In Part 1 of this two-part article, we discussed commonly used large-vessel embolic agents and clinical scenarios in which the agents might be used. We also discussed how to choose between the multitude of embolic agents that are currently commercially available. In this article, we will focus on embolic agents used to occlude small vessels and when to choose which agent.
It is vital to recognize one important characteristic of all embolic agents: regardless of composition, the smaller the agent, the greater the likelihood of organ ischemia. Most organs have some duplication of vascular supply; this collateralization is vital to organ survival following a proximal vascular event. If very small embolic agents are used, the effective level of the embolization is distal to where the collateral vessels join the main feeding artery, thereby effectively occluding inflow from both the primary and collateral circulations. Therefore, for most organ systems, small agents cause much greater ischemia than larger agents.
COMMONLY USED SMALL-VESSEL EMBOLIC AGENTS
Particles
Particles are currently the most commonly used agents for permanent small-vessel occlusion (Table 1). Particle sizes range between 100 and 1200 μm. Particles are radiolucent, but when mixed with contrast can be gently injected with a small syringe (1 to 10 mL) using fluoroscopic guidance. Use of small particles, especially in the solid organs such as the liver, may lead to focal necrosis and predispose to abscess formation.
Table 1.
Technical Pearls for Using Particulate Embolics
| Agent | Pearl |
|---|---|
| PVA, polyvinyl alcohol. | |
| Polyvinyl alcohol particles | Frequent agitation or addition of albumin (rarely used today) may prevent clumping, which is a common problem due to PVA's natural buoyancy. |
| Particle size should be matched to the delivery catheter used to prevent catheter occlusion (generally microcatheters are used for particles <700 μm). | |
| Forceful injection during the procedure may result in reflux of particles into a nontarget vessel due to “explosive” delivery. | |
| Trisacryl microspheres | Forceful injection through an undersized catheter may result in sphere cracking. |
Polyvinyl alcohol (PVA) is a nonuniform mixture of amorphous plastic particles commercially available in sizes ranging from 45 to 1180 μm. Generally, each vial contains a 200-μm size range (i.e., 300 to 500 μm) as a dry, coarse radiolucent powder that is reconstituted in contrast immediately prior to use. PVA particles cause a significant inflammatory response, which may add to the permanence of their occlusion.
Extruded PVA particles (Boston Scientific, Natick, MA) are a newer agent and are compressible and regular in shape. In theory, these particles are less likely to cause catheter occlusion. Trisacryl gelatin microspheres (Embospheres; Biosphere Medical, Boston, MA) are made from trisacryl gelatin; these spheres are round, homogeneous, and compressible. In theory, they provide improved delivery and vessel occlusion due to decreased clumping. Bead Block (Biocompatibles UK Ltd., Franham, UK) are compressible and hydrophilic PVA hydrogel particles with theoretical decreased risk of clumping. They are available suspended in sterile water in prepackaged syringes. Although FDA-approved as an embolic agent, Bead Block is now frequently used as a drug-eluting bead for transcatheter chemoembolization procedures.
Liquid Embolic Agents
Cyanoacrylates (glue) are tissue adhesive materials that polymerize rapidly once exposed to an ionic environment such as saline or blood, forming a cast of the vessel in which polymerization occurs (Table 2). An inflammatory reaction often accompanies the polymerization process. Ethiodol (Savage Laboratories, Melville, NY) is mixed with the glue to render cyanoacrylate radiopaque (Fig. 1).
Table 2.
Technical Pearls for Using Liquid Embolics
| Agent | Pearl |
|---|---|
| Cyanoacrylate | Nonionic solutions must be used during preparation to prevent polymerization and delivery catheter blockage; delivery catheter must be flushed with a nonionic solution, such as D5W, before and after every use; separate “nonionic table” is usually set up; glove changes are required before use; and immediate preuse mixing are all helpful techniques. |
| Ethiodol (Savage Laboratories, Melville, NY) mixture ratio is used to modulate the polymerization time per operator's preference (1:1–1:6), with higher concentrations of lipiodol conferring prolonged polymerization times. | |
| Ethylene-Vinyl Alcohol Copolymer (Onyx) Liquid Embolic System (Micro Therapeutics, Plymouth, MN) | Vials must be placed in a shaker for at least 20 min prior to the procedure, with continued agitation just prior to the delivery. |
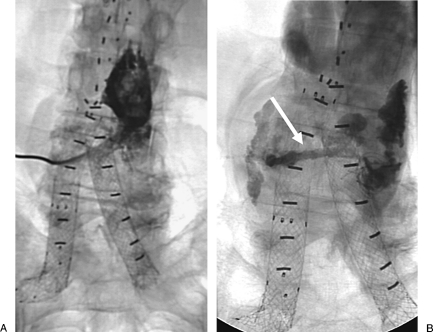
Figure 1.
A 67-year-old man status post–aortic endograft with type 2 endoleak. (A) Fluoroscopic image of translumbar sac injection shows sac. (B) Fluoroscopic image after glue embolization shows cast filling endoleak. Note “tail” of glue injected as the catheter is removed from the aortic sac.
Ethylene Vinyl Alcohol Copolymer (Onyx) Liquid Embolic System (Micro Therapeutics, Plymouth, MN) is a foamlike adhesive substance that solidifies upon contact with ionic materials. Onyx solidifies from the periphery toward the center, resulting in a spongy substance that forms a cast of the vessel (e.g., the “skin” forms first, and the cast fills from the inside, analogous to filling a water balloon).
Sclerosants are liquid embolization agents that produce nearly immediate and complete thrombosis and inflammation at the very small-vessel and tissue level (Table 3). Knowledge of vascular anatomy and prevention of embolization to nontarget locations is of utmost importance. Absolute alcohol is extremely toxic to living tissues, leading to complete vessel thrombosis/occlusion as well as direct tissue ablation and cell death. Essentially, whatever alcohol touches, it kills. Sodium tetradecyl sulfate (1 to 3%) is a mild sclerosant most often used for varicose vein, spermatic vein and renal ablations, pelvic congestion syndrome, and preoperative arteriovenous malformations. Boiling contrast has fallen out favor due to side effects (severe pain).
Table 3.
Technical Pearls for Using Sclerosants
| Issue | Pearl |
|---|---|
| Balloon inflation | Balloon inflation proximal to the injection site and aggressive aspiration with a large syringe prior to balloon deflation will prevent retrograde embolization. |
| Volume determination | Contrast injection with an occlusion balloon inflated will help to estimate the volume of sclerosant needed. |
| Analgesia | Patients usually sense an intense pain immediately after alcohol injection, and deep sedation or general anesthesia is usually recommended. |
| Alcohol radiopacity | When using alcohol, a 4:1 mixture with lipiodol will render alcohol radiopaque. |
Ethiodol is an inert oil-based contrast agent (Table 4). Once injected, oil droplets break into smaller globules to ∼50 μm in diameter, leading to a distal functional-level embolization. Lipiodol has an affinity to hepatocellular carcinoma, increasing the selectivity of the embolization.
Table 4.
Additional Technical Pearls
| Agent | Pearl |
|---|---|
| Ethiodol (Savage Laboratories, Melville, NY) | A hard plastic connector should be used during Ethiodol injection to prevent dissolution of the catheter hub by Ethiodol. |
| Gelfoam powder (Ethicon, Somerville, NJ) | Gelfoam powder tends to clump during injection, resulting in a more proximal (larger-vessel) occlusion (50–500 μm) than anticipated. |
| Thrombin | Complete thrombosis may take several minutes—allow this time to pass before deciding to inject again. Off-label for intravascular use. During intravascular catheter injection, devastating nontarget embolization may occur. |
Powder Substances
There are numerous powder embolics. Gelfoam (Ethicon, Somerville, NJ) powder is supplied in 1-g aliquots of sterile powder with each particle measuring ∼50 μm. Like Gelfoam sponge, Gelfoam powder embolization results in temporary occlusion lasting from days to several weeks. Microfibrillated collagen (Avitene, Davol Inc.; Cranson, RI; Angiostat) is a temporary agent very similar to Gelfoam in its properties. It is available in both “sheet” (analogous to sponge) and “flour” (analogous to powder) forms, representing proximal and distal agents, respectively. Thrombin is a natural enzyme that converts fibrinogen to fibrin as one of the end steps in clot formation for both the intrinsic and extrinsic coagulation cascades. Thrombin is most often used for direct-puncture injection of postcatheterization pseudoaneurysms to facilitate thrombosis. It is also used at the authors' institution, mixed with Gelfoam, for embolization of endoleaks following endovascular aneurysm repair. Starch microspheres are 50-μm porous spheres made of biodegradable starch that dissolve within minutes and are mainly utilized for distal small-artery temporary occlusion situations such as intra-arterial chemotherapy delivery.
CLINICAL SCENARIOS
Small-Vessel Permanent Occlusion
This clinical situation occurs when the abnormal vessel is too small to distinctly visualize angiographically, and a permanent occlusion is indicated. It is these scenarios, a third question must be answered by the operator, “Is tissue death desirable?” In situations in which end-organ or tissue death is not acceptable or desired (Fig. 2), such as some selective tumor embolizations, uterine fibroid embolization, bronchial artery embolization, or small-vessel gastrointestinal bleeding, particles are typically used. As a rule of thumb, the larger the particle size, the less likely the risk of ischemia. Larger particles (>300 μm) are typically used in these patient populations. In situations in which end-organ or tissue death is a desired end point, such as arteriovenous malformations (Fig. 3), superselective tumor embolizations (Fig. 4), and transcatheter renal ablation procedures, sclerosant liquid agents (absolute alcohol, sodium tetradecyl sulfate) and glues (cyanoacrylates, Onyx) are used. Ethiodol and thrombin may also be used as they cause occlusion at the very small-vessel level.
Figure 2.
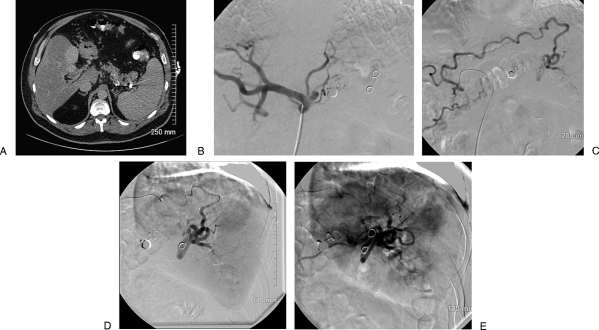
A 60-year-old man with long-standing cirrhosis and portal vein thrombosis. He has suffered multiple episodes of upper-gastrointestinal hemorrhage due to variceal bleeding, the most recent of which was life-threatening. He is not a candidate for transjugular intrahepatic portosystemic shunt due to his long-standing portal vein thrombosis. He underwent a proximal splenic embolization procedure 13 months previously in an attempt to decrease his portal blood flow to improve his portal hypertension. He presents now for a repeat embolization procedure. (A) Contrast-enhanced computed tomography scan of the abdomen, venous phase, demonstrating splenomegaly, fatty replacement of his liver, and multiple coils in his proximal and midsplenic artery. (B) Digital subtraction angiography of the celiac axis demonstrating complete occlusion of the proximal splenic artery. (C) Digital subtraction angiography of a hypertrophied right gastric artery, which has recanalized the distal splenic artery via short gastric arteries. (D) Delayed imaging of digital subtraction right gastric angiogram following embolization demonstrates normal splenic vascular blush. (E) Digital subtraction angiogram following embolization of the right gastric artery with trisacryl gelatin microspheres (700 to 900 μm) demonstrates significant diminution of flow to the normal splenic distribution. Because cell death was not a goal of the embolization, large particles were used. Notice also the reversal of flow in other collateral vessels supplying the splenic artery; this reversal of flow occurs due to the lack of outflow from the newly embolized splenic artery distribution.
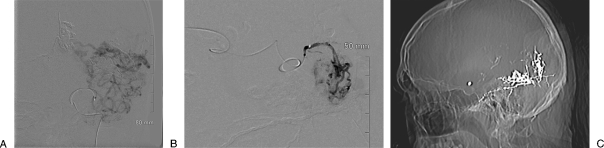
Figure 3.
A 41-year-old with Spetzler-Martin grade 6 arteriovenous malformation involving the mesial temporal and occipital regions. He presented with headache, right-sided numbness, paresthesias, and seizures (case courtesy of David Kumpe, M.D.). (A) Digital subtraction angiography of a branch artery demonstrates a large posterior cortical arteriovenous malformation. Multiple feeding arteries are supplying the nidus (or multiple nidi) of the arteriovenous malformation. Embolization at the current level of the catheter would occlude too many arteries, placing the underlying brain at risk from nontarget embolization. (B) Digital subtraction angiography following advancement of the catheter to a position closer to the nidus of the arteriovenous malformation. Embolization with a liquid embolic agent from here should minimize the risk of nontarget embolization. (C) Scout image from a computed tomography scan following multiple embolization procedures demonstrates the relatively large amount of radiopaque n-butyl cyanoacrylate used during this multistage embolization procedure.
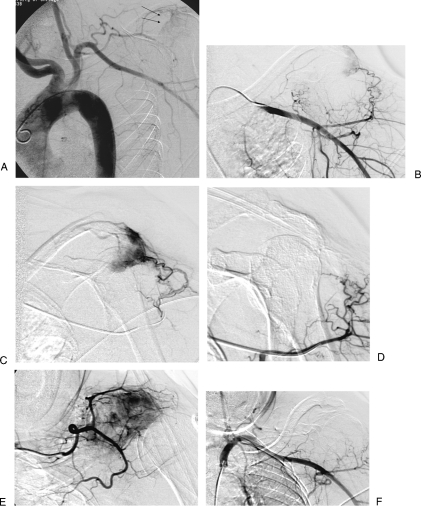
Figure 4.
A 68-year-old woman with renal cell carcinoma metastasis to left scapula. (A) Aortogram shows tumoral blush of right scapula (black arrows). (B) Selective axillary angiogram shows tumoral vessels. (C) Superselective angiogram through microcatheter shows tumor. (D) Superselective angiogram after embolization with trisacryl beads shows occlusion of tumoral vessels. (E) Superselective angiogram of second tumoral feeding vessel shows tumoral blush. (F) Final subclavian angiogram after additional particle embolization shows occlusion of tumoral vessels.
Small-Vessel Temporary Occlusion
This clinical situation is the least commonly encountered in this algorithm. Rarely used, small-vessel temporary occlusions may cause tissue ischemia but are designed to allow vessel recanalization. Such clinical settings typically involve tumor embolizations, where the operator knows that they are going to return for another round of treatment. Such agents include Gelfoam powder, starch microspheres, and Avitene.
APPROACH AND ALGORITHM
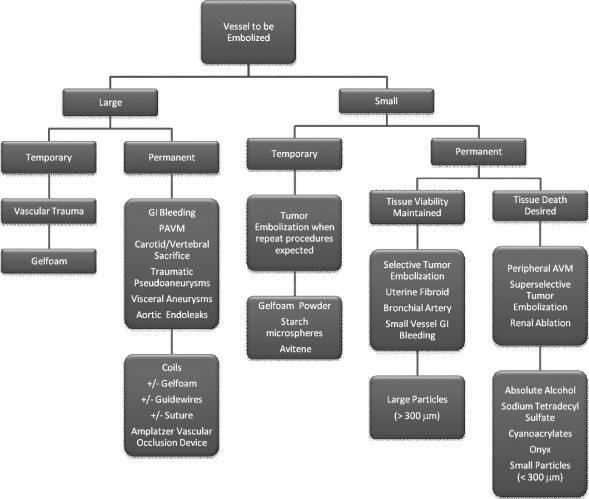
As discussed in Part 1 of this two-part article, to choose the most appropriate embolic agent, three questions must be considered (once it is decided that the vessel can be safely embolized, without putting the downstream organ[s] at risk; see Fig. 3):
How large is the vessel to be embolized? For the sake of this discussion, any vessel that can be seen angiographically is considered large. Vessels smaller than the resolution of digital subtraction angiography are considered small.
How long should the vessel remain occluded following embolization? Temporary occlusion implies occlusion of the vessel for hours to weeks; permanent occlusion is self-explanatory.
Should the embolized tissue remain viable after embolization? Ischemia is often a result (intended or unintended) of embolization. Intentional organ or cellular death implies using specific embolic agents.
By answering each of these questions (Fig. 5), the operator will progressively narrow down the appropriate agents to a much more manageable list.
Figure 5.
Decision algorithm for embolic choice. AVM, arteriovenous malformation; GI, gastrointestinal; PAVM, pulmonary arteriovenous malformation.
CONCLUSIONS
With the wide range of commercially available embolic agents, confusion is the rule rather than the exception on choosing an appropriate embolic. By taking a standardized, structured approach to each clinical situation, the operator can usually choose an appropriate agent. Asking the three simple questions outlined in this article—what size vessel, permanent or temporary occlusion, and whether organ death is desirable—will help the interventional radiologist decide.
SUGGESTED READINGS
- Loffroy R, Guiu B, Cercueil J P, Krausé D. Endovascular therapeutic embolisation: an overview of occluding agents and their effects on embolised tissues. Curr Vasc Pharmacol. 2009;7:250–263. doi: 10.2174/157016109787455617. [DOI] [PubMed] [Google Scholar]
- Abada H T, Golzarian J. Gelatine sponge particles: handling characteristics for endovascular use. Tech Vasc Interv Radiol. 2007;10:257–260. doi: 10.1053/j.tvir.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Laurent A. Microspheres and nonspherical particles for embolization. Tech Vasc Interv Radiol. 2007;10:248–256. doi: 10.1053/j.tvir.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Howington J U, Kerber C W, Hopkins L N. Liquid embolic agents in the treatment of intracranial arteriovenous malformations. Neurosurg Clin N Am. 2005;16:355–363, ix–x. doi: 10.1016/j.nec.2004.08.013. [DOI] [PubMed] [Google Scholar]