ABSTRACT
Renal vascular abnormalities included in this review are renal artery aneurysms (RAA) and renal arteriovenous malformations (AVM). The clinical presentation, diagnosis, and principles of management with emphasis on endovascular techniques are discussed.
Keywords: Renal artery aneurysm, renal arteriovenous malformation, embolization
RENAL ARTERY ANEURYSMS
Renal artery aneurysms (RAAs) are rare with an estimated incidence of 0.09%.1 They may be classified based on their morphology, etiology, or anatomic location. The morphologic subtypes include saccular, fusiform, and dissecting aneurysms and microaneurysms. The aneurysms may be congenital or degenerative or may occur secondary to trauma (including iatrogenic trauma such as biopsy, nephrostomy, etc.), infection, vasculitis (Fig. 1), neurofibromatosis, and Kawasaki's disease. A true aneurysm contains all three layers of the vascular wall, whereas the wall of a pseudoaneurysm is partially made up of the tissues surrounding the vessel. RAAs may be parenchymal or extraparenchymal in location. The extraparenchymal RAAs are more common in the mid and distal third of the renal artery and tend to affect the points of vessel bifurcation. In fact, 60% of RAAs occur at the bifurcation of the main renal artery.2 Parenchymal aneurysms can affect proximal lobar arteries as well as deep small arterioles close to the calyces. RAAs, either extraparenchymal, parenchymal, or combined, may be associated with renal artery stenosis, fibromuscular dysplasia, or renal cell carcinoma.1,3,4,5,6
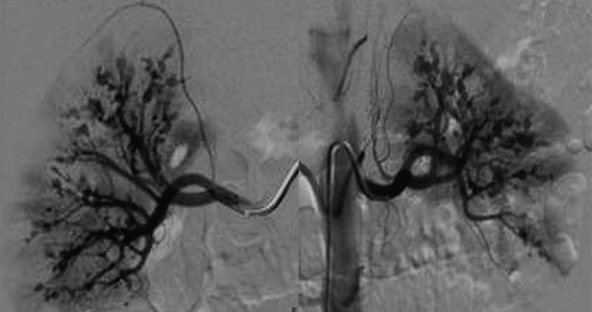
Figure 1.
Panoramic view of bilateral renal angiograms demonstrating multiple small renal arterial aneurysms in both kidneys in a patient with polyarteritis nodosa.
Pathogenesis
The pathogenesis of RAA is well described. Degeneration of elastic fibers and mediolysis lead to weakening of vessel wall. This results in expansion of the vessel from high intraluminal pressure. Marked arteriosclerotic changes are not commonly seen in RAAs. Arterial fibrodysplasia is the most prevalent vascular disease associated with RAAs in one series by Henke et al.2
Clinical Presentation
Most of these aneurysms are discovered incidentally. A few patients may present with uncontrolled hypertension, hematuria, or shock secondary to rupture of the aneurysm.
Natural History of Renal Artery Aneurysms
The natural history and clinical significance of RAAs are still uncertain. As described in the previous section, most RAAs do not pose any significant clinical problems and are likely to be discovered incidentally on examinations performed for unrelated conditions. The incidence of rupture is rare7,8,9 in the general population. However, it is high in pregnant women especially during the third trimester and is associated with high mortality.
The indications for treatment of these aneurysms are still controversial; most physicians accept that the indications for treatment are high risk of rupture, uncontrolled hypertension, presentation with rupture, or other symptoms such as pain or hematuria. Women tend to have a higher incidence of rupture; therefore, intervention is more common among women, particularly pregnant women and those expecting to conceive. Size does not have a direct correlation with rupture. Rupture of small RAAs (<2 cm) is well described in the literature.10,11
The cause of secondary hypertension in a RAA is unclear. Possible explanations contributing to high blood pressure in these cases are mechanical kinking of the renal artery, segmental renal parenchymal ischemia, flow turbulence, or coexistent renal artery stenosis. However, the blood pressure response and the reduction in antihypertensive medications appear similar in patients treated for a RAA with associated renal artery stenosis and those without coexisting stenosis.2
Diagnosis
RAAs are mostly discovered incidentally on imaging obtained for unrelated reasons (Fig. 2). They are also found during workup of uncontrolled hypertension. A RAA can be evaluated by duplex ultrasound, spiral computerized tomography (CT), three-dimensional (3D) contrast-enhanced magnetic resonance imaging (MRI), or conventional arteriography. Duplex ultrasound is an inexpensive, noninvasive technique in the demonstration of a RAA. The duplex ultrasound images show sonolucent lesions with turbulent flow. Ultrasound has pitfalls, however; aneurysms may be misinterpreted as cysts or dilated renal veins. Further, there may be limitations of visibility from body habitus and the examination is highly operator dependable.12,13 Multidetector spiral CT is widely available and has been established as a useful imaging modality for the diagnosis of RAAs with resolutions comparable to conventional arteriography.14 Focal dilatation of renal artery is the key feature, which is better demonstrated in thick slab multiplanar reformations and volume-rendered images. CT carries the risk of exposure to ionizing radiation and nephrotoxicity from iodine injection. Contrast-enhanced MR angiography (MRA) has also shown promise in the demonstration of RAA,15 but it is costly and has relatively limited availability.
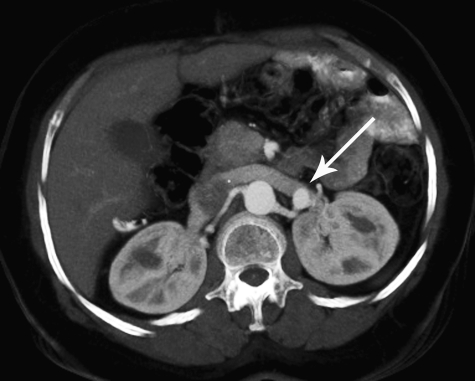
Figure 2.
Axial computed tomography (CT) image obtained in a patient with low back pain demonstrates an incidental small left renal artery aneurysm (arrow).
Digital subtraction arteriography has remained the gold standard in diagnosis and planning for endovascular intervention. Oblique views and or rotation angiography may be needed in the evaluation of the neck of the aneurysm and the relation of the aneurysm to branch vessel origins. This is a relatively invasive procedure compared with other imaging modalities described above. Currently, it is mainly used in planning an intervention rather than for the diagnosis of RAAs.
Management
The primary goal of RAA intervention is to exclude the aneurysm from high intraarterial pressure. This can be achieved by various means and each method is selected depending on the location and size of the aneurysm.
Rundback et al16 categorized RAAs based on their morphology and location into three types. This classification provides guidelines for management. Type 1 includes saccular aneurysms arising from the main renal artery or a large segmental branch. Type 2 includes fusiform aneurysms and type 3 includes intralobar artery aneurysms.
Type 1 aneurysms can be treated by both surgical methods as well as endoluminal techniques. Many authors have reported a high rate of success with endoluminal techniques.17,18,19,20,21,22,23,24,25 The extraparenchymal saccular aneurysm with a narrow neck can be isolated from the circulation by deployment of coils into the aneurysm, therefore promoting thrombosis. The coils remain constrained in the aneurysm due to the narrow neck. The saccular aneurysms with wide neck can be treated with bare metal stents with additional coil deployment in the aneurysm through the interstices of the stent. The interstices of the stent act as a narrow neck and prevent the coils from escaping into the circulation. Another method to exclude saccular aneurysms is through stent grafts (Fig. 3).26 Stent grafts require a normal-sized arterial segment both in the proximal and distal aspects of the aneurysm. Stent grafts cannot usually be placed at bifurcations unless Y-shaped stent grafts are used to extend the graft into the branches from the parent artery. An aneurysm at a branching point is difficult to treat without causing occlusion of end arterial circulation. A combination of stent graft extending into one of the branches and coiling and occlusion of the other branch has been successfully performed in select cases.2
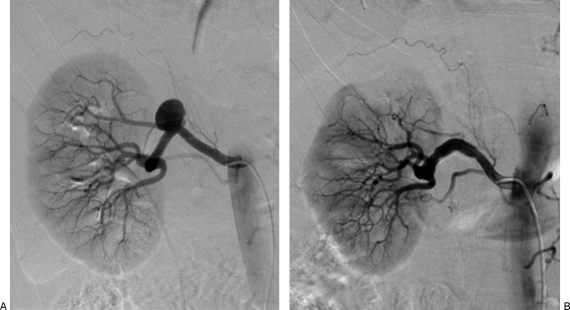
Figure 3.
Renal artery aneurysm in an asymptomatic 65-year-old woman. (A) Saccular right renal artery aneurysm on digital subtraction arteriogram. (B) The aneurysm was successfully excluded with a stent graft.
Type 2 fusiform aneurysms in the main renal artery or large segmental artery are best treated by surgery at specialized centers. Covered stent grafts have also been described in treating these aneurysms. When large fusiform aneurysms are seen in branch arteries, they could be excluded by stent graft placement in the parent artery from which the branch artery arises.
Type 3 interlobar and/or intra-parenchymal RAAs are similarly treated by endovascular techniques, but are usually accompanied by thrombosis of end arteries and resultant infarction. Super-selective embolization limits the infarction of renal parenchyma.27,28,29,30,31,32 This can be achieved with the use of 3-French microcatheters, which can be navigated coaxially to the site of the aneurysm, thereby limiting the number of vessels being occluded. Various embolic agents have been successfully used for occluding intraparenchymal aneurysms. Such embolic agents include regular/detachable coils, detachable balloons, gelatin sponge, alcohol, thrombin, and autologous blood clot.
Complications of endoluminal management are less common, but include injuries to renal vascular network, infarction, increase in hypertension, nontarget embolization (migration of coils into the main renal artery and systemic circulation), thrombosis of the stent grafts, and failure of exclusion with enlargement of the aneurysm, flank pain, and infection. Careful selection of patients is mandatory to avoid potential complications in this end-arterial vascular network.
Although no proper guidelines exist for follow-up of these aneurysms after surgical or endoluminal therapy, it is generally recommended that the patients are followed initially at 6 months and then yearly. Clinical, laboratory, and imaging evaluation should be performed to assess blood pressure, renal function status and reperfusion of the aneurysm. Follow-up evaluation with CT may be limited by streak artifacts from metallic coils. In such cases, color Doppler evaluation and catheter angiography under digital subtraction system may be helpful.
ABNORMAL RENAL ARTERIOVENOUS COMMUNICATIONS
Renal vascular malformations are abnormal communications between the arteries and veins in the kidneys. Generally, two types are described. Renal AVMs are congenital and represent a developmental abnormality wherein the artery and the vein communicate through a network of abnormal vessels (often referred to as “nidus”). A renal arteriovenous fistula (AVF) refers to a direct communication between an artery and a vein. These are often acquired in nature. AVFs constitute the majority of these vascular malformations and usually result from iatrogenic causes such as renal biopsy, percutaneous nephrostomy, or accidental trauma. AVMs can be classified into cavernous or cirsoid types. A cavernous AVM has a single arterial feeder, whereas a cirsoid AVM is fed through multiple arterial feeders and has a corkscrew appearance. The cirsoid type is more common than the cavernous AVMs. An AVM can also be secondary to malignancy in which angiogenic factors from the tumor are thought to stimulate formation of abnormal vascular communications. Bilateral AVMs are uncommon.
Clinical Presentation
An AVM is usually symptomatic with gross hematuria due to rupture of small venules into the calyces from abnormally increased intravascular pressure. Patients may present with flank pain from obstruction of renal collecting system by blood clots or with hypertension due to stimulation of the renin-angiotensin pathway or with cardiac failure due to high-output state. A few patients may be minimally symptomatic with microscopic hematuria. The AVMs may be detected in asymptomatic individuals during physical examination based on a frank bruit in the flank or abdomen. Sometimes, AVMs may be incidentally detected on imaging studies performed for other reasons.
Diagnosis
Ultrasound with color Doppler assessment may be used initially to detect clinically suspected AVMs. These appear as cystic lesions with high-velocity turbulent flow on color Doppler ultrasound.33 A renal AVM could be suggested by turbulent flow in the IVC or renal vein. If the AVM is in the left kidney, abnormal dilatation of the left renal vein also suggests the diagnosis. As in the diagnosis of renal artery aneurysm, the multidetector CT and contrast-enhanced MRA have roles in the diagnosis of renal AVMs. Large-diameter tortuous flow voids in T2-weighted MRIs represent dilated vessels with high flow and suggest underlying vascular malformation or fistula (Fig. 4). Dilated enhancing vessels with attenuation or signal similar to the arteries may lead to suspicion of these abnormal vascular malformations or fistulas in contrast-enhanced CT or MRI. The accurate evaluation of the multiple feeders into the AVM might be difficult by cross-sectional imaging due to crowding of vessels in the renal hilum. Capability for selective opacification of small arterial branches gives digital subtraction angiography (DSA) a distinct advantage in complete evaluation of feeding vessels and planning of the management in the AVMs. High-flow AVMs and AVFs are best assessed with DSA using a high frame rate. The diagnostic features include an abnormal dilatation of nidus with early opacification of venous outflow.
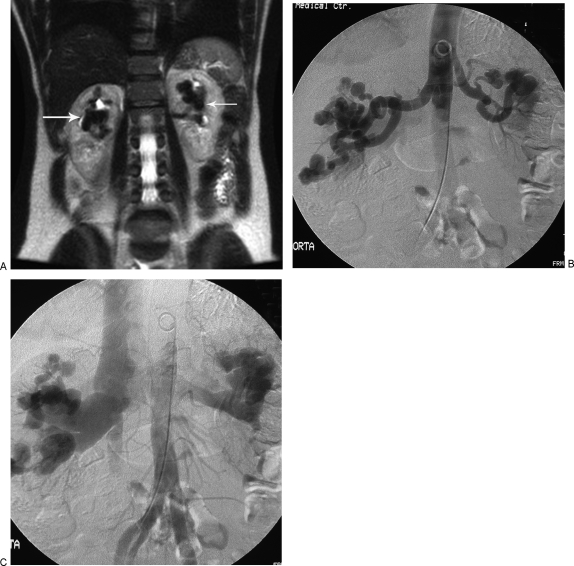
Figure 4.
Bilateral renal arteriovenous malformations (AVMs). (A) T2-weighted coronal magnetic resonance imaging of abdomen in a patient with vague abdominal pain. White arrows point to the tortuous flow voids suspicious for bilateral renal AVMs. (B) Digital subtraction aortogram confirming bilateral renal AVMs. (C) Note early filling of the renal veins and inferior vena cava.
Management
Surgical management is now reserved for cases associated with malignancy and in cases where endovascular methods failed or are deemed too risky or technically difficult. In general, nephron-sparing surgeries are preferred to total nephrectomy.
The principle of the successful exclusion of AVMs is based on destruction of the nidus and blocking all the arterial feeders. The agent of choice to achieve these objectives is absolute alcohol. It denatures proteins and causes sloughing of the endothelium, arterial spasm, and perivascular necrosis, thus stimulating intense thrombus formation. This usually results in complete and permanent occlusion.34 Alcohol ablation of the renal artery also has a long-lasting antihypertensive effect when the juxtaglomerular apparatus is destroyed, abolishing renin production.35
Alcohol may be mixed with metrizamide to visualize alcohol injection on fluoroscopy.36 Complications may result from reflux of alcohol into nontarget areas. Some drowsiness may be seen when injected alcohol leaks into the venous circulation.
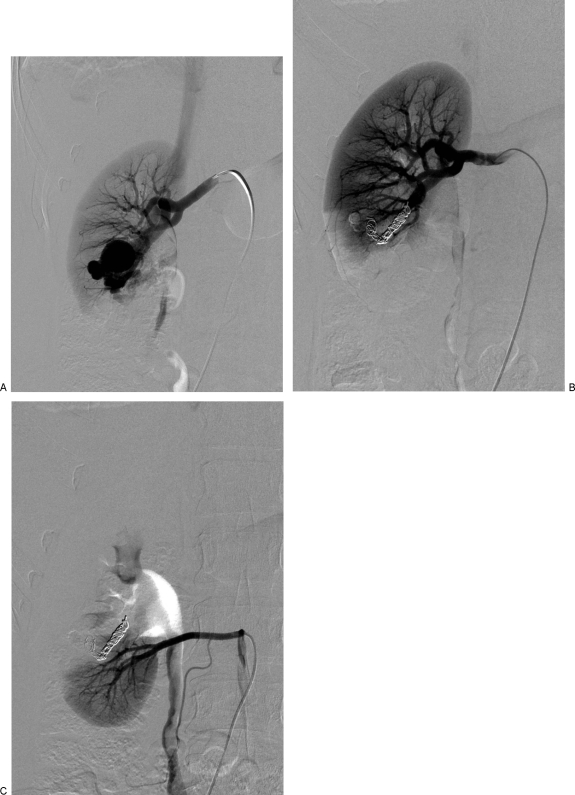
Larger AVMs can also be treated with coil embolization. This can be achieved by super-selective catheterization and coil-embolization of all the feeders (Fig. 5). Complications could arise from rapid shunting of coils into the venous circulation. In such cases, the coils may embolize to the pulmonary circulation or to the left side of the heart if interatrial communication exists. It is important for the angiographer to be familiar with retrieval techniques if such a complication unfolds (Fig. 6). Blocking the venous output of the AVM can help in preventing this complication. In an AVM with a single arterial feeder, occlusion of the feeding artery by stent graft or coils might result in thrombosis of the AVM (Fig. 7).
Figure 5.
Congenital right renal arteriovenous fistula (AVF) in a 25-year-old asymptomatic woman. (A) Right main renal artery angiogram shows a large AVF with an aneurysmal venous outflow. Note early opacification of the inferior vena cava. (B) Postembolization, there is successful occlusion of the AVF with coils. No early opacification of the venous outflow is seen. (C) The inferior pole was supplied by an accessory renal artery, which did not contribute to the fistula.
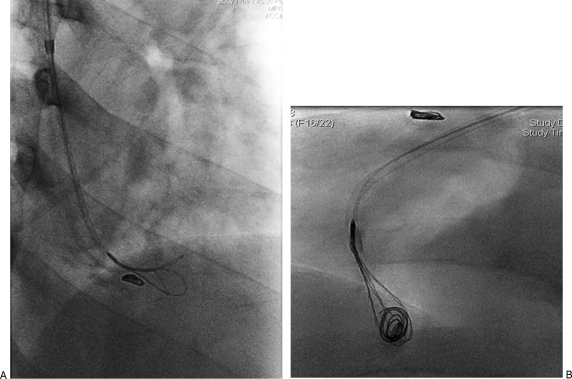
Figure 6.
Embolization coils in the pulmonary circulation. (A) Oblique view of right lower thorax demonstrating attempts at retrieval of coil in the pulmonary arterial circulation. This coil has slipped through the right renal arteriovenous fistula during attempted occlusion of the feeding artery. (B) Lateral view demonstrating successful attempts at retrieval of one of the two misdirected coils.
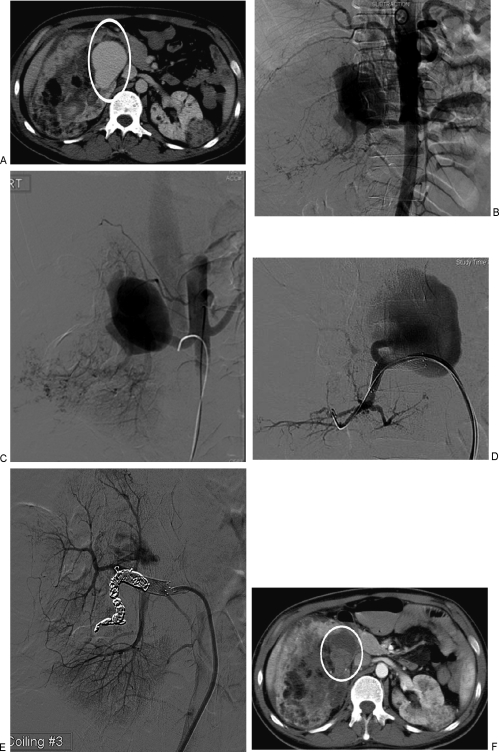
Figure 7.
Renal arteriovenous malformation (AVM). (A) Axial computed tomography (CT) scan with bilateral angiomyolipoma in a patient with tuberous sclerosis. Area in white ellipse shows the AVM in the right kidney. (B) Aortogram demonstrates the right renal AVM and classic angiographic findings of angiomyolipoma in the lower right renal arterial branches. (C) Selective right renal arteriogram demonstrates the AVM and early filling of the inferior vena cava. (D) Super-selective arteriogram confirms a single feeder into the AVM from a segmental renal artery branch. A stent has been placed in the main renal artery to assist in coiling of the feeder. (E) Successful coil embolization of the feeder to the AVM. Note minimal residual flow in the AVM. (F) Follow-up CT the next day demonstrates complete thrombosis of the AVM (in white circle).
Follow-up of these vascular malformations is similar to that described in the management of renal artery aneurysms, by contrast-enhanced CT, MRI, duplex ultrasound, and conventional arteriography.
CONCLUSION
Renal artery aneurysms and arteriovenous malformations can be successfully managed with endovascular techniques in most cases.
REFERENCES
- Stanley J C, Rhodes E L, Gewertz B L, Chang C Y, Walter J F, Fry W J. Renal artery aneurysms. Significance of macroaneurysms exclusive of dissections and fibrodysplastic mural dilations. Arch Surg. 1975;110(11):1327–1333. doi: 10.1001/archsurg.1975.01360170067009. [DOI] [PubMed] [Google Scholar]
- Henke P K, Cardneau J D, Welling T H, III, et al. Renal artery aneurysms: a 35-year clinical experience with 252 aneurysms in 168 patients. Ann Surg. 2001;234(4):454–462. discussion 462–463. doi: 10.1097/00000658-200110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman J H, Smith R F, Szilagyi E, Elliott J P. Aneurysms of the renal artery: problems of prognosis and surgical management. Surgery. 1978;84(4):563–572. [PubMed] [Google Scholar]
- Barrett J M, Dean R H, Boehm F H. Rupture of a renal artery aneurysm during pregnancy. South Med J. 1981;74(12):1549–1550. doi: 10.1097/00007611-198112000-00037. [DOI] [PubMed] [Google Scholar]
- De Bakey M E, Lefrak E A, Garcia-Rinaldi R, Noon G P. Aneurysm of the renal artery. A vascular reconstructive approach. Arch Surg. 1973;106(4):438–443. doi: 10.1001/archsurg.1973.01350160056009. [DOI] [PubMed] [Google Scholar]
- Hafez K S, Krishnamurthi V, Campbell S C, Novick A C. Contemporary management of renal cell carcinoma with coexistent renal artery disease: update of the Cleveland Clinic experience. Urology. 2000;56(3):382–386. doi: 10.1016/s0090-4295(00)00691-9. [DOI] [PubMed] [Google Scholar]
- Henriksson C, Björkerud S, Nilson A E, Pettersson S. Natural history of renal artery aneurysm elucidated by repeated angiography and pathoanatomical studies. Eur Urol. 1985;11(4):244–248. doi: 10.1159/000472506. [DOI] [PubMed] [Google Scholar]
- Henriksson C, Lukes P, Nilson A E, Pettersson S. Angiographically discovered, non-operated renal artery aneurysms. Scand J Urol Nephrol. 1984;18(1):59–62. doi: 10.3109/00365598409182164. [DOI] [PubMed] [Google Scholar]
- Tham G, Ekelund L, Herrlin K, Lindstedt E L, Olin T, Bergentz S E. Renal artery aneurysms. Natural history and prognosis. Ann Surg. 1983;197(3):348–352. doi: 10.1097/00000658-198303000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupp T, Allenberg J R, Post K, et al. Renal artery aneurysm: surgical indications and results. Eur J Vasc Surg. 1992;6:477–486. doi: 10.1016/s0950-821x(05)80620-6. [DOI] [PubMed] [Google Scholar]
- Reiher L, Grabitz K, Sandmann W. Reconstruction for renal artery aneurysm and its effect on hypertension. Eur J Vasc Endovasc Surg. 2000;20(5):454–456. doi: 10.1053/ejvs.2000.1216. [DOI] [PubMed] [Google Scholar]
- Shonai T, Koito K, Ichimura T, Hirokawa N, Sakata K, Hareyama M. Renal artery aneurysm: evaluation with color Doppler ultrasonography before and after percutaneous transarterial embolization. J Ultrasound Med. 2000;19(4):277–280. doi: 10.7863/jum.2000.19.4.277. [DOI] [PubMed] [Google Scholar]
- Hantman S S, Barie J J, Glendening T B, Eisenberg M N, Rapoport K D. Giant renal artery aneurysm mimicking a simple cyst on ultrasound. J Clin Ultrasound. 1982;10(3):136–139. doi: 10.1002/jcu.1870100311. [DOI] [PubMed] [Google Scholar]
- Sabharwal R, Vladica P, Law W P, Lau H, Patel M. Multidetector spiral CT renal angiography in the diagnosis of giant renal artery aneurysms. Abdom Imaging. 2006;31(3):374–378. doi: 10.1007/s00261-005-0079-0. [DOI] [PubMed] [Google Scholar]
- Browne R F, Riordan E O, Roberts J A, et al. Renal artery aneurysms: diagnosis and surveillance with 3D contrast-enhanced magnetic resonance angiography. Eur Radiol. 2004;14(10):1807–1812. doi: 10.1007/s00330-004-2341-1. [DOI] [PubMed] [Google Scholar]
- Rundback J H, Rizvi A, Rozenblit G N, et al. Percutaneous stent-graft management of renal artery aneurysms. J Vasc Interv Radiol. 2000;11(9):1189–1193. doi: 10.1016/s1051-0443(07)61362-1. [DOI] [PubMed] [Google Scholar]
- Karkos C D, D'Souza S P, Thomson G J, Chomal A, Matanhelia S S. Renal artery aneurysm: endovascular treatment by coil embolisation with preservation of renal blood flow. Eur J Vasc Endovasc Surg. 2000;19(2):214–216. doi: 10.1053/ejvs.1999.0946. [DOI] [PubMed] [Google Scholar]
- Kohei N, Kawanishi H, Sasaki M, Okada T, Ono H, Miyamoto S. [Superselective endovascular treatment of renal artery aneurysms with detachable microcoils] Hinyokika Kiyo. 2003;49(1):43–46. [PubMed] [Google Scholar]
- Gandini R, Spinelli A, Pampana E, Fabiano S, Pendenza G, Simonetti G. Bilateral renal artery aneurysm: percutaneous treatment with stent-graft placement. Cardiovasc Intervent Radiol. 2006;29(5):875–878. doi: 10.1007/s00270-004-8209-6. [DOI] [PubMed] [Google Scholar]
- Klein G E, Szolar D H, Breinl E, Raith J, Schreyer H H. Endovascular treatment of renal artery aneurysms with conventional non-detachable microcoils and Guglielmi detachable coils. Br J Urol. 1997;79(6):852–860. doi: 10.1046/j.1464-410x.1997.00157.x. [DOI] [PubMed] [Google Scholar]
- Dib M, Sedat J, Raffaelli C, Petit I, Robertson W G, Jaeger P. Endovascular treatment of a wide-neck renal artery bifurcation aneurysm. J Vasc Interv Radiol. 2003;14(11):1461–1464. doi: 10.1097/01.rvi.0000096765.74047.68. [DOI] [PubMed] [Google Scholar]
- Saltiel A A, Matalon T AS, Patel S K. Embolization of a giant renal arterial aneurysm. J Urol. 1990;144(5):1227–1228. doi: 10.1016/s0022-5347(17)39700-8. [DOI] [PubMed] [Google Scholar]
- Savastano S, Feltrin G P, Miotto D, Chiesura-Corona M. Renal aneurysm and arteriovenous fistula. Management with transcatheter embolization. Acta Radiol. 1990;31(1):73–76. [PubMed] [Google Scholar]
- Nosher J L, Needell G S, Bialy G, Zatina M. Catheter occlusion of a mycotic renal artery aneurysm with cure of associated renovascular hypertension. Cardiovasc Intervent Radiol. 1989;12(6):310–312. doi: 10.1007/BF02575427. [DOI] [PubMed] [Google Scholar]
- Routh W D, Keller F S, Gross G M. Transcatheter thrombosis of a leaking saccular aneurysm of the main renal artery with preservation of renal blood flow. AJR Am J Roentgenol. 1990;154(5):1097–1099. doi: 10.2214/ajr.154.5.2108549. [DOI] [PubMed] [Google Scholar]
- Bui B T, Oliva V L, Leclerc G, et al. Renal artery aneurysm: treatment with percutaneous placement of a stent-graft. Radiology. 1995;195(1):181–182. doi: 10.1148/radiology.195.1.7892464. [DOI] [PubMed] [Google Scholar]
- Liguori G, Trombetta C, Bucci S, Pozzi-Mucelli F, Bernobich E, Belgrano E. Percutaneous management of renal artery aneurysm with a stent-graft. J Urol. 2002;167(6):2518–2519. [PubMed] [Google Scholar]
- Kaufman S L, Martin L G, Zuckerman A M, Koch S R, Silverstein M I, Barton J W. Peripheral transcatheter embolization with platinum microcoils. Radiology. 1992;184(2):369–372. doi: 10.1148/radiology.184.2.1620829. [DOI] [PubMed] [Google Scholar]
- Huppert P E, Duda S H, Erley C M, et al. Embolization of renal vascular lesions: clinical experience with microcoils and tracker catheters. Cardiovasc Intervent Radiol. 1993;16(6):361–367. doi: 10.1007/BF02603141. [DOI] [PubMed] [Google Scholar]
- Beaujeux R, Saussine C h, al-Fakir A, et al. Superselective endo-vascular treatment of renal vascular lesions. J Urol. 1995;153(1):14–17. doi: 10.1097/00005392-199501000-00007. [DOI] [PubMed] [Google Scholar]
- Tshomba Y, Deleo G, Ferrari S, Marina R, Biasi G M. Renal artery aneurysm: improved renal function after coil embolization. J Endovasc Ther. 2002;9(1):54–58. doi: 10.1177/152660280200900110. [DOI] [PubMed] [Google Scholar]
- Miller D C, Forauer A, Faerber G J. Successful angioembolization of renal artery pseudoaneurysms after blunt abdominal trauma. Urology. 2002;59(3):444. doi: 10.1016/s0090-4295(01)01595-3. [DOI] [PubMed] [Google Scholar]
- Cisternino S J, Malave S R, Neiman H L. Congenital renal arteriovenous malformation: ultrasonic appearance. J Urol. 1981;126(2):238–239. doi: 10.1016/s0022-5347(17)54459-6. [DOI] [PubMed] [Google Scholar]
- Yakes W F, Haas D K, Parker S H, et al. Symptomatic vascular malformations: ethanol embolotherapy. Radiology. 1989;170(3 Pt 2):1059–1066. doi: 10.1148/radiology.170.3.2916057. [DOI] [PubMed] [Google Scholar]
- Iaccarino V, Russo D, Niola R, et al. Total or partial percutaneous renal ablation in the treatment of renovascular hypertension: radiological and clinical aspects. Br J Radiol. 1989;62(739):593–598. doi: 10.1259/0007-1285-62-739-593. [DOI] [PubMed] [Google Scholar]
- Zak M B, Szof C A, Munzenberger P J. Metrizamide-ethanol for treatment of symptomatic vascular malformations. Am J Hosp Pharm. 1994;51(23):2964–2969. [PubMed] [Google Scholar]