. Rupture, though a potential complication seems rare. The cause of death in these patients is the coexistence of cerebral tumoral lesions.11
Ehlers-Danlos syndrome (EDS) is a rare, variably inherited disorder affecting connective tissue. The clinical triad of skin hyperelasticity, joint hypermobility, and increased skin fragility is diagnostic. The vascular system is affected specifically in EDS type IV, which accounts for 4% of cases. Pathologically, there is reduction or absence of type III collagen within the tunica media of the large elastic arteries. Patients are susceptible to both aneurysm formation and spontaneous rupture of nonaneurysmal vessels resulting from extreme vessel fragility. Patients present with abdominal pain and hypertension. Attributable to friability of native vessels, surgical repair is seldom recommended due to the risk of dehiscence and false aneurysms at the anastomotic sites even with modified surgical techniques.12 Endovascular treatment of these aneurysms, is therefore, highly desirable.
Periarteritis nodosa is a progressive disease of small- and medium-sized arteries presenting with small aneurysms of the renal, mesenteric, hepatic, and other arteries in the early course of the disease. The pathogenesis is fibrinoid necrosis of the small- and medium-sized arteries, likely related to immune complex disease. This pathologic process may result in edema and inflammation causing transmural necrosis of the arterial wall with stenosis, aneurysm formation and subsequent healing, resulting in disruption of blood supply to the abdominal organs leading to ischemia, perforation, or hemorrhage. Patients usually present with abdominal pain, melena, or hematemesis. The renal artery is most frequently involved. Aneurysmal rupture with perinephric hematoma presents with poor prognosis. Hepatic artery is the next most common associated artery, which may be complicated by free intraperitoneal rupture. Hematomas limited by the hepatic capsule may be observed if small and nonexpanding. The larger or expanding hematomas should be treated operatively on an emergent basis. Arteriography is invaluable in confirming the diagnosis and assessing the presence of additional aneurysms (Fig. 6). Regression of aneurysms after treatment with cytotoxic or immunosuppressive treatment is well documented. Repeat arteriography in 3 to 4 months can ascertain this regression. The diagnosis needs to be confirmed in any patient with massive hemorrhage or with a history of fever, arthralgia, weakness, abdominal pain, pleuritic chest pain or other clinical manifestation of periarteritis nodosa.13
Figure 6.
(A) Repeat angiography of the patient in Fig. 1 due to recurrent upper abdominal pain demonstrates multiple small aneurysms in the celiac axis, hepatic, left gastric, splenic arteries. (B) Aneurysmal dilatation of the right renal artery is noted. (C) Multiple aneurysms are noted along the inferior mesenteric artery branches. Clinicopathologic correlation confirmed polyarteritis nodosa.
Marfan's syndrome is an inherited connective tissue disorder that classically affects the skeleton, eyes, and cardiovascular structures. Patients with this syndrome almost invariably have medionecrosis of the aorta, which weakens its wall and causes dilatation of the aorta rather than a saccular aneurysm. Multiple visceral artery aneurysms are associated.
Wegener's granulomatosis is also associated with small microaneurysms of the visceral arterial arcade. This is extremely rare. Exceedingly rare aneurysms involving developmentally anomalous visceral arteries have been reported. These include celio-mesenteric anomaly and spleno-mesenteric trunk.14,15 Arterial degeneration of visceral arteries, particularly the middle colic artery has been associated with α-1 antitrypsin deficiency with reduced elastin content in the arterial aneurysmal walls. Only 20 such cases are reported in the English literature.16 Very rarely, focal absence of arterial media, similar to, but much more extensive that intracranial berry aneurysms has been identified, without evidence of inflammation or vasculitis. Embryological ischemia has been attributed to medial agenesis. When such pathologic information is documented, a diligent search for associated aneurysms with visceral, peripheral, cerebral, and coronary arteriography is advised.17
Technique of Percutaneous Transcatheter Embolization
Procedures are usually performed in the endovascular suite under local anesthesia and conscious sedation. The femoral artery is the preferential access site. Brachial artery is an option when femoral artery is inaccessible. A 5- or 6-French sheath is placed in the accessed artery. The celiac and superior mesenteric arteriograms are performed by injecting 30 to 50 mL of iodinated contrast material at the rate of 5 to 6 mL/second. A triaxial endovascular system is recommended with a 3-French microcatheter specifically designed for deployment of glue and 0.018” intravascular coils. In such a system, a sheath or guiding catheter is placed into the visceral vessel of interest. A 4-French or 5-French catheter is telescoped through the sheath, through which a microcatheter is used for glue/coil deployment. If glue polymerizes within the catheter, rapid exchange of the microcatheter is possible without losing access. The microcatheter is also useful for interventions at the distal aspect of the arterial bed and for selective embolization of a VAA/VAPA sac, with preservation of the parent artery.18
The U.S. Food and Drug Administration (FDA) has approved the use of N-butyl cyanoacrylate (NBCA) for preoperative ablation of the intracranial arteriovenous malformations, and there is documented benefit for extension of its use for the treatment of VAAs and endoleaks after endoluminal stent grafting. Depending on the desired rate of polymerization, NBCA is diluted with ethiodized oil, a polymerization retardant. When embolizing a vessel with high flow rates, where rapid in vivo polymerization is desired, a ratio of 1:1 oil to NBCA is used. In cases where the microcatheter tip is positioned distant from the desired site of polymerization, a greater volume of ethiodized oil is added, 2:1 or 3:1. The glue is used often, when access to the aneurysm's outflow vessel is unattainable. Liquid embolization is beneficial when ablating aneurysms with multiple outflow vessels or in a setting of persistent aneurysm flow despite coiling. It also allows for more controlled embolization of the entire diseased arterial segment. However, one must be aware, that liquid embolic agents have potential for distal embolization of nontarget sites, if the injection rate is too rapid or if polymerization time is prolonged. Other complications of transcatheter glue embolization include splenic abscess, rupture of the spleen, pneumonia, and septicemia.18 Glue embolization has been reported as an effective treatment for hemobilia.7
Another option for successful embolization of an aneurysm while preserving distal organ perfusion is stent-assisted coiling of the aneurysm. An uncovered metallic stent is selectively placed across the neck of the aneurysm to preserve flow within a visceral vessel. The aneurysm is cannulated through the interstices of the stent and embolized with microcoils. The stent also helps exclude the coils placed in the aneurysm from encroaching and compromising a nondiseased parent artery. Rarely, stents may be used to treat a hemodynamically significant dissection resulting from visceral artery cannulation. Postembolization angiography of both the SMA and CA is performed to ensure successful exclusion of the aneurysmal segment.
Postoperative imaging usually consists of CT angiography (CTA), MR angiography (MRA), duplex or a combination of these. MRA is the preferred modality, as image artifacts after coil embolization are less pronounced than on CT. Scan intervals at 1, 3, and 6 months after embolization are recommended. Postoperative imaging is assessed for sac flow, aneurysm size change, and adequacy of organ perfusion.
SPLENIC ARTERY ANEURYSMS
VAAs involve the splenic artery in 60 to 80% of cases.2 Since variations in hormonal milieu contribute to extracellular matrix degeneration, splenic artery aneurysms (SAAs) are more common in women.7 Most aneurysms are small (2 to 4 cm), saccular, asymptomatic and located in the mid to distal third of the splenic artery.2 Rarely noted, is aneurysmal degeneration of the entire artery (cirsoid aneurysm). Although there are multiple causes of SAAs, most are degenerative. Association with portal hypertension and pregnancy are well documented. Estrogen and progesterone receptors in the arterial wall and additive effects of relaxin, as well as the high-flow state associated with pregnancy further contribute to the deleterious effects.2 Pseudoaneurysms associated with pancreatitis and pancreatic pseudocysts are a frequent cause of SAAs. With regard to splenic artery pseudoaneurysms, size of the aneurysm sac is a poor predictor of rupture. Rates of rupture in a SAA is 2%, with a mortality rate of 36%.19 Rupture of an SAA during pregnancy, most often in the third trimester, is a catastrophic event, with reported maternal and fetal mortality rates of 70% and 90%, respectively.2
Patients with SAAs rupture usually present with left upper quadrant pain radiating to the subcapsular region, often resulting in hypotension. Initial rupture is contained within the lesser sac, followed by penetration of the lesser sac and free rupture into the peritoneal cavity—often known as the “double rupture phenomenon.” Therefore, open surgery is justified for treatment of symptomatic as well as aneurysms associated with pregnancy.2
Surgical treatment for splenic artery aneurysms includes splenectomy and aneurysmectomy. Distal pancreatectomy may be performed when the aneurysm is deeply embedded in the pancreatic tissue. Splenic preservation without vascular reconstruction carries a risk of splenic infarction or abscess formation. Aneurysms of the proximal vessel may be treated with aneurysmectomy and end-to-end anastomosis or simple ligation/exclusion without arterial reconstruction. Splenectomy must be performed when aneurysms are located at the splenic hilus. When possible, arterial reconstruction is preferred to ligation/ exclusion.1 When splenectomy is performed, heterotopic splenic autotransplantaion into the omentum may be performed to prevent the risk of postsplenectomy infection.4
Occasional reports of stent graft placement in the SA have been reported. Favorable situations for stent graft placement include a wide neck aneurysm and proximal location of the aneurysm. This offers the benefit of maintaining splenic perfusion, while excluding the aneurysm, thereby eliminating the risk of aneurysm rupture.20 In addition, treatment with stent grafts allows future access into the splenic artery if selective embolization of the spleen is required for hypersplenism. The natural history of true splenic artery aneurysms involves elongation and increasing tortuosity of the vessel, thereby limiting the delivery of a covered stent to the mid or distal aspect of the splenic artery, due to the existing device limitations.7 In such cases, coil embolization of the aneurysm may be performed.
A concern for splenic insufficiency exists after main splenic artery embolization. Infarcts and splenic atrophy are noted in as much as 40% of cases after distal or hilar splenic artery ablation, however with minimal clinical sequelae.7
HEPATIC ARTERY ANEURYSMS
Hepatic artery aneurysms (HAAs) are the second most common VAAs, and are more common in men.2 Most HAAs are solitary and involve the hepatic artery outside the liver. They make up 20% of all visceral artery aneurysms, with false aneurysms accounting for half of them.4 Aneurysm location often helps predict both cause and treatment strategy. Intrahepatic branch aneurysms are most frequently the result of trauma, iatrogenic injury from biopsy or intervention, infection, or vasculitis. In contrast, extrahepatic aneurysms are most often degenerative or dysplastic. Hepatic artery pseudoaneurysms are usually due to liver transplantation, general surgical procedures, percutaneous liver interventions, and trauma, as well as hepatocellular cancer.2
Rupture of a HAA may occur in the hepatobiliary or gastrointestinal tract as well as the peritoneal cavity. Hemobilia occurs in traumatic intrahepatic pseudoaneurysms. The triad of epigastric pain, hemobilia, and obstructive jaundice (Quincke triad) is seen in one-third of symptomatic patients.2 In 80% of patients with a HAA, rupture is the reason for first medical consultation.19 Rupture occurs with equal frequency into the peritoneal cavity and biliary tract. Rupture may also occur in the duodenum, gallbladder, portal vein, or stomach.19 Aneurysms of nonatherosclerotic etiology have a higher risk of rupture. Overall mortality rate with HAA rupture ranges from 21 to 35%. New recommendations have emerged regarding a selective approach to treatment of a HAA. Although symptomatic and ruptured aneurysms will be invariably treated, nonemergent intervention in asymptomatic patients, may be limited to aneurysms of nonatherosclerotic origin and for multiple hepatic aneurysms. Aneurysms of atherosclerotic etiology may be treated if enlarging or symptomatic.3 Intrahepatic aneurysms, which previously required hepatic resection, are now easily treated with transcatheter coil occlusion or embolization. Collateral blood flow and oxygen delivery from the portal vein minimize ischemic complications. Aneurysms of the CHA may be surgically ligated or trapped with coils. Collateral flow through the gastroduodenal artery and its branches provides ample blood flow to the liver. Patients with extensive atherosclerosis or previous gastrointestinal surgery may lack collateral blood supply to the PHA. Aneurysms of the PHA (distal to the gastroduodenal artery) require surgical ligation and bypass surgery owing to the limited potential for collateral blood flow to the liver.2 Alternatively, endovascular treatment with placement of a covered stent across the aneurysm isolates the aneurysm, while preserving adequate flow to the liver.
Endovascular treatment of a HAA is associated with potential short-term complications like hepatic ischemia, hepatic abscess, and cholecystitis. Long-term effects of aneurysmal recanalization are a concern.
GASTRODUODENAL, PANCREATICODUODENAL, AND PANCREATIC ARTERY ANEURYSMS
They constitute 6% of all VAAs; almost 90% of these present with rupture. Pancreatitis, peptic ulcer disease, and increasing blood flow through these vessels, such as with CA occlusion, are common etiological factors.4 These aneurysms are also seen after pancreatic surgery. Most patients are symptomatic at the time of diagnosis, presenting with epigastric pain radiating to the back. Aneurysmal rupture manifests as gastrointestinal hemorrhage through the stomach, duodenum, and less often through the biliary or pancreatic ductal system.19 Boudghene et al20 reported procedural success in all 32 pancreaticoduodenal aneurysms in which transcatheter embolization was attempted. It must be emphasized that, when treating aneurysms in the pancreaticoduodenal distribution, a careful search for and occlusion of collateral supply to these aneurysms is essential before ending the procedure.2 Interval follow-up, often including repeat arteriography, is necessary to ensure aneurysm occlusion. When aneurysms are associated with pseudocyst formation, cyst decompression should be undertaken.4
CELIAC ARTERY ANEURYSMS
Celiac artery aneurysms (CAAs) account for 4% of VAAs, and are frequently symptomatic. Rupture, seen in up to 13% of cases, is associated with intraperitoneal hemorrhage, though communication with the gastrointestinal tract may occur.19 A CAA is often degenerative, reportedly being associated with aortic aneurysms in 20% of cases and with other VAAs in 40%.2 Aneurysmectomy with primary reanastomosis of the CA is sometimes possible.4 When this is not possible, aorto-celiac synthetic prosthesis bypass or autologous vein bypass may be performed.4 Because the aneurysms usually involve the proximal portion of the celiac trunk, absence of a proximal landing zone limits endovascular treatment with coils.2
SUPERIOR MESENTERIC ARTERY ANEURYSMS
SMA aneurysms account for 5.5% of all visceral artery aneurysms, most often presenting as abdominal pain. Of these, 38 to 50% present with rupture.19 SMA aneurysms can be saccular or fusiform and are commonly found in the proximal 5 cm of the artery. Causes of SMA aneurysm include inflammation, vasculitis, trauma, arterial dissection, and dysplastic and degenerative aneurysms.2 These lesions are frequently mycotic in origin, associated with bacterial endocarditis. SMA aneurysms are often symptomatic, manifesting with acute, colicky upper abdominal pain, nausea, or vomiting. Because most aneurysms contain mural thrombus, symptoms may arise from aneurysm embolization of the peripheral vascular bed or narrowing of the arterial lumen.2 A palpable mass has been reported in up to 50% of patients. With the risk of rupture reported to approach 50%, treatment is indicated for most SMA aneurysms.
Treatment options depend on the patient's hemodynamic status and surgical risk. Surgical options include aneurysmectomy, aneurysmorrhaphy, or ligation, with or without arterial reconstruction. Simple ligation of both CAAs and SMA aneurysms is often possible because of the extensive collateral circulation between the celiac artery and SMAs through the pancreatic arcades.4 Revascularization with venous or synthetic graft or direct arterial reconstruction is deemed necessary in patients with ruptured aneurysm and bowel ischemia or preoperative signs or symptoms of intestinal ischemia.2,21
Because these aneurysms usually involve the proximal portion of the SMA, the absence of a proximal landing zone limits endovascular treatment with coils.2
INFERIOR MESENTERIC ARTERY ANEURYSMS
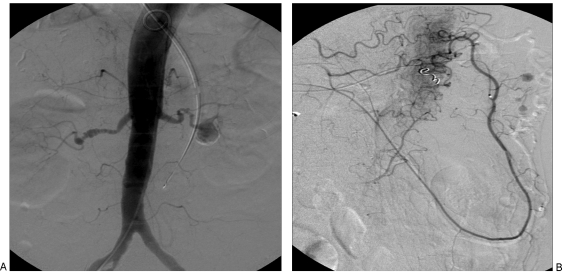
Incidence of inferior mesenteric artery (IMA) aneurysms is 1% of all VAAs. These are located in the most proximal segment of the artery and are usually asymptomatic. Increased blood flow, resulting from occlusion or stenosis of both the celiac axis and SMA arteries is considered the cause of these aneurysms. In these cases, the entire blood flow is via the IMA and a dilated marginal artery of Drummond. A “jet disorder” phenomenon is postulated as the cause of aneurysm formation in these vessels unadapted for high flow. Subsequent ostial stenosis of the IMA is often noted (Fig. 7).22 Revascularization of the CA and SMA is undertaken before IMA aneurysms can be treated endovascularly or surgically, but caution is necessary to avoid compromising colonic perfusion.23 Treatment has historically been resection, ligation, or reimplantation of the IMA into the aorta.22
Figure 7.
Patient with fibromuscular dysplasia. Angiography revealed ostial occlusion of the celiac axis and superior mesenteric artery. (A) Classic “string of beads” appearance of the right renal artery with aneurysms of the left renal artery. (B) Multiple aneurysms are identified along branches of the marginal artery.
CONCLUSION
Endovascular management is suitable for patients with aneurysm rupture and hemodynamic instability. It is the desirable method of treatment in patients with multiple comorbidities, especially malignancy.3 It is also associated with a decreased length of hospital stay in the elective setting. Also, failure of primary treatment can be managed with repeat percutaneous procedures. It is the ideal method of management in surgically hard-to-access intrapancreatic and peripancreatic territories. Open surgical repair of VAAs in hostile environments of pancreatitis and abdominal sepsis, is technically difficult and elevates the morbidity of surgical repair. In these situations, endovascular intervention provides a distinct advantage over conventional repair.7 The majority of the pseudoaneurysms can be successfully treated with endovascular repair. In congenitally abnormal and fragile arteries, where surgical dehiscence is likely, endovascular treatment may present a less traumatic option. Surgical intervention is difficult in cases involving bowel adhesions and anatomic variation involving previous abdominal surgery where endovascular repair maybe an alternative.1
Endovascular treatment of VAAs and VAPAs is an alternative to conventional surgery with lower procedural mortality and morbidity, reducing aneurysm-related symptoms with high technical success rates.1
REFERENCES
- Huang Y-K, Hsieh H-C, Tsai F-C, Chang S H, Lu M S, Ko P J. Visceral artery aneurysm: risk factor analysis and therapeutic opinion. Eur J Vasc Endovasc Surg. 2007;33(3):293–301. doi: 10.1016/j.ejvs.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Nosher J L, Chung J, Brevetti L S, Graham A M, Siegel R L. Visceral and renal artery aneurysms: a pictorial essay on endovascular therapy. Radiographics. 2006;26(6):1687–1704. quiz 1687. doi: 10.1148/rg.266055732. [DOI] [PubMed] [Google Scholar]
- Sachdev U, Baril D T, Ellozy S H, et al. Management of aneurysms involving branches of the celiac and superior mesenteric arteries: a comparison of surgical and endovascular therapy. J Vasc Surg. 2006;44(4):718–724. doi: 10.1016/j.jvs.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Chiesa R, Astore D, Guzzo G, et al. Visceral artery aneurysms. Ann Vasc Surg. 2005;19(1):42–48. doi: 10.1007/s10016-004-0150-2. [DOI] [PubMed] [Google Scholar]
- Ikeda O, Tamura Y, Nakasone Y, Iryou Y, Yamashita Y. Nonoperative management of unruptured visceral artery aneurysms: treatment by transcatheter coil embolization. J Vasc Surg. 2008;47(6):1212–1219. doi: 10.1016/j.jvs.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Pulli R, Dorigo W, Troisi N, Pratesi G, Innocenti A A, Pratesi C. Surgical treatment of visceral artery aneurysms: A 25-year experience. J Vasc Surg. 2008;48(2):334–342. doi: 10.1016/j.jvs.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Tulsyan N, Kashyap V S, Greenberg R K, et al. The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg. 2007;45(2):276–283. discussion 283. doi: 10.1016/j.jvs.2006.10.049. [DOI] [PubMed] [Google Scholar]
- Friedman S G, Pogo G J, Moccio C G. Mycotic aneurysm of the superior mesenteric artery. J Vasc Surg. 1987;6(1):87–90. doi: 10.1067/mva.1987.avs0060087. [DOI] [PubMed] [Google Scholar]
- McCready R A, Bryant M A, Fehrenbacher J W, Rowe M G. Infected splenic artery aneurysm with associated splenic abscess formation secondary to bacterial endocarditis: case report and review of the literature. J Vasc Surg. 2007;45(5):1066–1068. doi: 10.1016/j.jvs.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Hyare H, Desigan S, Brookes J A, Guiney M J, Lees W R. Endovascular management of major arterial hemorrhage as a complication of inflammatory pancreatic disease. J Vasc Interv Radiol. 2007;18(5):591–596. doi: 10.1016/j.jvir.2007.02.035. [DOI] [PubMed] [Google Scholar]
- Hassen-Khodja R, Declemy S, Batt M, et al. Visceral artery aneurysms in Von Recklinghausen's neurofibromatosis. J Vasc Surg. 1997;25(3):572–575. doi: 10.1016/s0741-5214(97)70270-2. [DOI] [PubMed] [Google Scholar]
- Parfitt J, Chalmers R TA, Wolfe J HN. Visceral aneurysms in Ehlers-Danlos syndrome: case report and review of the literature. J Vasc Surg. 2000;31(6):1248–1251. doi: 10.1067/mva.2000.105667. [DOI] [PubMed] [Google Scholar]
- Sellke F W, Williams G B, Donovan D L, Clarke R E. Management of intra-abdominal aneurysms associated with periarteritis nodosa. J Vasc Surg. 1986;4(3):294–298. doi: 10.1067/mva.1986.avs0040294. [DOI] [PubMed] [Google Scholar]
- Bailey R W, Riles T S, Rosen R J, Sullivan L P. Celiomesenteric anomaly and aneurysm: clinical and etiologic features. J Vasc Surg. 1991;14(2):229–234. doi: 10.1067/mva.1991.28728. [DOI] [PubMed] [Google Scholar]
- Settembrini P G, Jausseran J-M, Roveri S, et al. Aneurysms of anomalous splenomesenteric trunk: clinical features and surgical management in two cases. J Vasc Surg. 1996;24(4):687–692. doi: 10.1016/s0741-5214(96)70085-x. [DOI] [PubMed] [Google Scholar]
- Mitchell M B, McAnena O J, Rutherford R B. Ruptured mesenteric artery aneurysm in a patient with alpha 1-antitrypsin deficiency: etiologic implications. J Vasc Surg. 1993;17(2):420–424. [PubMed] [Google Scholar]
- O'Hara P J, Ratliff N B, Graor R A, Novick A, Beven E G. Medial agenesis associated with multiple extracranial peripheral and visceral arterial aneurysms. J Vasc Surg. 1985;2(2):298–306. doi: 10.1067/mva.1985.avs0020298. [DOI] [PubMed] [Google Scholar]
- Kim B S, Do H M, Razavi M. N-butyl cyanoacrylate glue embolization of splenic artery aneurysms. J Vasc Interv Radiol. 2004;15(1 Pt 1):91–94. doi: 10.1097/01.rvi.0000099537.29957.13. [DOI] [PubMed] [Google Scholar]
- Carr S C, Mahvi D M, Hoch J R, Archer C W, Turnipseed W D. Visceral artery aneurysm rupture. J Vasc Surg. 2001;33(4):806–811. doi: 10.1067/mva.2001.112320. [DOI] [PubMed] [Google Scholar]
- Boudghene F, L'Hermine C, Bigot J M. Arterial complications of pancreatitis: diagnostic and therapeutic aspects in 104 cases. J Vasc Interv Radiol. 1993;4(4):551–558. doi: 10.1016/s1051-0443(93)71920-x. [DOI] [PubMed] [Google Scholar]
- Arepally A, Dagli M, Hofmann L V, Kim H S, Cooper M, Klein A. Treatment of splenic artery aneurysm with use of a stent-graft. J Vasc Interv Radiol. 2002;13(6):631–633. doi: 10.1016/s1051-0443(07)61659-5. [DOI] [PubMed] [Google Scholar]
- Sallou C, Cron J, Julia P, Fabiani J N. Aneurysm of the inferior mesenteric artery: case report and review of the literature. Eur J Vasc Endovasc Surg. 1997;14(1):71–74. doi: 10.1016/s1078-5884(97)80229-8. [DOI] [PubMed] [Google Scholar]
- Mandeville K LD, Bicknell C, Narula S, Renton S. Inferior mesenteric artery aneurysm with occlusion of the superior mesenteric artery, coeliac trunk and right renal artery. Eur J Vasc Endovasc Surg. 2008;35(3):312–313. doi: 10.1016/j.ejvs.2007.07.018. [DOI] [PubMed] [Google Scholar]