ABSTRACT
Endovascular interventions (EVIs) are an important adjunct to open surgical management of peripheral vascular injuries. In appropriate situations, EVIs decrease operative time, estimated blood loss, and iatrogenic complications when compared with similar surgical cohorts by limiting surgical dissection in traumatized operative fields. In situations where definitive repair is not possible with EVIs, endovascular techniques permit control of hemorrhage or damage and facilitate open surgical repair. EVIs for peripheral vascular injury have proven effective in three anatomic regions: the neck, subclavian, and lower-extremity regions. The interventional radiologist should become familiar with the physical and personnel resources in the area preferred by the consulting trauma team to minimize unnecessary delays when acute intervention or angiography is requested. Clinical and radiographic surveillance for patency and compliance with antiplatelet or anticoagulation therapy is essential but has historically been poor in trauma patients.
Keywords: Endovascular, trauma, vascular injury, carotid artery, subclavian artery, femoral artery
GENERAL PRINCIPLES
Management of peripheral vascular injuries with endovascular techniques has increased in frequency during the last decade as trauma surgeons have become more familiar with endovascular capabilities and operating rooms (ORs) have been adapted to permit endovascular interventions in acute trauma patients. Endovascular interventions (EVIs), when appropriate, decrease OR time, estimated blood loss (EBL), and iatrogenic injury in the area of trauma but increase operating costs when compared with open surgical cohorts.1,2 There has been a steady increase in the use of endovascular interventions for peripheral trauma as more physicians experienced in these techniques become available. In a review of the National Trauma Data Bank, Reuben et al found an increase from 2.1% in 1994 to 8.1% in 2003 in the use of EVIs for vascular trauma. Fifty-five percent of vascular injuries are from blunt mechanisms, and 45% are secondary to penetrating trauma.1 The blood vessels that are most frequently injured in blunt trauma are the iliac, internal carotid, and brachial arteries and the thoracic aorta. The most frequently injured vessels in penetrating trauma are the brachial artery and the superficial femoral artery (SFA). EVIs are utilized to treat and manage venous traumatic injuries, but this review will focus on arterial injuries.3,4,5
The ideal candidate for EVI is a patient with a low-velocity injury (stab wound or handgun blast) in an anatomic region where surgical exposure may prolong ischemic or bleeding complications, or in a region with an increased risk of iatrogenic nerve injury during exposure of the vessel, such as the subclavian or internal carotid artery (ICA). Injuries that require surgical intervention, such as debridement for high-velocity gunshot wounds or contamination, embolectomy, or compartment syndrome, may not benefit as much from definitive endovascular repair. However, these injuries may benefit from angiography and proximal balloon occlusion to limit EBL. In addition to balloon occlusion, hemorrhage control interventions include embolization and deployment of a covered stent. Pseudoaneurysms and arteriovenous fistulas are excluded with either covered stents or coil embolization with or without a stent. Dissections have been managed with balloons, bare metal stents, or covered stents.
The only absolute contraindication to endovascular repair of an injury is the inability to cross a lesion with a wire unless the goal of the procedure is hemorrhage control with embolization. There are several relative contraindications that should be considered in conjunction with the consulting trauma service. Uncontrolled hemorrhage and hemodynamic instability have long been considered absolute contraindications to EVI, but this has been in the context of an endovascular suite that is remote from the OR. Hybrid ORs with endovascular capabilities may permit more liberal use of endovascular techniques in the OR in the event that immediate surgical access is necessary. The inability to use heparin is also an important consideration, but not an absolute contraindication because most open repairs would have the same limitation. The interventional radiologist (IR) must be aware specifically of two situations when considering heparin: multisystem trauma patients with associated intrathoracic or intra-abdominal injuries and blunt trauma victims with possible closed head injuries. Insufficient fixation points and obliged coverage of major arterial branches for stents or covered stents are additional considerations for EVIs. Placement of stents in areas of mobility such as the ICA, popliteal artery, common femoral artery, and axillary artery has also been considered a contraindication for EVI. With the exception of the popliteal artery, these anatomic regions are relatively accessible surgically, and the utility of an EVI can be discussed with the consulting service based on the aforementioned considerations.
In preparation for an integrated endovascular and open approach to the management of peripheral vascular injuries, it is essential that the IR become familiar with the physical environment where most acute trauma interventions will take place and the personnel experienced with EVIs. The OR may only have portable equipment, or a dedicated hybrid OR may have a fixed fluoroscopy unit. If portable equipment is used, the largest available OR is preferred. A critically important issue is the experience level of the OR staff, including the surgical technician, OR nurse, and radiology technician. This is a particularly important factor at night when designated endovascular personnel may not be available. If the OR staff are not familiar with the equipment (endovascular table, power injector, basic endovascular techniques, and so on) or the location of endovascular supplies, the IR may need to make arrangements to have an endovascular team available for acute interventions. These issues are vital when treating an acutely ill trauma patient as unnecessary delays may allow the lethal triad of hypothermia, coagulopathy, and acidosis to develop in the patient with uncontrolled hemorrhage. Considerations for endovascular trauma inventory to ensure versatility include the following: long (90- to 120-cm) sheaths and deployment platforms for stents, balloons, and covered stents; exchange length (>260-cm) wires; larger sheaths (9 or 10 French) for covered stents; monorail systems with coronary balloons and stents for tibial interventions; digital subtraction capability (if portable equipment is used); a power injection device for high-flow vessels; and compliant balloons for hemorrhage control of larger vessels to include veins. Although balloon-expandable stents and covered stents have been used successfully, self-expanding stents are preferred to minimize flexibility limitations in mobile arteries.6,7,8 Self-expanding stents are oversized by 10 to 20% of the diameter of the injured vessel to optimize apposition. Lastly, informing the trauma team of your facility's endovascular capabilities through presentations or participation in morning report is vital to ensuring that trauma patients have access to endovascular management of their injuries.
Follow-up for all trauma patient interventions is notoriously poor. Most studies, including those cited in this review, are retrospective with short follow-up periods by traditional surveillance standards. Facilities that perform EVIs should pursue diligent prospective surveillance, including duplex studies and ensuring continued antiplatelet therapy for prescribed periods, to maximize durability and reporting of complications for EVIs. Although a perceived disadvantage of endovascular repair of arterial injuries has been lack of durability, this has not been supported by case series on this subject. At the very least, appropriate endovascular interventions may serve as a damage control technique that will allow critically ill patients time to recover from acute systemic injuries and delay open surgical intervention. In addition to these general principles, this review article will address EVI in three common peripheral anatomic regions that each has specific considerations when approaching injuries in these areas.
LOWER-EXTREMITY AND ILIAC ARTERY INJURIES
Injuries to lower-extremity and iliac arteries have significant morbidity and mortality. Iliac artery injury has a reported 40% mortality.7 Penetrating and blunt injuries to the popliteal artery have mortalities of 10.5 and 27.5%, respectively, and injuries to the tibial arteries have an amputation rate of 38%.8,9 The popliteal and iliac arteries are most suitable for EVI based on their challenging surgical accessibility.
The most commonly injured peripheral artery, the SFA, is easily accessible via surgical exposure, which negates the major benefits of EVIs outlined above. Exceptions to open surgical management occur in situations with concomitant orthopedic injuries where hardware compromises surgical exposure or when an angiogram reveals a lesion that can be treated easily with endovascular techniques and facilitate an efficient transition to orthopedic fixation. Blunt SFA trauma complicates 10% of femur fractures with flow-limiting dissection or thrombosis, and a focal lesion that can be crossed with a wire successfully can be treated with a short self-expanding stent and be expected to have good long-term patency. Iliac artery injuries managed with EVI avoid surgical exposure of large retroperitoneal hematomas with a propensity for hemorrhage and iatrogenic risk to the ureters and underlying iliac veins.
Surgical exposure of injuries to the popliteal artery is performed typically through a medial approach with extensive dissection and risk of injury to the veins and peroneal nerve in addition to disruption of muscles and tendons. This risk and morbidity of this incision in addition to the saphenous vein harvest incision may be alleviated with EVIs. In a case report from 2007, a blunt popliteal artery occlusion with a pulseless extremity was repaired with a percutaneous thrombectomy with a distal cerebral protection device (Filterwire EZ; Boston Scientific, Natick, MA), balloon angioplasty of an intimal dissection through a 6-French sheath, and a procedure time of 1 hour without complications.9 The intervention offered to each patient will need to be individualized in consultation with the referring service. Occasionally, an open surgical intervention will still be the preferred means to treat an injury.
Injuries to tibial arteries with foot ischemia must involve repair of one of the tibial vessels with a tibial artery bypass to avoid amputation. EVI has been performed safely with 2-year patency in a patient with foot ischemia and thrombosis of the anterior tibial, peroneal, and posterior tibial arteries treated with self-expanding coronary stents to maintain patency of the posterior tibial artery. EVI in this case avoided surgical dissection posterior to the tibia in a surgical field with an open tibia fracture, hematoma, and orthopedic hardware to reduce the fracture.8
CAROTID
Patients with carotid and vertebral artery (VA) injuries (CVIs) are at risk for significant neurological morbidity and mortality. Blunt CVI carries a 20 to 40% mortality and 40 to 80% stroke rate if left untreated without anticoagulation, and penetrating CVI has a mortality of 31% and stroke rate of 23%.2,10 These concerns of neurological morbidity and mortality from embolic complications has led to a more aggressive treatment strategy for CVI when compared with other anatomic regions.
Most blunt CVIs are managed with anticoagulation unless contraindicated because of associated injuries and bleeding complications. The most common blunt injuries that require treatment are pseudoaneurysms associated with dissections and grade IV VA injuries (occluded without extravasation) in patients who cannot be anticoagulated.10,11,12 Pseudoaneurysms are treated with either stent grafts or stents with adjunctive coil embolization, and grade IV VA injuries are treated with coil embolization.
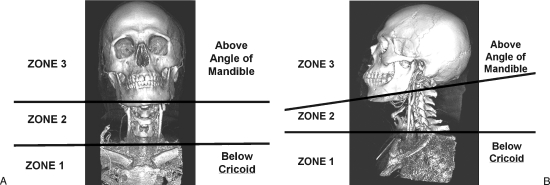
The neck is divided into anatomic zones to facilitate decision making in the management of penetrating neck injuries. The sternocleidomastoid muscle separates the neck into anterior and posterior triangles. The major vascular structures in the neck are within the anterior triangle. The neck is further divided into three horizontal zones (Fig. 1). Zone 1 extends superiorly from the thoracic inlet up to the inferior border of the cricoid cartilage. The major vascular structures within this zone include the subclavian and innominate vessels, the proximal common carotid, and lower vertebral arteries. Zone 2 extends from the cricoid cartilage superiorly up to the angle of the mandible. The distal common carotid artery and its bifurcation along with the vertebral arteries are within this zone. Zone 3 extends from the angle of the mandible up to the skull base. This zone contains the external carotid artery branches as well as the internal carotid and distal vertebral arteries. The vessels within zone 2 are relatively easy to expose. Injured vessels within zones 1 and 3 may be very difficult to identify and control. For this reason, vascular injuries to zones 1 and 3 often require an arteriogram to evaluate and plan further interventions.
Figure 1.
Zones of the neck. Anterior (A) and lateral (B) views of a 3-D reconstruction of a computed tomographic angiogram of the neck demonstrate the three anatomic zones. Zone I extends from the thoracic inlet up to the inferior border of the cricoid cartilage. Zone II extends from the cricoid cartilage to the angle of the mandible. Zone III extends from the angle of the mandible up to the skull base. Injured vessels within zones I and III may be very difficult to identify and control, and may require an arteriogram to evaluate and plan further interventions.
Vascular injuries in zone 1 and zone 3 are difficult to efficiently expose surgically and warrant consideration for endovascular management. Penetrating carotid artery injuries in zone 2 of the neck (between the cricoid cartilage and angle of the mandible) are accessible via standard surgical approaches and are generally approached with open surgical management. In addition to penetrating zone 2 injuries, relative contraindications to endovascular management include aerodigestive injury, infected injuries, inability to cross a lesion with a wire, fresh clot in the region of injury, and the inability to anticoagulate the patient during the procedure. Uncontrolled hemorrhage is also a contraindication; however, balloon occlusion in some instances of zone 1 and 3 injuries will permit more efficient control of the bleeding even if surgical management is anticipated. For this reason, an arteriogram may be appropriate in patients who are hemodynamically unstable to obtain vascular control of a bleeding vessel. In contrast to blunt CVI, most penetrating injuries require deployment of stent grafts because of hemorrhage complications and will thus require a larger sheath than that required for bare metal stents or coil embolization.
In addition to general endovascular principles that are applied to management of all peripheral injuries, CVI management has some specific considerations and techniques. The ability to heparinize a patient undergoing an endovascular carotid intervention is essential, and this should be clarified with the consulting trauma service before proceeding. Life-threatening hemorrhage in a surgically inaccessible area is a conceivable exception to the need for adjunctive anticoagulation. Other injury complications, such pseudoaneurysms and arteriovenous fistula, may be postponed until anticoagulation is feasible. Cerebral protection devices (CPDs) should be considered in situations where an intraluminal thrombus is visualized but the 0.014-inch wire will not support a more rigid stent-graft deployment system. If a CPD is used, a buddy wire support with a stiff 0.018-inch wire or long 9- or 10-French sheath may be necessary to deploy the stent graft. The buddy wire may be the only option (if a CPD is used) for proximal left common carotid injuries because the long sheath will not have sufficient purchase across the ostium of the left common carotid artery. It is critical that carotid artery interventions are performed by an experienced IR, even more so than with axillosubclavian or lower-extremity injuries.
Data on the durability of CVI endovascular interventions are scarce, but available data show that selective use of these techniques is safe and durable. Du Toit et al examined a series of 19 zones 1 and 3 penetrating carotid injuries treated with stent grafts over a 10.5-year period. The technical success rate was 100% with one stroke within 30 days of the procedure. Of the 14 patients who had a mean follow-up of 44 months, there were no stent-graft-related strokes or deaths. One asymptomatic occlusion was detected in follow-up.2 DuBose et al reviewed 31 studies that examined stent placement for carotid artery injuries between 1994 and 2007. The postprocedural stroke rate for 113 patients was 3.5%, occlusion rate was 9.7%, and leak rate was 5.3% for a follow-up period of 2 weeks to 2 years.10 Donas et al reported an 11% stroke rate and 0% mortality in 63 patients treated for carotid artery dissection (28 related to trauma).12 Cox et al treated 10 pseudoaneurysms from military injuries in the carotid and vertebral arteries with no neurological morbidity; however, one of the two stent grafts occluded during follow-up.13
AXILLOSUBCLAVIAN ARTERY AND VENOUS INJURIES
Injuries to blood vessels in the thoracic outlet account for 5 to 10% of civilian and military vascular injuries.14,15 Morbidity is significant with a brachial plexus injury rate of 20 to 43%.16 Mortality of arterial and venous injuries is estimated to be 40 and 50%, respectively.5,17 Forty to 50% of penetrating axillosubclavian trauma is amenable to endovascular management.17
Emergent surgical exposure of axillosubclavian injuries is challenging and fraught with potential iatrogenic injury to neurovascular structures, blood loss, and prolonged operative times. Paraclavicular vascular exposures, including clavicle resection, imperil the vagus, phrenic, and recurrent laryngeal nerves; the brachial plexus; the thoracic duct and the underlying pleura, especially in the presence of a large hematoma or hemorrhage. Proximal surgical control may also necessitate a median sternotomy. Remote access to these injuries with endovascular techniques may decrease the morbidity associated with surgical exposure. In addition to femoral access, axillosubclavian injuries are accessible through retrograde brachial access, which allows for a direct approach to the injury while other injuries are being treated by the trauma team. Endovascular management, where feasible, has shown a significant reduction in operating room time and estimated blood loss when compare with similar surgical cohorts.14
The only absolute contraindication to endovascular management of axillosubclavian injuries is the inability to cross the injury with a wire, unless the goal is to embolize the area of injury to stop hemorrhage. In these situations, balloon occlusion can serve as proximal control while surgical exposure is obtained. Relative contraindications specific to this anatomic region include the need for VA coverage to exclude an injury with a covered stent, injury to the third portion of the axillary artery, and compartment syndrome. VA coverage is an acceptable consequence of endovascular management for a patient in extremis, as is placement of a covered stent in the distal axillary artery, where the concern is stent fracture in a mobile area of the upper extremity. Compartment syndrome does not preclude endovascular techniques, but the fact that surgical exposure is necessary diminishes the potential advantages of endovascular management.
Inadvertent percutaneous cannulation of the brachiocephalic arteries during attempts at central venous line placement is particularly suitable for controlled endovascular management and deserves mention in a trauma discussion. Placement of large-bore (>7-French) lines into arteries complicates 0.1 to 0.8% of attempted central line placements. Removal of these lines with external compression has a higher complication rate (to include stroke) than either surgical or endovascular repair. Guilbert et al undertook a case series and literature review of this subject. Fifteen of 24 (62.5%) and 7 of 7 (100%) patients with carotid and subclavian artery cannulation, respectively, had significant complications, whereas 1 of 14 (7.1%) patients treated with surgical exploration and 0 of 12 (0%) with endovascular management had complications. The relative risk was 17.86 favoring noncompression management (P < 0.001) compared with compression alone. Injuries to zone 1 of the neck and intrathoracic arteries and the subclavian arteries are preferentially managed percutaneously when large-bore catheters are inadvertently placed.18
Several recent series have shown the feasibility of endovascular management of axillosubclavian injuries. Xenos et al identified 27 such injuries between 1996 and 2002, of which 12 (42%) were deemed suitable for endovascular treatment. Seven injuries were managed with a variety of endovascular techniques, and these cases showed a significant reduction in OR time (132 ± 15 minutes versus 193 ± 15 minutes, P = 0.04) and estimated blood loss (70 ± 12.2 mL versus 220 ± 56.1 mL, P = 0.01) when compared with patients who underwent surgical repair. One-year patency rates were similar: 5 of 5 (100%) open repairs remained patent, 1 of 7 (14.3%) of the covered stents occluded with resultant arm claudication, and 2 of 7 (28.6%) (iatrogenic injuries) died secondary to complications of primary disease within 8 months.14 In a retrospective examination of axillosubclavian injuries, Danetz et al found that a similar proportion of injuries are appropriate for endovascular techniques. Excluding emergency room deaths, 17 of 40 (43%) penetrating injuries were potentially treatable. Approximately one-third of remaining injuries were deemed unsuitable for endovascular management due to hemodynamic instability because endovascular interventions were performed in interventional suites remote from the OR.17 Carrick et al identified 15 patients with penetrating subclavian artery injuries between 2004 and 2005, of which 4 of 10 (40%) survivors were managed successfully with endovascular techniques (covered stents) in an interventional suite separate from the OR. Eight of 10 (80%) patients underwent angiography, and 2 of 10 (20%) patients were taken directly to the OR secondary to hemodynamic instability. One failed endovascular attempt occurred during treatment of a pseudoaneurysm near the origin of the dominant VA where stent-graft fixation was compromised to preserve VA flow.16 Exclusion of the VA is a relative contraindication for EVI; nevertheless, it may be considered an acceptable consequence of endovascular management when faced with a patient with active hemorrhage.
CONCLUSION
EVIs have contributed significantly to the management of peripheral vascular injuries, proving to be effective in definitive repair, damage control, and hemorrhage control in critically ill trauma patients. In the few instances of comparison to similar open surgical cohorts, EVIs have had lower OR times and lower EBL and have trended toward decreased iatrogenic injuries in the area of trauma. The benefits of these improvements must be weighed against the additional costs of EVIs. The IR's familiarity with the physical environment and personnel in the OR will assist in anticipating potential challenges in the event that an EVI is planned to treat a peripheral vascular injury. Prospective surveillance protocols will maximize the effectiveness and durability of endovascular interventions.
ACKNOWLEDGMENTS
There has been no financial support received for writing this article.
REFERENCES
- Reuben B C, Whitten M G, Sarfati M, Kraiss L W. Increasing use of endovascular therapy in acute arterial injuries: analysis of the National Trauma Data Bank. J Vasc Surg. 2007;46:1222–1226. doi: 10.1016/j.jvs.2007.08.023. [DOI] [PubMed] [Google Scholar]
- du Toit D F, Coolen D, Lambrechts A, de V Odendaal J, Warren B L. The endovascular management of penetrating carotid artery injuries: long-term follow-up. Eur J Vasc Endovasc Surg. 2009;38:267–272. doi: 10.1016/j.ejvs.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Kumar V. Endovascular treatment of penetrating injury of axillary vein with Viabahn endoprosthesis. J Vasc Surg. 2004;40:1243–1244. doi: 10.1016/j.jvs.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Sam A D, II, Frusha J D, McNeil J W, Olinde A J. Repair of a blunt traumatic inferior vena cava laceration with commercially available endografts. J Vasc Surg. 2006;43:841–843. doi: 10.1016/j.jvs.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Tillman B W, Vaccaro P S, Starr J E, Das B M. Use of an endovascular occlusion balloon for control of unremitting venous hemorrhage. J Vasc Surg. 2006;43:399–400. doi: 10.1016/j.jvs.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Marin M L, Veith F J, Panetta T F, et al. Transluminally placed endovascular stented graft repair for arterial trauma. J Vasc Surg. 1994;20:466–472. discussion 472–473. doi: 10.1016/0741-5214(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Katsanos K, Sabharwal T, Carrell T, Dourado R, Adam A. Peripheral endografts for the treatment of traumatic arterial injuries. Emerg Radiol. 2009;16:175–184. doi: 10.1007/s10140-008-0771-9. [DOI] [PubMed] [Google Scholar]
- Alvarez-Tostado J, Tulsyan N, Butler B, Rizzo A. Endovascular management of acute critical ischemia secondary to blunt tibial artery injury. J Vasc Surg. 2006;44:1101–1103. doi: 10.1016/j.jvs.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Hutto J D, Reed A B. Endovascular repair of an acute blunt popliteal artery injury. J Vasc Surg. 2007;45:188–190. doi: 10.1016/j.jvs.2006.08.067. [DOI] [PubMed] [Google Scholar]
- DuBose J, Recinos G, Teixeira P GR, Inaba K, Demetriades D. Endovascular stenting for the treatment of traumatic internal carotid injuries: expanding experience. J Trauma. 2008;65:1561–1566. doi: 10.1097/TA.0b013e31817fd954. [DOI] [PubMed] [Google Scholar]
- Stein D M, Boswell S, Sliker C W, Lui F Y, Scalea T M. Blunt cerebrovascular injuries: does treatment always matter? J Trauma. 2009;66:132–143. discussion 143–144. doi: 10.1097/TA.0b013e318142d146. [DOI] [PubMed] [Google Scholar]
- Donas K P, Mayer D, Guber I, Baumgartner R, Genoni M, Lachat M. Endovascular repair of extracranial carotid artery dissection: current status and level of evidence. J Vasc Interv Radiol. 2008;19:1693–1698. doi: 10.1016/j.jvir.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Cox M W, Whittaker D R, Martinez C, Fox C J, Feuerstein I M, Gillespie D L. Traumatic pseudoaneurysms of the head and neck: early endovascular intervention. J Vasc Surg. 2007;46:1227–1233. doi: 10.1016/j.jvs.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Xenos E S, Freeman M, Stevens S, Cassada D, Pacanowski J, Goldman M. Covered stents for injuries of subclavian and axillary arteries. J Vasc Surg. 2003;38:451–454. doi: 10.1016/s0741-5214(03)00553-6. [DOI] [PubMed] [Google Scholar]
- Fox C J, Gillespie D L, O'Donnell S D, et al. Contemporary management of wartime vascular trauma. J Vasc Surg. 2005;41:638–644. doi: 10.1016/j.jvs.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Carrick M M, Morrison A C, Pham H Q, et al. Modern management of traumatic subclavian artery injuries: a single institution's experience in the evolution of endovascular repair. Am J Surg. 2009 doi: 10.1016/j.amjsurg.2008.11.031. June 10 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Danetz J S, Cassano A D, Stoner M C, Ivatury R R, Levy M M. Feasibility of endovascular repair in penetrating axillosubclavian injuries: a retrospective review. J Vasc Surg. 2005;41:246–254. doi: 10.1016/j.jvs.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Guilbert M C, Elkouri S, Bracco D, et al. Arterial trauma during central venous catheter insertion: case series, review and proposed algorithm. J Vasc Surg. 2008;48:918–925. discussion 925. doi: 10.1016/j.jvs.2008.04.046. [DOI] [PubMed] [Google Scholar]