ABSTRACT
Trauma continues to be the leading cause of death in the young population. Uncontrolled bleeding is a major factor in early mortality after trauma, contributing to 30 to 40% of trauma-related deaths. Transcatheter embolization techniques play a significant role in the comprehensive modern treatment of traumatic vascular injuries to solid organs and extremities. The purpose of this article is to review current principles and techniques in the use of embolization for the treatment of traumatic arterial injuries of solid organs and extremities.
Keywords: Trauma, transcatheter embolization, embolization
Embolization is an established endovascular technique useful in the emergency treatment of many traumatic injuries.1 Embolization is a lifesaving procedure that can control bleeding in an expeditious and minimally invasive manner with less disruption of normal tissues than standard surgical intervention. Embolization in trauma is the intentional and controlled occlusion of vessels to stop hemorrhage. Most of the embolization agents work by creating a mechanical occlusion of the vessels by providing a framework for thrombus formation. Other mechanisms involve an inflammatory reaction of the vessel or direct destruction. In general, liquid and small-particle agents create a more distal (capillary) occlusion with a higher risk of ischemia and necrosis. These agents are mainly indicated in the treatment of tumors and arteriovenous malformation and are rarely used in trauma. Most of the embolization agents require a relatively intact coagulation cascade. It is imperative that embolization is performed before severe coagulopathy develops. Massive transfusion of blood products in the setting of multiorgan injuries are commonly complicated by a severe coagulopathy.2 It is estimated that a third of all patients admitted after severe trauma have coagulopathy and almost half are hypothermic.3
The widespread use of multidetector computed tomography (MDCT) scanners in the emergency department has decreased significantly the need of exploratory angiography.4 MDCT images are invaluable to guide the angiography when searching for areas of suspected injuries with selective angiograms. In the majority of cases, selective or superselective angiograms are always required to rule out any injuries and active contrast extravasation. For example, selective catheterization of each internal iliac (hypogastric) artery is almost always required to adequately rule out any pelvic arterial injuries that may not be very evident in a nonselective pelvic angiogram. A complete angiogram includes the evaluation of the presence of collaterals, potential anatomic variants, and status of the runoff vessels.
FACTORS TO BE CONSIDERED FOR EMBOLIZATION
Arterial access is usually obtained using a common femoral approach generally on the side opposite to pelvic or lower-extremity injuries. In a patient with poor or nonpalpable pulses, ultrasound guidance is very helpful to obtain arterial access.
Vessels in the generally young trauma population tend to spasm frequently.
Size of vessels and duration of occlusion. In general, large vessels require coils; smaller vessels can be treated with particles, gelatin sponge (Gelfoam, Upjohn, Kalamazoo, MI) or microcoils.
Need for an expedited treatment of injuries. In cases of massive bleeding in an unstable patient, nonselective embolization may be required when multiple areas of contrast extravasation are seen; examples of this situation include multiple arterial injuries of the pelvic vessels or the liver. In these cases, a temporary agent like Gelfoam is used to allow potential for future recanalization. If the trauma patient is relatively stable, superselective embolization is always recommended to preserve organ tissue and avoid tissue ischemia or necrosis.
Organs with dual vascular supply such as the liver or organs with extensive collateral circulation like the pelvis, the upper gastrointestinal tract, and pancreas tolerate well relatively large areas of embolization.
Extensive collateral circulation can provide distal circulation to areas of vascular injuries resulting in continuous bleeding. This phenomenon is most commonly seen in the liver, the pelvis, and muscular branches of the extremities. To avoid distal reconstitution of the injured vessels, distal and proximal embolization (so called “sandwich technique”) is recommended when possible. This is accomplished by placing coils distal and proximal to the site of vessel injury (Fig. 1). The technique requires crossing the area of injury, which is feasible when dealing with a pseudoaneurysm (PSA), but may be not possible or wise to do in cases of vessel transection. Due to the small size of many of the vessels in solid organs, distal embolization with particles or Gelfoam followed by proximal embolization with coils is an effective option.
Some organs like the spleen can tolerate proximal embolization as collateral circulation will serve to preserve the organ parenchyma. End arterial organs such the kidney have poor collateral circulation, and embolization will result in parenchymal loss. Superselective embolization is always indicated.
Nonexpendable axial branches of the upper (subclavian, axillary, brachial) and lower (iliac, common and superficial femoral, popliteal) extremities cannot be sacrificed with embolization and require surgical reconstruction or stent-graft placement to preserve distal flow.
In the presence of traumatic arteriovenous fistulas (AVFs), the potential for coil migration and pulmonary embolism should be considered.
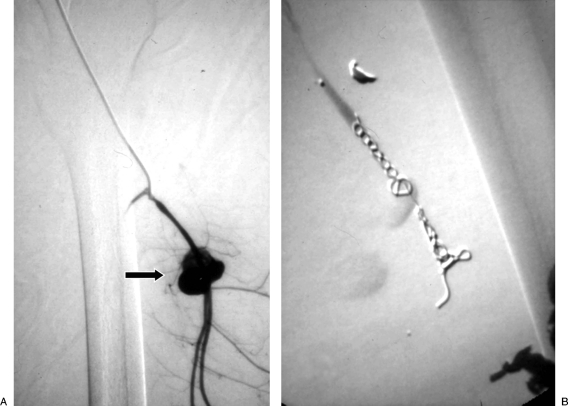
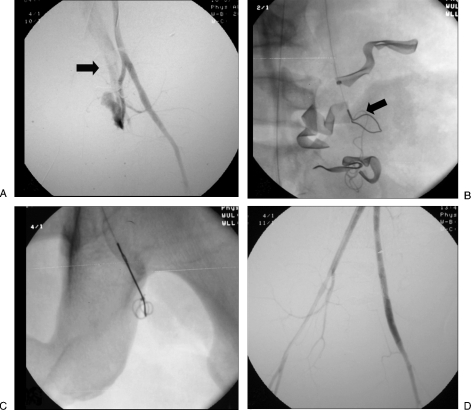
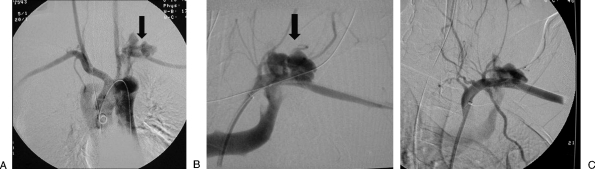
Figure 1.
“Sandwich” technique. (A) Digital subtraction angiogram shows a pseudoaneurysm (PSA) of the profunda femoris artery (arrow). (B) After distal and proximal embolization with multiple microcoils, there is successful exclusion of the PSA.
EMBOLIZATION AGENTS
Embolization agents are usually classified as temporary or permanent agents. Temporary agents include autologous blood clots, fat, dura, muscle and fascia (which are rarely used and have only an historical interest), and Gelfoam. Permanent agents include metallic coils and embolization particles.5 Most liquid agents including absolute alcohol and other sclerosing agents have no role in the treatment of traumatic injuries due to a significant risk of tissue necrosis. Some liquid agents such as N-butyl cyanoacrylate (Trufill NBCA, Cordis Neurovascular, Miami Lakes, FL) and ethylene vinyl alcohol copolymer (Onyx, Micro Therapeutics, Inc., Irvine, CA), have been used in the treatment of selected traumatic injuries especially in patients with coagulopathy. Thrombin is used extensively for the treatment of iatrogenic access site PSA.
Principles of Gelfoam and Particle Embolization
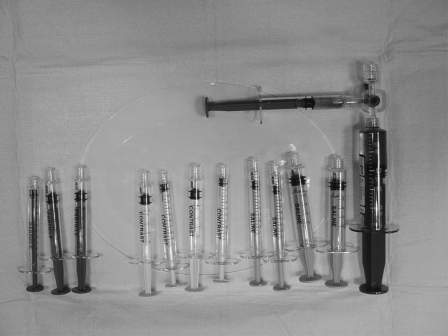
Table setup: a separate table with all the embolization agents and separate bowls for saline and contrast is recommended. Specially marked syringes are also recommended, with small red syringes marked as “embolization” or “particles,” and other colored syringes marked as “contrast” and “saline” (Fig. 2). Because many of the particles will lodge in the hub of a microcatheter, the syringes used for particle injection should not be used to inject contrast for postembolization angiograms as residual particles commonly stay in the walls and hubs of the syringes.
After selective catheterization of the bleeding vessel, a test injection of contrast is performed to ensure proper catheter position and potential reflux into nontarget areas.
A small amount of particles or Gelfoam is slowly injected with small “puffs” of the slurry or particles that are previously opacified with contrast. Many times this mixture is difficult to visualize, especially in obese patients. A digital subtraction angiogram using the same embolization rate and same small “puffs” will facilitate seeing if stasis is achieved or if reflux of the mixture is occurring.
After the particles or Gelfoam is injected, the catheter is flushed with normal saline. This is usually performed under fluoroscopic guidance until you no longer see the opacified agent; this implies that the system is “purged” or clean of the embolization material.
The particles or Gelfoam can clump together and occlude the catheter. This is not uncommon as the particles tend to aggregate at the hub of the microcatheter. This can usually be prevented by frequently flushing the system with normal saline after each particle injection.
If severe resistance is noted during the embolization, clean the system with a small volume of normal saline (better results are obtained with a 1-mL syringe) and avoid injecting a large volume of saline. A common mistake is to forcefully inject the saline in an effort to clear the system; this may result in significant reflux of the embolization agent in nontarget areas once the obstruction is relieved.
The speed and the dynamics of the vascular flow change as the embolization progresses. Fast-flowing distal vessels will become stagnant and additional embolization can easily reflux into proximal areas.
Consider that the system has a relatively large volume of embolization agent in the lumen of the catheters. This becomes critical in the last part of the embolization once stasis is accomplished and the system is “full” of the agent. If the target vessel is occluded and the catheter is still full of particles, removing or purging the catheter can cause accidental embolization of other organs.
Figure 2.
Photograph shows components of the embolization table. Note that different-colored syringes marked as “particles,” “saline,” and “contrast” are shown. Particles are mixed with contrast using a three-way stopcock.
Gelfoam
Gelfoam is one of the most commonly used agents in trauma. This gelatin sponge is a temporary agent derived from a biologic substance made of purified skin gelatin that has been used for many years as the primary embolization agent. Gelfoam is also commonly used to provide relatively distal target embolization followed by more proximal embolization with coils. Gelfoam is also used after placement of coils to reinforce thrombosis, especially in patients with coagulopathy where coils alone may not provide vessel occlusion.2 The agent has a highly porous structure with no intrinsic hemostatic action but induces thrombosis due to its close contact with platelets.6 Advantages of this agent include that it is readily available, is inexpensive, and provides temporal embolization. Animal studies have shown that the peak foreign body reaction within a vessel can be reached after 20 days, with the cellular reaction disappearing by 45 days.7 In most clinical instances, vessel recanalization after Gelfoam is seen within 2 weeks but may range from 3 weeks to 4 months.6,8 In rare instances, the use of Gelfoam can lead to permanent occlusion, which may be observed after dense packing with the agent and with the use of smaller fragments.6,9 The main disadvantages are that the sizes of the particles are not uniform, and that disruption of the clot with rebleeding is possible.
Gelfoam Preparation and Tips
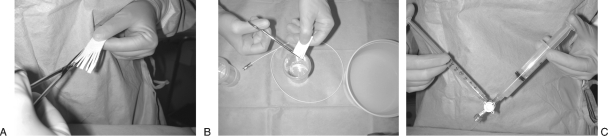
Gelfoam is available in a powder form with particle size of 40 to 60 um. Gelfoam powder provides a very distal capillary-level occlusion and it is not recommended in trauma applications. The most commonly used form is the sheet or block of Gelfoam that is cut with scissors in small 1- to 2-mm cubes that are then mixed with contrast medium in a 3- to 5-mL syringe using a three-way stopcock until a slurry is created (Fig. 3). When Gelfoam sheets are cut with scissors into cubes, the particles will have a size distribution ranging between 500 and 2000 um.6,10 When the agent in pumped forcefully through the stopcock, there is a significantly higher number of <500-μm particles created. A faster preparation method is to cut a sheet into larger 1 × 1-cm pieces by hand and to squeeze these into a 5-mL syringe. The compressed Gelfoam is then mixed with 3 to 5 mL of contrast using the stopcock and a second syringe. The resulting slurry is very concentrated, and usually smaller amounts are required to achieve vessel occlusion. The pumping method seems to produce smaller particles that are less uniform in size than the cutting method. Occasionally, a single larger piece or “torpedo” is injected to provide more proximal embolization; however, a larger piece is difficult to load in the system and can result in catheter occlusion. If a microcatheter is used for particles or Gelfoam injection, a larger-lumen (0.027 to 0.028 inches) microcatheter is recommended. Also, the stopcock can be place into a partially closed position to force the slurry into a more liquid form to prevent occlusion of the microcatheter lumen. If the use of microcoils is also planned, the regular 0.021-inch microcatheter lumen should be used to avoid having the coils “jammed” in the lumen of the larger (0.027- to 0.028-inch) microcatheter.
Figure 3.
Photographs of Gelfoam preparation steps. (A) The agent is cut into small cubes using sterile scissors. (B) The cubes are placed into a small cup to mix them with contrast. (C) The mixture is pumped using a three-way stopcock to create a slurry.
Particles
Principles of particle embolization are similar to Gelfoam; however, the main differences are that the sizes of the particles are more uniform and that particles produce a permanent occlusion of the vessel. Particles produce vessel occlusion by adherence to the wall, mechanical occlusion, and inflammatory reaction. Particles include polyvinyl alcohol (PVA), tris-acryl gelatin microspheres (TAGM), and hydrogel particles, among others. The industry standard today is to make calibrated sizes that typically span a 200-μm range: 100 to 300 μm, 300 to 500 μm, 500 to 700 μm, 700 to 900 μm, and 900 to 1200 μm. The particles are prepared by mixing them with contrast material, usually by adding 5 mL of contrast to the 2- to 5-mL prefilled saline. The air is carefully removed, and the syringe is gently inverted several times. Then, using a three-way stopcock attached to a smaller 1- to 5-mL syringe, the solution is further mixed (Fig. 2). The smaller syringe is used to inject small aliquots of the particles under careful fluoroscopic guidance. Constant agitation and remixing of the agent is very important to prevent particle aggregation. A higher-contrast material concentration usually results in precipitation of the particles with a higher tendency to conglomerate and form clusters. The solution tends to become more stable with time, and premixing several minutes in advance is important. Adding saline makes the solution more homogeneous but visualization of the injections under fluoroscopy may be difficult especially in obese patients or when motion artifacts are present.
PVA Particles
PVA was originally used as a sponge sheet shaved into small fragments, then as noncalibrated particles that were not very uniform in caliber, and more recently as calibrated particles that are more uniform in size (Contour SE Boston Scientific, Natick, MA). Particle aggregation with occlusion of the hub of the catheter was a significant problem with the noncalibrated particles. Also, the extent of embolization appeared to be more diffuse with the noncalibrated particles because the produced aggregates caused more proximal embolization of larger vessels and remained occlusive over time.
TAGM
TAGM spheres (Embospheres, Biosphere Medical, Rockland, MA) are made from an acrylic polymer matrix impregnated and embedded with porcine gelatin. The particles are packaged in a 20-mL syringe prefilled with 2 mL of normal saline. A hydrophilic surface and uniformly spherical shape prevents aggregation.
Bead Block Microspheres
Bead Block microspheres (Biocompatibles, distributed by Terumo, Somerset, NJ) are also produced from a biocompatible microporous PVA hydrogel. The particles tolerate 20 to 30% deformation and are highly flexible and compressible. The behave more like the TAGM particles that the PVA particles.
There are differences in the physical properties between the different types of spheres that may explain the different levels of occlusion of observed in vivo. Animal studies have shown that for the same size of particles, PVA spheres blocked significantly more distally in the vessels than TAGM spheres. PVA-based spheres (Contour SE) behave like a macroporous sponge that releases water under compression, and Embospheres or Bead Block spheres act as polymeric hydrogels that can be deformed without releasing water.11 Bead Block spheres have more deformation with slightly deeper penetration than Embospheres, but significantly less distal penetration than the Contour SE spheres.
Embozene Microspheres
Embozene microspheres are newer particles (CeloNova Biosciences, Newnan, GA) made of a hydrogel core covered with a Polyzene agent that helps lubricate the surface and have high compressibility. Each size is color coded. They have the tightest size distribution with most particles within a 50-μm range. They are available in close range of sizes: 40, 100, 250, 400, 500, 700, and 900 μm. In theory, there is less tendency to clump, aggregate, or cause catheter occlusion. Further clinical studies are needed to evaluate if these narrow-range particles have significant advantages over the conventional spheres.
Quadra Spheres
Quadra spheres are polyvinyl alcohol sodium acrylate spheres (Biosphere Medical) that expand to 4 times their dry-state diameter within 10 minutes when in contact with blood, nonionic contrast medium, or saline solution. In theory, the sphere expansion allows for secondary conformability to the vessel architecture, resulting in an increase in surface area contact for a more complete vessel occlusion. These particles have been used for drug delivery and uterine fibroid embolization; experience in trauma is currently limited.
Liquid Embolization Agents
The use of liquid agents is limited in trauma. Absolute alcohol or other sclerosing agents are not used in trauma due to the potential for tissue necrosis. N-butyl cyanoacrylate (Trufill NBCA, Cordis Neurovascular) and ethylene vinyl alcohol copolymer (Onyx, Micro Therapeutics, Inc.) may facilitate successful embolization independent of the patient's coagulation status. They have been used successfully in the treatment of selected trauma patients with coagulopathy. The disadvantages of n-butyl cyanoacrylate and ethylene vinyl alcohol copolymer include the excessive cost of these agents and the need for extensive experience by the operator to prevent serious complications.12
Thrombin
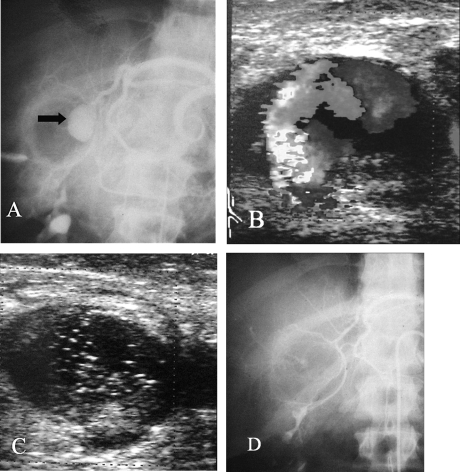
Thrombin has been used extensively for the treatment of femoral iatrogenic PSA after catheterization. It is derived from bovine thrombin with the rare potential of allergic reactions. Thrombin directly activates fibrinogen and converts it into fibrin monomers. Being a liquid agent, there is the potential for accidental distal embolization with serious consequences. Main uses in trauma include the percutaneous treatment of postcatheterization PSA and the direct percutaneous puncture and embolization of peripheral PSA where the catheterization of the parent vessels is impossible. It has been successfully used in the treatment of traumatic PSA of the liver, spleen, lower extremities (Fig. 4). Thrombin is used a liquid agent usually in a dilution of 1000 in 1 mL, injecting a small volume until thrombosis of the PSA is seen. At times, thrombin is combined with collagen for a more viscous and stable solution.
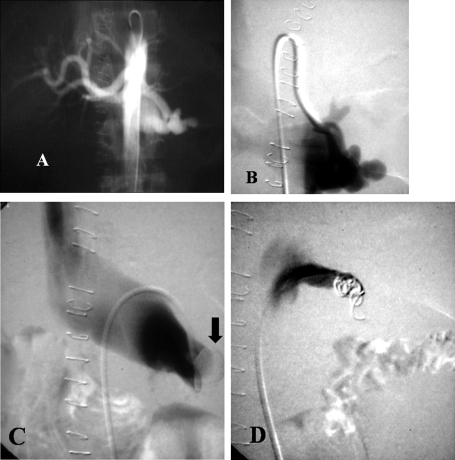
Figure 4.
Patient with a stab wound to the liver resulting in hemobilia. (A) Angiogram shows a large pseudoaneurysm (PSA) of a replaced left hepatic artery (arrow). Superselective catheterization was not possible due to severe spasm of the vessels. Percutaneous puncture with injection of thrombin under ultrasound guidance was performed. (B) Duplex ultrasound demonstrates flow in the PSA. (C) Duplex ultrasound after thrombin injection shows thrombosis of the PSA. (D) Angiogram after the embolization shows no filling of the PSA.
Metallic Coils
A coil is basically a piece of wire that is looped in different shapes and sizes. Coils provide a frame for clot formation and have the addition of some fibers (made of wool, nylon fibers, polyester, silk) to increase thrombogenicity. Bare coils require relatively dense packing to produce vessel occlusion. Coils are made of steel or platinum. Platinum is more expensive but is very radiopaque and more malleable than steel. Coils are available in multiple configurations. For trauma embolization, the main types of coils used are the macrocoils in 0.038- and 0.035-inch sizes and available in diameters from 3 to 15 mm. Microcoils are inserted through microcatheters usually with a 0.021-inch lumen and available in sizes from 1 to 20 mm. Most of the coils are deployed by pushing them with a wire or a special wire pusher (pushable coils). Other coils have some mechanism with controlled deployment that allows reposition before final release of the coil (detachable coils).5
Tips and Tricks for Coils
Regular coils are available in 0.038- or 0.035-inch thickness. The 0.038-inch coils would get stuck inside the lumen of a regular 0.035-inch catheter. To avoid confusion with the lumen of the catheters, it is more practical to keep a stock of only 0.035-inch coils that would fit through both 0.035-inch and 0.038-inch catheter lumens.
Microcoils are usually of 0.018-inch thickness and deployed through a microcatheter with a 0.021-inch (0.53-mm) lumen. Many of the pushable microcoils and some of the detachable coils (Interlock) may become “jammed” inside the lumen of a larger diameter (0.027-inch) microcatheter when the wire used to push the coil out passes over the proximal end of the coil. When this occurs, deployment may be impossible, or the coil can be deployed in a nonintended area.
Ensure the catheter position is stable. This is of paramount importance because it is not uncommon for the coil to push back the catheter into the parent vessel, resulting in nontargeted embolization. A good support system is very important to force the coil into a dense packing. Most of the time a regular 4- to 5-French diagnostic catheter is used to deploy the coils, or a microcatheter is advanced through the diagnostic catheter to deploy a microcoil. In some cases, a guiding catheter or a long sheath are used to further secure the 4- or 5-French diagnostic catheter with the microcatheter, forming a more stable triaxial system.
If the coil is kicking back the catheter, firmly hold the system to force the coil to form in the vessel. Some of the detachable coils can be used as a wire to obtain a more distal access if the coil is pushing the system back into the parent vessel. Once the microcatheter is in a more distal position, an attempt to redeploy the coil can be made. If the problem still persists, using a smaller and shorter coil will often resolve this issue.
If there are doubts about the stability of the catheter, a wire test is recommended. Simply use the access wire to determine if advancing it a few centimeters into the target area will result in the catheter pushing back too much. If there are doubts about the stability of the system and the catheter is close to a critical vessel, it is advisable to use a detachable coil system that will allow repositioning before final release.
Always use an introducer sheath for arterial access in the event the coil becomes stuck in the catheter, so that both the catheter and the coil can be removed without losing arterial access.
Never use a catheter with side holes, because the coil may become wedged inside the side hole and it will not deploy.
Always make sure that the coil delivery system is well positioned inside the hub of the catheter to avoid deploying part of the coil in the hub of the catheter, which will result in catheter occlusion or coil damage.
Correct vessel sizing is critical. In general, coils should be sized about the size of the vessel. Some softer coils can be oversized 20 to 30%, and other stiffer coils only allow less than 1-mm oversizing without remaining elongated once deployed.
A coil that is too small for the vessel may migrate distally. A coil that is too large may push the catheter back and cause nonintentional proximal embolization or migration of the coil into a critical vessel (Fig. 5).
A small coil can also embolize through an AVF into the pulmonary arteries. A larger short coil can be placed first followed by smaller softer coils for dense packing (scaffold technique). Other alternatives to treat AVF include placement of an occlusion balloon in the venous side (Fig. 6), the use of an Amplatzer plug (AGA Medical, Plymouth, MN) (Fig. 7), or a detachable coil.
Compact coiling is very important to avoid vessel recanalization. Two classic techniques are the anchor technique and the scaffold technique. The former involves placing the initial coil in a small branch vessel to anchor the first coil. The scaffold technique consists of placing an initial larger and stiffer regular coil, followed by more dense packing with softer coils.
Complete vessel thrombosis usually takes less than 5 minutes. Although at times additional coiling may be required, waiting a few minutes and/or pulling the catheter back a few millimeters to avoid injecting the contrast medium inside the coils is all it takes to demonstrate or achieve vessel occlusion.
Regular coils are deployed using a regular guide wire; in our practice we routinely use a Bentson guide wire that has a very flexible and long distal tip portion that prevents the catheter from backing up when the coil is pushed. Specially designed coil pushers are commercially available to deploy microcoils.
It is very important to make sure the final portion of the coil is completely deployed, as removing the catheter may inadvertently pull the coil out of the target. Make sure the coil is completely deployed by advancing the guide wire past the distal end of the catheter, but only a few millimeters to prevent the coil being pushed distally by your wire.
Pushable coils cannot be repositioned if misplaced, and coil retrieval may be required in some cases. If the coil is not completely out of the catheter, sometimes the combination of coil and catheter can be gently advanced forward to try to engage the target vessel. If the coil is protruding from the tip of the catheter, it may be possible to remove both from the sheath as a unit. Careful fluoroscopic guidance is necessary to ensure that the coil is not pushed out of the catheter by the access sheath or guiding catheter.
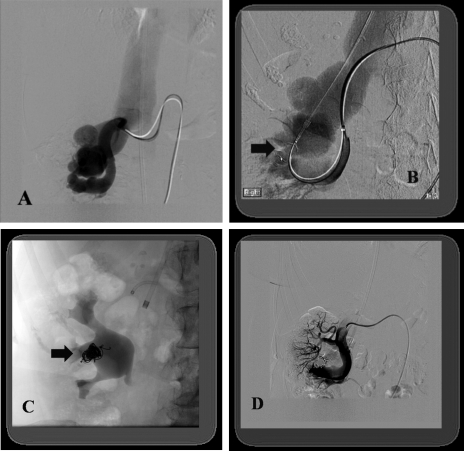
Figure 5.
(A) Digital subtraction angiogram shows a pseudoaneurysm (PSA) with arteriovenous fistula of the anterior trunk of the left hypogastric; note the draining vein (arrow). (B) Radiograph shows a misplaced coil protruding into the common iliac artery due to coil oversizing. A snare is also shown in an attempt to remove the coil that resulted in coil migration. (C) Radiograph shows the migrated coil being removed with the snare from the left common femoral artery. Part of a second coil was also protruding into the iliac artery. A noncovered stent was placed over the coil in the common-external iliac artery. (D) Digital subtraction angiogram shows successful exclusion of the PSA.
Figure 6.
Patient with a large left renal artery arteriovenous fistula (AVF) after a nephrectomy. (A) Angiogram shows an AVF of the stump of the left renal artery. (B) Selective left renal digital subtraction angiogram shows rapid filling of the left renal vein. (C) Digital subtraction angiogram venogram shows an occlusion balloon placed in the left renal vein to decrease flow and prevent coil migration. (D) Digital subtraction angiogram after embolization shows successful exclusion of the AVF.
Figure 7.
Patient with a massive long-standing renal arteriovenous fistula (AVF) that resulted in heart failure. (A) Digital subtraction angiogram shows large AVF massive dilatation of the renal vein. (B) Access was obtained in the arterial and venous systems. An Amplatzer plug was placed through the venous system (arrow) in the exact location of the fistula. (C) Radiograph shows multiple coils were placed inside the plug (arrow) to increase thrombosis. (D) Digital subtraction angiogram after embolization shows successful exclusion of the fistula. Note that the renal artery branches are now seen. Case courtesy of Barth Dolmatch, M.D., UT Southwestern, Dallas.
Detachable Coils
Detachable coils allow very precise deployment and embolization of different-sized vessels. The main advantage is that in case of initial misplacement, they can be retrieved. Disadvantages include that the setup takes more time and they are significantly more expensive. These coils were originally designed for the treatment of cerebral aneurysms; some of the manufacturers have developed similar versions for the peripheral use that are less expensive. These coils are mainly used for the embolization of vessels that are very close to parent vessels where very precise positioning is required. Table 1 shows the most commonly used detachable coils.
Table 1.
The Most Commonly Used Detachable Coils
| Brand Name | Sizes | Deployment System | Price | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Interlock Synthetic fibers (Boston Scientific) | 3- to 14-mm diameter helicals; 2/3–2/6 vortex | Interlocking | ++ | Highly visible due to platinum-tungsten alloy. Easy deployment. Very long coils allow compact packaging with one coil. | May accidentally deploy inside a 0.027-inch or larger lumen catheter. Lock may accidentally engage and retrieve the deployed coils if pushed inside the deployed coils. |
| Azur (Terumo) | 4–20 mm | Rapid electrolytic release. A light confirms that the detachment mechanism has been activated. | +++ | Platinum coil and an expandable hydrogel polymer. Coating undergoes limited expansion within the first 3 minutes, and fully expands within 20 minutes. In theory allows nearly 4–5 times more filling than regular platinum coils of the same size. | Moderately complex setup mechanism. |
| GDC (Boston) | Several sizes and shapes | Electrolytic detachment. A 1-mA current is used to detach the coil at the weld point. | ++++ | Nonfibered, extremely soft, platinum coil. | Require more operator experience, elaborate setup for delivery. |
| Trufill (Cordis) | 2–20 mm | Hydrostatic detachment. Coil is attached to the end of the microballoon. Releases when reaches 700 psi. | ++++ | Very soft, easy deployment. | Potential for coil motion during release. |
Amplatzer Vascular Plugs
Amplatzer vascular plugs (AVPs) are occlusion devices derived from septal occluders used in cardiology. These plugs consist of a noncovered nitinol mesh that should to be oversized 30 to 50% of the vessel diameter at the occlusion site. A guiding catheter or sheath is placed in position, and the plug is deployed by unsheathing it. The device can be resheathed if not in satisfactory position, and the plug is released by rotating the delivery wire counterclockwise. The main uses in trauma include the occlusion of relatively large vessels such the splenic artery, the renal artery, and peripheral AVF where using regular coils have the risk of accidental embolization (Fig. 7).13 Although the device is more expensive than the regular pushable coils, usually a single device is required, saving time and the cost of multiple coils. The main disadvantage of the AVP is that the device is relatively stiff compared with regular coils, and placing the guiding catheter or sheath inside tortuous vessels can be very difficult. Successful plug deployment into the target area may require using of triaxial system and additional manipulation. The first-generation AVP devices have diameters from 4 to 16 mm using 5- to 8-French sheaths. The second-generation AVP II devices have a three-disk configuration, which includes additional thinner disks attached at the ends of the plugs for additional cross-sectional material. These AVP II devices range from 3 to 22 mm using 4- to 7-French sheaths. These newer plugs are more thrombogenic.
COVERED STENTS IN TRAUMA
Although not embolization agents, covered stents can provide life- and limb-saving vascular hemostatis while keeping the vessel lumen patent. Covered stents allow treatment of PSA, AVF, and other injuries in the renal, iliac, subclavian, and axillary arteries and other nonexpendable vessels14,15,16 (Fig. 8). Custom-made devices were originally created by attaching a piece of saphenous vein or surgical graft over a bare metal stent. There are now several commercially available stent grafts. However, the use of metallic stents in trauma is not an approved indication, and it is an off-label applications of the stents that are marketed and FDA approved for biliary or tracheobronchial use. Potential complications of covered stent placement include stent occlusion, deformation and kinking, and loss of vessel branches after stent placement. Although rare, covered stents have also the potential of getting infected in case of bacteremia and sepsis. The use of large introducer sheaths and the cost of these devices are also a major drawback of the use of covered stents in trauma. Adequate sizing is also critical with stent grafts. Stent graft undersizing will result in an endoleak due to inadequate sealing of the vessel wall (Fig. 9). With the use of the Viabahn device (Gore, Flagstaff, AZ), stent oversizing can result in stent infolding. Table 2 shows the commercially available stent grafts that have been used in trauma. There is currently a lack of long-term data on the patency of covered stents in the treatment of injuries of younger patients. The use of covered stents in trauma patients is discussed in greater detail in the article “Endovascular Management of Peripheral Vascular Trauma” by Dr. Chatt Johnson included in this issue of Seminars in Interventional Radiology.
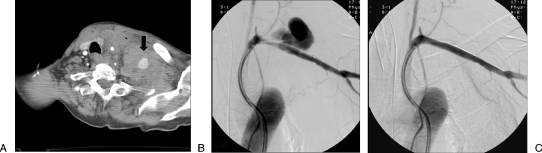
Figure 8.
(A) Axial computed tomography in an 80-year-old patient involved in a motor vehicle accident shows a large supraclavicular hematoma with active contrast extravasation (arrow). (B) Digital subtraction angiogram shows a large pseudoaneurysm (PSA) of the left subclavian artery. (C) Digital subtraction angiogram after placement of a covered stent shows successful exclusion of the PSA.
Figure 9.
Nonselective thoracic (A) and selective subclavian digital subtraction angiogram (B) show large subclavian artery pseudoaneurysm (PSA) with arteriovenous fistula (AVF; arrows). (C) Digital subtraction angiogram after stent-graft placement shows persistent filling of the PSA and AVF due to undersizing of the stent graft.
Table 2.
Commercially Available Stent Grafts
| Diameter (mm) | Length | Introducer sheaths | |
|---|---|---|---|
| Self-expandable | |||
| Fluency (Bard) | 6–10 | 4, 6, 8 cm | 8–9 French |
| Viabahn (Gore) | 5–13 | 2.5, 5,10,15 cm | 6–12 French |
| Balloon expandable | |||
| I Cast (Atrium) | 5–12 | 16,22,38,59 mm | 6–7 French |
| Jo Stent (Abbott) | 3–5 | 12,16,19, 26 mm | 6 French (7-French guiding catheter) |
GENERAL APPROACH TO SPECIFIC VASCULAR TERRITORIES
Extremity Trauma
Extremity injuries are commonly associated with orthopedic injuries. Any associated fracture or dislocation should be quickly reduced. Reduction alone may be adequate to restore perfusion. If the extremity is ischemic despite the reduction, urgent revascularization is indicated. Most cases of arterial disruption of the main arteries of an extremity mandate urgent surgical repair with surgical bypass. Occasionally, a transection can be recanalized and a stent graft placed, but most commonly an occlusion balloon is placed to provide temporary tamponade while surgical repair is undertaken.17 Nonvital arteries of the extremities can be safely embolized.
In the upper extremity, the muscular arterial branches in the arm can be safely occluded due to the extensive collateral supply. Isolated injuries of the radial, ulnar, or intereosseous arteries can be safely embolized if a distal palmar arch is intact. Usually coils are used, avoiding particles due to the risk of distal embolization in the hand. The brachial artery usually requires surgical reconstruction, although cases of repair of traumatic injuries to the brachial with covered stents have been reported. The small caliber of this vessel makes the use of covered stents in young patients a less attractive option. Surgical exposure of the axillary and subclavian arteries can be difficult. In cases of transaction of these vessels, temporary occlusion with a balloon is indicated while the patient goes to surgery.17 In some patients with a partial transaction, PSA, or AVF, especially in patients with multiple injuries, placement of a covered stents is a viable alternative (Fig. 8). Long-term data on the use of covered stents for traumatic extremity injuries is not available.
In the lower extremities, the common and external iliac, and the common and superficial femoral arteries are vital vessels that cannot be sacrificed. Traumatic injuries require repair with surgery or placement of covered stents.15 The branches of the profunda femoris artery are ideal targets for embolization, and the sandwich technique is recommended to avoid distal recanalization from multiple muscular collaterals (Fig. 1). The profunda femoris artery is a very important collateral; its main trunk should be preserved if possible, utilizing more selective distal embolization. The popliteal artery is commonly injured during knee trauma, and surgical repair is recommended. Embolization of one or two of the runoff vessels is technically possible as long as the plantar arch is supplied by the other vessels. Distal and proximal embolization with the sandwich technique is very important to avoid retrograde flow from the plantar arcades. Coils are the agent of choice18 (Fig. 10).
Figure 10.
Self-inflicted vascular injury in a 15-year-old male playing with a knife. (A) Digital subtraction angiogram shows a pseudoaneurysm of the anterior tibial artery with an arteriovenous fistula. (B) Digital subtraction angiogram shows multiple coils placed distal to the lesion using the sandwich technique. (C) Digital subtraction angiogram after embolization shows exclusion of the lesion; the anterior tibial is embolized. (D) Delay digital subtraction angiogram show retrograde filling of the anterior tibial artery up to the site of the distal coils.
Liver
Solid organ injuries are usually treated with surgical exploration if the patient is hemodynamically unstable. In stable patients or those where surgical control of hemorrhage is not possible, angiography with embolization is a lifesaving procedure. Most parenchymal liver injuries involve small or medium branches, and revascularization from distal branches after proximal embolization is not uncommon (Fig. 11). If superselective embolization with microcoils proximal and distal to the lesion is not possible, particle or Gelfoam embolization to achieve distal control followed by proximal coil embolization is recommended. Ideally, particle embolization should be performed distal to the cystic artery if possible. Extensive embolization is usually well tolerated thanks to the dual supply of the liver. However, in high-grade liver injuries, major hepatic necrosis, biliary and abscess complications are relatively frequent complications.19,20,21 Gallbladder infarction and biliary ischemia are also reported complications. Injuries to the common or proper hepatic arteries can be also coil embolized, but placement of covered stents is recommended if technically feasible. Biliary injuries with bile leakage may contribute to delayed hemorrhage that can present with hemobilia or arterioportal fistulas after high-grade liver injuries.2
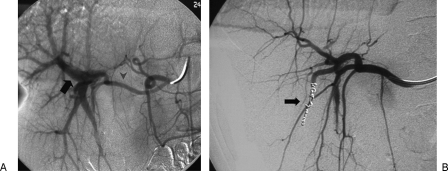
Figure 11.
Arterioportal fistula presenting with hemobilia after percutaneous liver biopsy. (A) Digital subtraction angiogram shows arteriovenous fistula (AVF); note the proper hepatic (arrowhead) and the portal vein (arrow). (B) Angiogram after distal and proximal embolization with microcoils shows successful exclusion of the AVF.
Spleen
The method of embolization of splenic injuries—proximal versus distal—is still a controversial issue. A shattered spleen with multiple areas of contrast extravasation is better treated with proximal embolization of the splenic artery using coils or Amplatzer plugs. Collateral circulation from the pancreatic, gastroduodenal, and gastric branches usually maintain distal splenic perfusion.22 Parenchymal injuries can be treated with selective distal coil or particle embolization, but if the injuries areas are too numerous, distal embolization may result in loss of as significant portion of the parenchyma and the procedure can be very time-consuming. Splenic infarction, splenic atrophy, and postprocedure bleeding are reported complications. Fever, pleural effusion, and partial splenic infarction are not uncommon.23,24 Splenic abscess formation is the most feared complication after embolization but is relatively infrequent. Rarely, extensive splenic artery embolization can result in pancreatitis.25
Kidneys
Injuries of the main renal artery are treated by surgical repair or placement of a stent graft. Penetrating or blunt renal injuries usually result in distal PSA or AVF. The renal arteries are true end arteries; renal parenchyma distal to the level of occlusion usually infarcts. Most of the time, distal recanalization from collateral vessels is not seen except in the rare case involving collaterals from capsular vessels.2 Superselective embolization of peripheral lesions usually with microcoils is recommended (Fig. 12).15,26,27 For very distal injuries, the use of Gelfoam or particles is also a viable alternative.
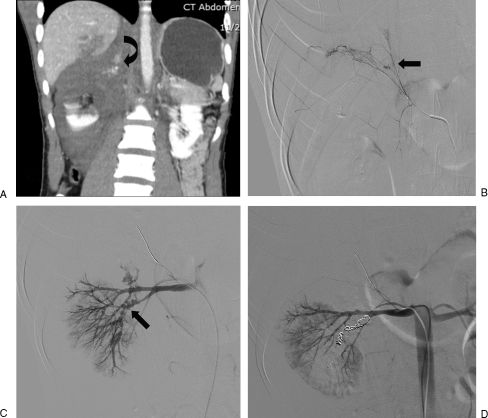
Figure 12.
(A) Coronal computed tomography image shows a large suprarenal hematoma with active extravasation (curve arrow). (B) Selective right adrenal angiogram shows active extravasation of contrast (arrow). (C) Digital subtraction angiogram renal angiogram shows active extravasation from lower pole branches. (D) Digital subtraction angiogram after adrenal and selective renal artery embolization shows no evidence of active extravasation.
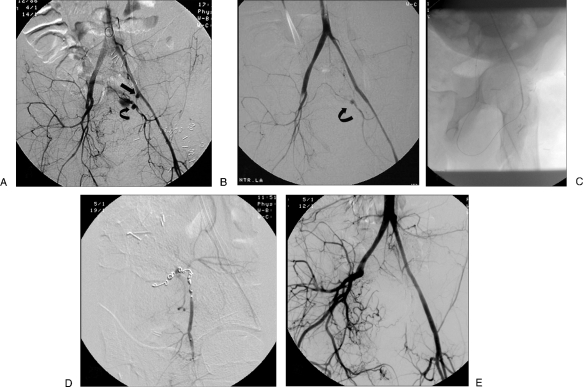
Pelvis
Retroperitoneal hemorrhage after pelvic fractures is difficult to control with surgery, and packing is often performed. Usually these patients are managed with external pelvic fixation using towels, sheets, or specially designed binders and pelvic C-clamps or external pins. Pelvic fixation can successfully arrest venous and smaller arterial bleeding as well as bleeding from bony surfaces.28 Embolization is commonly used to control arterial bleeding.29,30 Selective angiograms with injection of each hypogastric artery is recommended. Superselective embolization using Gelfoam or particles, or more commonly microcoils, is recommended. The potential for distal bleeding from collateral circulation can be a significant problem (Fig. 13). In patients who are hemodynamically unstable with multiple areas of contrast extravasation, nonselective proximal embolization of the anterior, posterior, or sometimes the hypogastric artery with Gelfoam can be a lifesaving procedure. Serious complications have been reported after pelvic embolization including uterine and bladder necrosis, paresis, buttock ischemia, and impotence.31
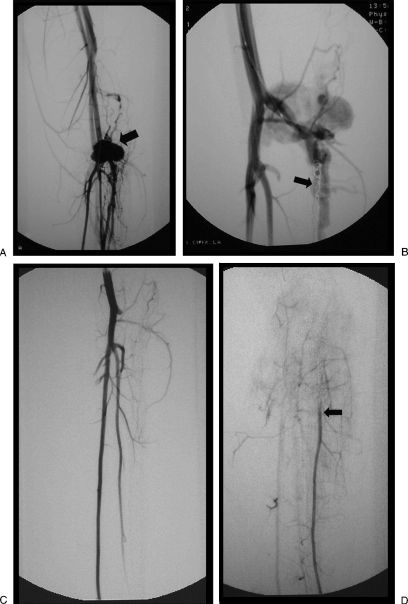
Figure 13.
Patient with massive bleeding after a gunshot wound to the pelvis initially treated with surgery. Following ligation of the right external iliac and left internal iliac (hypogastric) arteries, the patient continued with massive blood loss. (A) Digital subtraction angiogram pelvic angiogram. Delay image shows a stump of the left hypogastric (arrow); reconstitution of the left hypogastric is seen with a pseudoaneurysm (PSA; curve arrow). (B) Digital subtraction pelvic angiogram shows a stent graft placed over the stump of the left hypogastric; there is persistent filling of the PSA though collaterals. (C) Radiograph shows retrograde filling involving distal collaterals from the circumflex femoral artery into the area of PSA. (D) Digital subtraction angiogram shows multiple microcoils placed distal and proximal to the lesion. (E) Pelvic angiogram after the embolization shows successful exclusion of the PSA. (Case courtesy of Arturo Gonzalez, M.D., LSU, Lafayette, LA.)
CONCLUSION
Transcatheter arterial embolization is a minimally invasive procedure with an established role for the management of selected traumatic injuries of the solid organs and extremities. Multidisciplinary trauma teams with established protocols that help to decide when embolization and/or surgery are required are a critical part of the modern management of arterial injuries. Embolization should be performed early in the control of arterial bleeding before severe coagulopathy develops. There are multiple embolization agents and materials that require familiarization and practice to provide an expedited and safe control of hemorrhage of traumatic injuries.
REFERENCES
- Bauer J R, Ray C E. Transcatheter arterial embolization in the trauma patient: a review. Semin Intervent Radiol. 2004;21:11–22. doi: 10.1055/s-2004-831401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn D R, Cerny V, Coats T J, et al. Task Force for Advanced Bleeding Care in Trauma Management of bleeding following major trauma: a European guideline. Crit Care. 2007;11:R17. doi: 10.1186/cc5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert S, Dahl B, Lönn L. Update on the roles of angiography and embolisation in pelvic fracture. Injury. 2008;39:1290–1294. doi: 10.1016/j.injury.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Doody O, Given M F, Lyon S M. Extremities—indications and techniques for treatment of extremity vascular injuries. Injury. 2008;39:1295–1303. doi: 10.1016/j.injury.2008.02.043. [DOI] [PubMed] [Google Scholar]
- Vaidya A, Tozer K R, Chen J. An overview of embolic agents. Semin Intervent Radiol. 2008;25:204–215. doi: 10.1055/s-0028-1085930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abada H T, Golzarian J. Gelatine sponge particles: handling characteristics for endovascular use. Tech Vasc Interv Radiol. 2007;10:257–260. doi: 10.1053/j.tvir.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Barth K H, Strandberg J D, White R L. Long term follow-up of transcatheter embolization with autologous clot, oxycel and gelfoam in domestic swine. Invest Radiol. 1977;12:273–280. doi: 10.1097/00004424-197705000-00012. [DOI] [PubMed] [Google Scholar]
- Laurent A. Microspheres and nonspherical particles for embolization. Tech Vasc Interv Radiol. 2007;10:248–256. doi: 10.1053/j.tvir.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Jander H P, Russinovich N A. Transcatheter gelfoam embolization in abdominal, retroperitoneal, and pelvic hemorrhage. Radiology. 1980;136:337–344. doi: 10.1148/radiology.136.2.6967615. [DOI] [PubMed] [Google Scholar]
- Katsumori T, Kasahara T. The size of gelatin sponge particles: differences with preparation method. Cardiovasc Intervent Radiol. 2006;29:1077–1083. doi: 10.1007/s00270-006-0059-y. [DOI] [PubMed] [Google Scholar]
- Laurent A, Wassef M, Saint Maurice J P, et al. Arterial distribution of calibrated tris-acryl gelatin and polyvinyl alcohol microspheres in a sheep kidney model. Invest Radiol. 2006;41:8–14. doi: 10.1097/01.rli.0000188027.34400.f3. [DOI] [PubMed] [Google Scholar]
- Mavili E, Donmez H, Ozcan N, Akcali Y. Endovascular treatment of lower limb penetrating arterial traumas. Cardiovasc Intervent Radiol. 2007;30:1124–1129. doi: 10.1007/s00270-007-9142-2. [DOI] [PubMed] [Google Scholar]
- Widlus D M, Moeslein F M, Richard H M., III Evaluation of the Amplatzer vascular plug for proximal splenic artery embolization. J Vasc Interv Radiol. 2008;19:652–656. doi: 10.1016/j.jvir.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Piffaretti G, Tozzi M, Lomazzi C, et al. Endovascular treatment for traumatic injuries of the peripheral arteries following blunt trauma. Injury. 2007;38:1091–1097. doi: 10.1016/j.injury.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Burdick T R, Hoffer E K, Kooy T, et al. Which arteries are expendable? The practice and pitfalls of embolization throughout the body. Semin Intervent Radiol. 2008;25:191–203. doi: 10.1055/s-0028-1085925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi J C, Schönholz C, Ferreira L M, Bergan J. Endovascular stent-graft treatment of traumatic arterial lesions. Ann Vasc Surg. 1999;13:121–129. doi: 10.1007/s100169900230. [DOI] [PubMed] [Google Scholar]
- Scalea T M, Sclafani S J. Angiographically placed balloons for arterial control: a description of a technique. J Trauma. 1991;31:1671–1677. doi: 10.1097/00005373-199112000-00018. [DOI] [PubMed] [Google Scholar]
- Lopera J E, Suri R, Cura M, Kroma G, El-Merhi F. Crural artery traumatic injuries: treatment with embolization. Cardiovasc Intervent Radiol. 2008;31:550–557. doi: 10.1007/s00270-008-9309-5. [DOI] [PubMed] [Google Scholar]
- Monnin V, Sengel C, Thony F, et al. Place of arterial embolization in severe blunt hepatic trauma: a multidisciplinary approach. Cardiovasc Intervent Radiol. 2008;31:875–882. doi: 10.1007/s00270-007-9277-1. [DOI] [PubMed] [Google Scholar]
- Mohr A M, Lavery R F, Barone A, et al. Angiographic embolization for liver injuries: low mortality, high morbidity. J Trauma. 2003;55:1077–1081. discussion 1081–1082. doi: 10.1097/01.TA.0000100219.02085.AB. [DOI] [PubMed] [Google Scholar]
- Dabbs D N, Stein D M, Scalea T M. Major hepatic necrosis: a common complication after angioembolization for treatment of high-grade liver injuries. J Trauma. 2009;66:621–627. discussion 627–629. doi: 10.1097/TA.0b013e31819919f2. [DOI] [PubMed] [Google Scholar]
- Bessoud B, Denys A, Calmes J M, et al. Nonoperative management of traumatic splenic injuries: is there a role for proximal splenic artery embolization? AJR Am J Roentgenol. 2006;186:779–785. doi: 10.2214/AJR.04.1800. [DOI] [PubMed] [Google Scholar]
- Raikhlin A, Baerlocher M O, Asch M R, Myers A. Imaging and transcatheter arterial embolization for traumatic splenic injuries: review of the literature. Can J Surg. 2008;51:464–472. [PMC free article] [PubMed] [Google Scholar]
- Wu S C, Chen R J, Yang A D, Tung C C, Lee K H. Complications associated with embolization in the treatment of blunt splenic injury. World J Surg. 2008;32:476–482. doi: 10.1007/s00268-007-9322-x. [DOI] [PubMed] [Google Scholar]
- Hamers R L, Van Den Berg F G, Groeneveld A B. Acute necrotizing pancreatitis following inadvertent extensive splenic artery embolization for trauma. Br J Radiol. 2009;82:e11–e14. doi: 10.1259/bjr/92246530. [DOI] [PubMed] [Google Scholar]
- Brewer M E, Jr, Strnad B T, Daley B J, et al. Percutaneous embolization for the management of grade 5 renal trauma in hemodynamically unstable patients: initial experience. J Urol. 2009;181:1737–1741. doi: 10.1016/j.juro.2008.11.100. [DOI] [PubMed] [Google Scholar]
- Breyer B N, McAninch J W, Elliott S P, Master V A. Minimally invasive endovascular techniques to treat acute renal hemorrhage. J Urol. 2008;179:2248–2252. discussion 2253. doi: 10.1016/j.juro.2008.01.104. [DOI] [PubMed] [Google Scholar]
- Verbeek D, Sugrue M, Balogh Z, et al. Acute management of hemodynamically unstable pelvic trauma patients: time for a change? Multicenter review of recent practice. World J Surg. 2008;32:1874–1882. doi: 10.1007/s00268-008-9591-z. [DOI] [PubMed] [Google Scholar]
- Demetriades D, Karaiskakis M, Toutouzas K, Alo K, Velmahos G, Chan L. Pelvic fractures: epidemiology and predictors of associated abdominal injuries and outcomes. J Am Coll Surg. 2002;195:1–10. doi: 10.1016/s1072-7515(02)01197-3. [DOI] [PubMed] [Google Scholar]
- Agolini S F, Shah K, Jaffe J, Newcomb J, Rhodes M, Reed J F., III Arterial embolization is a rapid and effective technique for controlling pelvic fracture hemorrhage. J Trauma. 1997;43:395–399. doi: 10.1097/00005373-199709000-00001. [DOI] [PubMed] [Google Scholar]
- Hare W S, Holland C J. Paresis following internal iliac artery embolization. Radiology. 1983;146:47–51. doi: 10.1148/radiology.146.1.6849068. [DOI] [PubMed] [Google Scholar]