ABSTRACT
Within vascular anomalies, vascular malformations are those present at birth that grow with the patient and exhibit abnormal dilated vascular channels lined by mature endothelium. Vascular tumors, the other group of vascular anomalies, demonstrate endothelial hypercellularity. Vascular malformations are further divided into low-flow varieties (capillary, venous, and lymphatic malformations) and high-flow varieties (arteriovenous malformation and fistula). All malformations exhibit a predictable group of clinical patterns that vary in severity and rate of progression. The interventional radiologist must incorporate this clinical data with characteristic ultrasound and magnetic resonance findings to arrive at a diagnosis. One must then decide in a multidisciplinary fashion, based on objective clinical criteria and image-based morphology, if the patent is a candidate for intervention. Sclerotherapy is a technique used to treat vascular malformations whereby an endothelial-cidal agent is introduced into the endoluminal compartment to initiate vascular closure. The high flow rate of an arteriovenous malformation requires the incorporation of superselective transarterial, direct, and transvenous access with flow reduction techniques to deliver adequate dose of sclerosant and embolic to the nidus. Satisfactory outcomes are seen in over half of all malformations patients. Similar treatment-related complications are seen between malformations but are lowest in lymphatic and highest in arteriovenous malformations.
Keywords: Vascular malformation, vascular anomalies, sclerotherapy, embolization, venous malformation, lymphatic malformation, arteriovenous malformation, radiology, hemangioma, classification
Over the last decade, there have been increasing signs of relatively wide acceptance of the concept that a large group of predominantly vascular pathological states are in fact the expression of a much smaller group of vascular entities, collectively known as vascular anomalies. Improved understanding of the classification, clinical behavior, pathology, and molecular biology of vascular anomalies, along with simultaneous technological advances in soft-tissue imaging diagnosis and percutaneous interventional techniques, have created a fertile and expanding domain that is particularly well suited to the practice of interventional radiology. However, like many new fields in interventional radiology, ground can be lost if not approached with the goal of providing a complete and integrated clinically oriented service that is founded on up-to-date knowledge, full pre- and postprocedural patient care, and multidisciplinary consultation throughout the process.1,2,3,4,5 This article is intended as a step-by-step practical synopsis explaining the classification, diagnostic issues, and percutaneous techniques in the treatment of vascular malformations and the requisites and workings of a sustainable clinically oriented service.
THE IMPORTANCE OF A MULTIDISCIPLINARY APPROACH
As a mandatory prerequisite before beginning or expanding a minimally invasive vascular anomaly practice, a core multidisciplinary team of relevant surgeons, internists, dermatologists, hematologists, nurses, physiotherapists, medical photographers, and patient coordinators should be assembled.3 This group must communicate effectively, meet or round regularly, and be uniformly familiar with the clinical pathways of their various colleagues' disciplines in arriving at a diagnosis and optimized management for the vascular anomaly patient2,3
UNDERSTANDING THE CURRENT VASCULAR ANOMALY CLASSIFICATION
In a 1982 landmark article, Mulliken and Glowacki6 put forth a vascular anomaly classification scheme based on endothelial cell characteristics. Vascular anomalies were ultimately divided into either vascular tumors or vascular malformations. Vascular tumors (e.g., the infantile hemangioma) demonstrated rapid neonatal growth, endothelial hypercellularity, increased cellular turnover, and in the case of infantile hemangiomas, a later involutive phase.6,7 Vascular malformations were described as lesions present at birth, growing commensurately or pari passu with the child, and consisted of vascular spaces lined with normal flattened “mature” endothelium. These lesions were further divided into capillary malformations (CMs), venous malformations (VMs), lymphatic malformations (LMs), arteriovenous malformations (AVMs), and combined lesions.
In 1993, a complementary classification scheme was developed by Jackson and colleagues8 based on flow characteristics, which has evolved to divide vascular malformations into high-flow lesions (AVMs and fistulae) and low-flow lesions (VMs and LMs). Based on the lesion predominant cavity size, LMs have since been further divided into macrocystic, microcystic, and mixed varieties. In 1996, The International Society for the Study of Vascular Anomalies (ISSVA) arrived at a Mulliken-based nosological consensus that forms current vascular anomaly classification framework (Table 1).
Table 1.
| Tumors | Vascular Malformations |
|||
|---|---|---|---|---|
| Simple | Combined | |||
| ISSVA, International Society for the Study of Vascular Anomalies. | ||||
| Based on Scientific Committee of the 11th Meeting of the International Society for the Study of Vascular Anomalies, Rome, Italy, 1996. | ||||
| Adapted from Enjolras O. Classification and management of the various superficial vascular anomalies: hemangioma and vascular malformation. J Dermatol 1997;24:701–710. | ||||
| Adapted from Garzon MC, Huang JT, Enjolras O, Frieden IJ. Vascular malformations: part I. J Am Acad Dermatol Mar 2007;56(3):353–370; quiz 371–354. | ||||
| Adapted from: Jackson IT, Carreno R, Potparic Z, Hussain K. Hemangiomas, vascular malformations, and lymphovenous malformations: classification and methods of treatment. Plast Reconstr Surg 1993;91:1216–1230. | ||||
| Adapted from Vaidya S, Cooke D, Kogut M, Stratil P, Bittles M, Sidhu M. Imaging and percutaneous treatment of vascular anomalies. Semin Intervent Radiol 2008;25(3):216–233. | ||||
| Infantile hemangioma | Low Flow | Capillary malformation (CM) | High Flow | Arteriovenous fistula (AVF) |
| Congenital hemangioma | Lymphatic malformation (LM) | Arteriovenous malformation (AVM) | ||
| Tufted angioma | Venous malformation (VM) | CVM | ||
| Kaposiform hemangioendothelioma | CLVM | |||
| Hemangiopericytoma | LVM | |||
| Pyogenic granuloma | CAVM | |||
| Spindle-cell hemangioendothelioma | CLAVM | |||
BASIC PATHOLOGICAL CHARACTERISTICS OF VASCULAR MALFORMATIONS
Grossly, malformations can be single, multiple, focal, or infiltrative across multiple soft tissue planes and spaces involving, muscle, fat, bone, or neurovascular structures or joints. Microscopically, all vascular malformations demonstrate irregularly dilated, variably thickened vascular channels lined with mature endothelial cells.6,9,10,11
VMs are often deficient in an internal elastic lamina, with intermittent absence of smooth muscle within the vessel wall that likely leads to progressive ectasia.11,12 Localized intravascular coagulopathy is common in VMs,13,14 and luminal thrombi can form phleboliths or papillary fronds (Masson's endothelial hyperplasia).10,11,12,15
LMs demonstrate endothelial monolayer-lined small or microscopic channels (microcystic) or enlarged spaces (macrocystic) that contain few lymphocytes, proteinaceous fluid, and even erythrocytes.10,11 The larger spaces may be separated by fibrous septae creating a multiseptate appearance. Given a close embryological origin, LMs may contain significant venous or CM components as in the Klippel-Trenaunay syndrome.11
AVMs demonstrate variable histology that may reveal capillaries, venules, and arterioles that may exhibit hypertrophy, wall thickening, and possibly dystrophic calcification, all within a densely fibrous or fibromyxomatous background.10,11 The point of an AVM at which rapid arterial shunting passes into an arterialized vein acting as a low pressure cell is termed the nidus.16 The increased shunting into the nidus leads to arterial and venous hypertrophy that can compress, erode, infiltrate, or destroy adjacent normal structures.17,18
Capillary and mixed malformations are beyond the scope of this discussion and will not be further addressed in this article.
THE TYPICAL CLINICAL PRESENTATION OF VASCULAR MALFORMATIONS
The diagnosis of a vascular malformation can usually be made correctly on the basis of clinical history and examination alone; however, diagnosis is nearly always confirmed with some form of imaging or rarely biopsy. Although all vascular malformations are present at birth, if located deep or if slow growing, they may not be detected until adolescence or even early adulthood after somatic growth has slowed or ceased.11,19,20 With a more detailed history, the patient often can often recall symptoms referable to the region earlier in life.16
Forty percent of VMs are located in the head and neck, 40% in the extremities, and 20% in the trunk.21 The lesions often present with symptoms of pain and swelling related to (1) local mass effect or compression of adjacent structures, (2) venous engorgement secondary to activity or stasis, (3) local hemorrhage, (4) local muscular dysfunction or contracture, or (5) intralesional thrombosis.13,21,22,23 On exam, when relatively superficial or involving the dermis, these lesions can present as soft, rubbery, serpiginous, ecchymotic, or faintly blue-tinged structures that can change in size based on dependant positioning or Valsalva.20,24
LMs occur in the head and neck (48%), in the trunk and extremities (42%) and in the intrathoracic or abdominal compartment (10%) and can present very similarly to a VM as a mass with variable symptoms.25 Lesions can be solitary or multifocal and usually grow slowly but can rapidly expand due to intralesional hemorrhage or infection.26 Varying degrees of disturbed local lymphatic development can be observed, ranging from skin texture changes and discoloration to lymphedema and hyperkeratosis. In the case of some syndromes, local CMs or VMs can be observed.
AVMs most commonly occur in the extremities and pelvis, and given their more overt high-flow physiology, 40% are detectable at birth.27,28 The Schobinger classification outlines the progressive clinical course of an AVM if left untreated.29 Lesions progress from stage I (“quiescence”) to stage II (“expansion”) with increasing pulse and thrill. Stage III is characterized by local “destruction” associated with pain, ischemia, and necrosis. If left untreated, AVMs can progress to stage IV (“decompensation”) with high-output cardiac failure.
ROLE OF INITIAL NONINVASIVE IMAGING IN THE WORKUP OF A SUSPECTED VASCULAR MALFORMATION
All imaging modalities including plain film radiography, ultrasound, computed tomography (CT)/CT angiography (CTA), magnetic resonance imaging (MRI), and angiography can provide variable degrees of diagnostic and information for preprocedural planning in the workup of vascular malformations. However, to avoid noncontributory imaging and delayed or erroneous diagnosis, a logical imaging algorithm should be followed. This algorithm must be considered in context of the degree of probability that the lesion in question is in fact a vascular malformation. In other words, if a patient presents with an atypical chronological history (lesion presenting acutely or the lesion has not been present for years), or if any imaging findings are nonspecific, equivocal, or more compatible with a nonvascular malformation process, an alternative diagnosis must be considered, which may prompt further imaging or even biopsy.2
ULTRASOUND AS THE FIRST STEP IN THE IMAGING WORKUP
Although the typical patient often presents to the vascular anomaly center with a variable degree of imaging workup already performed, the first examination to be considered is ultrasound and Doppler examination.2,30 In the setting of a typical malformation history, this examination will allow immediate differentiation between a low-flow (VM or LM) and high-flow (AVM) lesion. In the low-flow category, 98% of VMs demonstrate heterogeneous echotexture with 82% being hypoechoic, 10% hyperechoic, and 8% isoechoic.31 Occasionally, anechoic tubular structures (4 to 50%) and pathognomonic echogenic phleboliths with acoustical shadowing (16%) are identified.21,31 Contrary to common belief, although the vast majority (84%) will demonstrate monophasic (78%) or biphasic (6%) flow on Doppler, a significant minority (16%) of VMs will demonstrate no detectable flow31 (Fig. 1).
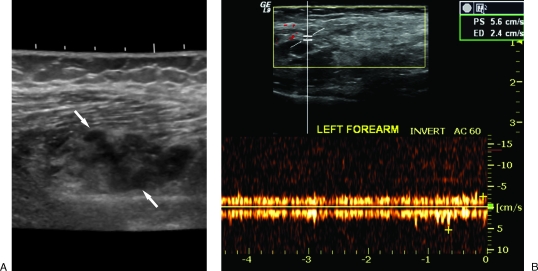
Figure 1.
Ultrasound of venous malformations (VMs). (A) Typical sonographic appearance of a VM (arrows) demonstrating heterogeneous echotexture. (B) Doppler evaluation revealing monophasic venous waveform.
Also within the low-flow category, macrocystic LMs can be readily differentiated from VMs sonographically by possessing variably enlarged cystic anechoic spaces that may contain septations and/or debris.30 Obviously, these spaces do not demonstrate detectible Doppler flow; however, septations and adjacent interstitium can demonstrate some Doppler flow.30 Microcystic LMs, on the other hand, may be sonographically confused with the small minority of VMs because their multiple small cystic cavity interfaces may create a hyperechoic echotexture with no significant detectable Doppler flow22,32 (Fig. 2).
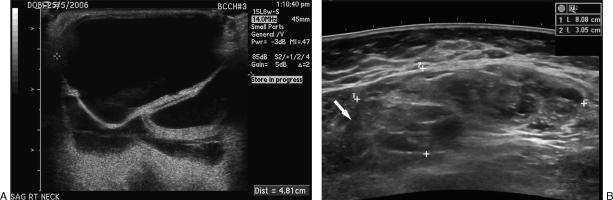
Figure 2.
Ultrasound of lymphatic malformations (LMs). (A) Macrocystic LM with large anechoic spaces and internal septations (arrow). (B) Microcystic LM (outlined by measurement markers) with diffuse complex heterogeneous echotexture that can easily be confused with a venous malformation.
AVMs, as previously described, reveal high systolic flow, shunting, and spectral broadening within a region of high vessel density due to localized arterial and venous hypertrophy22 (Fig. 3).
Figure 3.
Ultrasound of arteriovenous malformations. Expected Doppler appearance of rapid arteriovenous shunting with arterialized venous waveform and spectral broadening.
MRI FOR LESION CONFIRMATION, CHARACTERIZATION, AND TREATMENT PLANNING
If history and ultrasound findings are consistent with a low-flow malformation, an MRI is typically performed. If the history and ultrasound findings are consistent with an AVM, MRI with or without CTA is indicated. If the clinical history is atypical and/or ultrasound findings are equivocal due to the presence of intermediate flow or suspicions of an aggressive lesion, alternate diagnosis must be considered and the workup must proceed accordingly.2
MRI has become the imaging modality of choice in the confirmation, characterization, and differentiation between vascular malformations and their subtypes and allows for treatment planning and objective imaging follow-up posttherapy.2,33,34,35 VMs usually present as single or multiple lobulated, cavitary, serpiginous, or infiltrative masses that are usually isointense or hypointense on T1-weighted and hyperintense on T2-weighted or Short Tau Inversion Recovery (STIR) images.21,22,35,36,37,38 Signal voids can indicate thrombosed vessels, phleboliths, or fibrous structures imaged transversely and can be clarified by contrast-enhanced gradient echo imaging.35 Fluid-fluid levels indicate prior hemorrhage or proteinaceous material.9 Contrast imaging reveals homogeneous or heterogeneous enhancement with dynamic contrast-enhanced MR exhibiting a “contrast rise time” of ∼50 to 100 seconds, with a threshold of greater than 30 seconds as being highly sensitive and specific for low-flow malformations39,40 (Fig. 4).
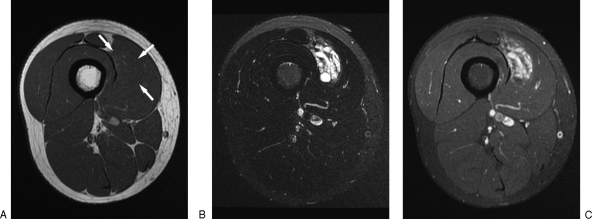
Figure 4.
Magnetic resonance imaging of venous malformations (VMs). (A) Axial T1-weighted non-fat-suppressed image revealing barely perceptible signal change within the vastus medialis due to a VM (arrows) (B) T2-weighted fat-suppressed image reveals markedly increased lesion conspicuity. (C) T1-weighted fat-suppressed image reveals significant VM enhancement with gadolinium.
Macrocystic LMs are usually easily differentiated from VMs by their predominantly cavitary lobulated and septated appearance that is hypointense on T1-weighted and hyperintense on T2-weighted and STIR images.22,33,37,41 Unlike VMs, macrocystic lesions will not enhance except in possible “rings and arcs” within cyst wall or septae.33,35,39 Macrocystic lesions are more likely to demonstrate fluid-fluid levels. Microcystic lesions are differentiated from macrocystic varieties by their relative absence of dominant cystic spaces and can be differentiated from VMs by their usual lack of contrast enhancement33,35 (Fig. 5).
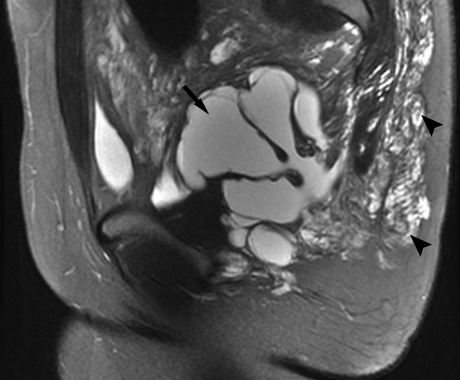
Figure 5.
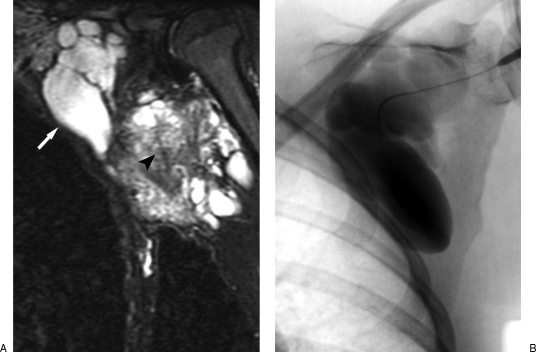
Magnetic resonance imaging of lymphatic malformations (LMs). Sagittal T2-weighted fat-saturated image of the pelvis reveals a mixed lymphatic malformation with macrocystic (arrow) and microcystic (arrowheads) components.
AVMs have a dramatically different appearance on MRI than their low-flow counterparts, with high-flow physiology leading to a focal or infiltrative tangle of flow voids seen on both T1- and T2-weighted imaging.22,35 The lesion can be associated with fatty hypertrophy or muscular atrophy.33,42 Unlike other vascular malformations and many hypervascular tumors, there is usually a very characteristic lack of mass effect in AVMs on MRI.33,43 As expected, contrast administration does not reveal enhancement of a distinct mass, and dynamic contrast studies reveal “contrast rise times” of less than 20 seconds as being highly specific for a high-flow lesion39,42 (Fig. 6).
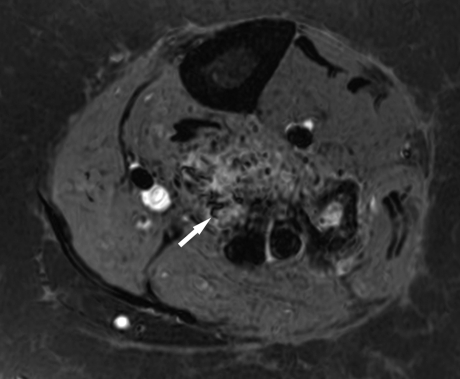
Figure 6.
Magnetic resonance imaging of arteriovenous malformations (AVMs). T1-weighted fat-saturated gadolinium-enhanced imaging of a trifurcation AVM within the calf reveals multiple flow voids (arrow).
THE SPECIFIC USE OF CT AND CTA FOR AVMS
Occasionally, an equivocal MRI the setting of suspected AVM by history, exam, and ultrasound should prompt CTA or even catheter-directed angiography for confirmation.2 Aside from detection, the high spatial resolution of CTA allows for highly accurate measurement and mapping of feeding and draining structures and involvement of adjacent structures to assist in interventional radiologic or surgical planning44 (Fig. 7).
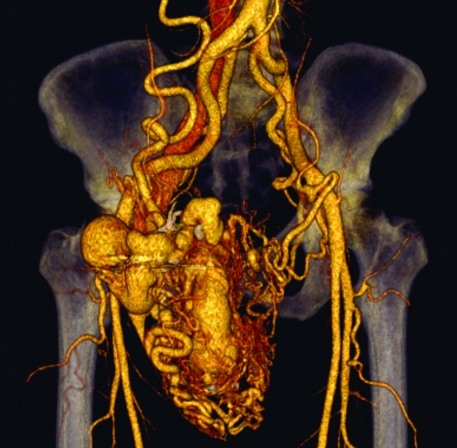
Figure 7.
Computed tomography angiography (CTA) of arteriovenous malformations (AVMs). Shaded-surface CTA reconstruction demonstrating typical hypertrophied and aneurysmal arterial and venous structures within an AVM. Such imaging is invaluable in providing quantitative information that can be used for procedure planning.
THE LIMITED ROLE OF OTHER IMAGING MODALITIES
Positive findings and clinical sequelae are often present on plain film radiography in the vascular malformation patient. Most notably, phleboliths can be identified on plain film and are highly specific for VMs, and AVMs can cause local destructive bony changes leading to pathological fracture or bony overgrowth17,18,21,22 (Fig. 8). Although dysmorphic or dysplastic bony phenomena that may be associated with the malformation may have significant long-term implications for the patient, they will otherwise usually have little bearing on the diagnostic workup and management decisions regarding potential percutaneous intervention.
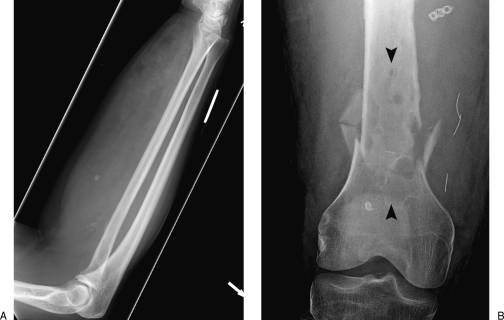
Figure 8.
Plain film findings of vascular malformations. (A) The presence of phleboliths (arrows) is a classic finding in venous malformations. (B) Arteriovenous malformation adjacent to the femur leading to permeative lysis within a region of bone (arrowheads) and subsequent pathological fracture.
Catheter arteriography, although possessing demonstrable findings, has no role in the diagnostic workup or percutaneous management of low-flow vascular malformations.2,16 The role of arteriography for high-flow lesions will be discussed under AVMs.
ARRIVING AT A CLINICAL-RADIOLOGICAL DIAGNOSIS
With rare exception, the combination of appropriate clinical history, physical examination, ultrasound, and MRI are sufficient rule in or out a diagnosis of vascular malformation and to differentiate between VM, macrocystic LM, microcystic LM, and AVM. However, if the history is not typical or ultrasound or MRI findings are not consistent with a particular malformation, other diagnoses must be considered, which may require additional imaging and/or biopsy. A synopsis of noninvasive imaging findings in vascular malformations can be found in Table 2.
Table 2.
Differential Imaging Characteristics of Vascular Malformations
| VM | Macro-LM | Micro-LM | AVM | |
|---|---|---|---|---|
| AVM, arteriovenous malformation; LM, lymphatic malformation; VM, venous malformation. | ||||
| Ultrasound | ||||
| Architecture | Heterogeneous | Cystic spaces, septations | Heterogeneous | High vessel density, vascular hypertrophy |
| Echogenicity | Hypoechoic >> hyperechoic > isoechoic | Largely anechoic | Hyperechoic | Variable |
| Flow | Monophasic >> none > biphasic | None | None | High shunt flow, spectral broadening |
| MRI | ||||
| Architecture | Heterogeneous, occasional voids from phleboliths | Large cystic spaces, septations | Heterogeneous | Vascular tangles, flow voids, lack of mass effect |
| T1 imaging | Isointense or hypointense | Hypo- or isointense | Hypointense | Turbulence, flow voids |
| T2 imaging | Very hyperintense | Very hyperintense | Very hyperintense | Turbulence, flow voids |
| Enhancement | Diffuse, hetero- or homogeneous | None | None or minimal | Yes |
| Flow measurement | Low | None | None | High |
THE PATIENT'S INTERVENTIONAL RADIOLOGIST OFFICE VISIT
Once a diagnosis of vascular malformation has been made, an in-person consultation between the patient and the interventional radiologist is required. This encounter should occur well away from the bustle of a busy interventional suite area and at a time when the radiologist can give his or her undivided attention to the relevant patient issues and questions, preferably in an office setting.
The directed clinical history and physical exam should also include accurate tape measurement and medial photography of the lesion and the nonneoplastic nature of the malformation, its indolent natural history, and the relative probability of growth, the implications of which should be discussed with the patient.2,16,45 Most importantly, the lesion's impact on quality-of-life issues must be assessed, as this is the major determining factor that must be weighed against potential therapeutic complications in arriving at the decision of whether or not to proceed with therapy.
Lee4 has comprehensively outlined five absolute and six relative indications for therapy in a vascular malformation. Absolute indications include (1) hemorrhage, (2) progressive high output cardiac failure, (3) complications of venous hypertension, (4) lesions in a life- or limb-threatening location, or (5) lesions threatening vital functions. Relative indications include (1) progressive disabling pain or discomfort, (2) functional impairment or disability affecting daily activity or quality of life, (3) severe cosmetic deformity and/or psychological disability and negative impact on quality of life, (4) vascular deforming bone syndrome, (5) location at site of high risk of secondary complications, and (6) recurrent infection/sepsis.
Often, patient reassurance that the mass that brought them to medical attention is nonneoplastic is sufficiently therapeutic, and only routine distant clinical follow-up is necessary.
Conservative management should be considered for the symptomatic patient (1) who does not meet the threshold for intervention by the above criteria, or (2) who does meet the criteria for intervention, but the lesion is not appropriate for intervention due to anatomic considerations, predicted complication rate, or patient apprehension, and/or (3) to compliment the care of a patient who is being considered for or currently undergoing percutaneous or surgical intervention. Conservative therapy includes the prescription of appropriately fitted pressure garments that can slow the progression of distention, pain, deformity, and ulceration and can also be used in conjunction with percutaneous therapy.12,19,21,46 Aspirin therapy has been successfully prescribed to treat paroxysmal pain secondary to recurrent subclinical lesion thrombosis, and in conjunction with pressure garments can be definitive therapy.12,21,27 Cessation of oral contraceptives may reduce paroxysmal pain.27,47 Given the abnormal thrombophilic state present in some patients with large VMs, prophylactic low-molecular-weight heparin during high thrombosis risk states, such as pregnancy, prolonged bed rest, surgery, and even sclerotherapy, have been discussed.13,19
If the patient is a candidate for percutaneous intervention, the lesion-specific technique, risks, postprocedural course, and likely need for multisession therapy must be discussed. Once informed consent is obtained, routine laboratory examinations, ancillary consultations, and, depending on lesion location and predicted level of procedure-related discomfort, anesthesia consultation can be obtained.
TECHNIQUE OF SCLEROTHERAPY FOR VENOUS MALFORMATIONS
Sclerotherapy involves the introduction of a substance into the lumen of the given malformation, which will induce by direct contact the death of the adjacent endothelial lining and initiate a cascade of thrombosis and fibrosis within the lumen and within the lesion as a whole.12,48,49 Several sclerosants are clinically available that vary in their mode of action, relative toxicity, complication rate, and therapeutic effect. These will be discussed later in context with procedure technique.
The treatment of a VM always begins with diagnostic phlebography, which serves as a final diagnostic confirmation of the suspected pathology and also demonstrates the particular malformations morphological characteristics, which, even more so than MR findings, have a strong impact on technique, complication rate, and outcome.21,50,51,52 The diagnostic phlebogram should be performed in an angiographic suite equipped with digital subtraction, “road map” and “overlay” capabilities, and real-time ultrasound. One should be able to review MRIs in the procedure room.2,16 In the small minority of cases were the procedure is merely diagnostic, local analgesia in the setting of local sterile drape and prep is sufficient. In the vast majority of cases where phlebography immediately precedes sclerotherapy, the patient will likely require additional conscious sedation and/or locoregional block or general anesthesia based on anticipated patient discomfort.2,16,53,54,55
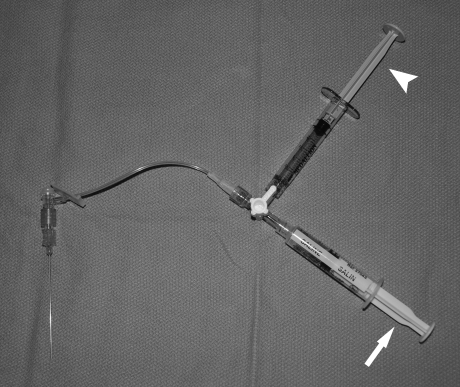
A 20- to 27-guage single-wall entry needle or butterfly needle is attached via low-volume tubing to a three-way stopcock, which is in turn attached to an in-line port syringe of saline and side-arm port of undilated iodinated contrast. A safe route to the lesion should be chosen that avoids neurovascular structures.56 To improve lesion size and conspicuity on imaging and aid access, tourniquets of pneumatic cuffs may be transiently placed beyond the lesion's venous outflow.2,16,55,57,58,59 While gently aspirating on the saline syringe, the needle is introduced into the lesion under either real-time ultrasound guidance or magnetic resonance correlated fluoroscopic guidance.21,60,61 Occasionally, if superficial, the lesion can be entered by direct visualization or palpation alone.55 If a “flash” of blood is seen, the stopcock is turned and contrast is very gently injected to confirm stable intraluminal access and evaluate the VM anatomy.21,55 If no “flash” can be elicited despite multiple passes and one is confidently within the substance of the lesion on ultrasound, a contrast injection should performed in any case and VM anatomy may be demonstrated (Fig. 9).
Figure 9.
Diagnostic phlebography apparatus for venous malformations. Dual saline (arrow) and contrast (arrowhead) syringes are attached to a stopcock to allow rapid detection of entry into a vascular space followed by immediate contrast injection for phlebography.
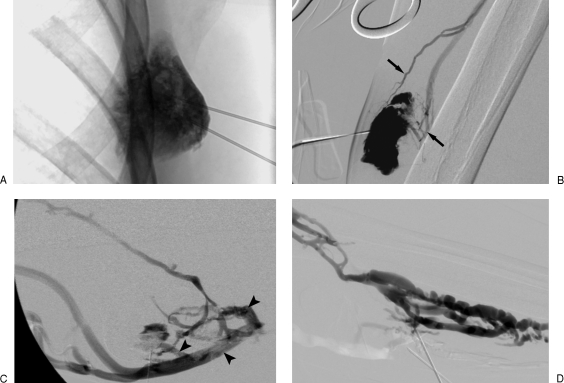
The VM should be evaluated for architecture, distribution, volume, flow rate, and type and rate of venous drainage, all the while confirming that stable intraluminal positioning is being maintained. Architecturally, VMs can demonstrate a spongy, cavitary, or dysmorphic appearance, with better results of sclerotherapy for cavitary and dysmorphic lesions being observed by some authors.21 One of the most important characteristics bearing an effect on sclerotherapeutic efficacy is that of venous drainage as described by Dubois and colleagues50 and Puig et al.51,52 Type I lesions are isolated malformations without phlebographically appreciable venous drainage. Type II and III lesions demonstrate normal-sized and enlarged venous drainage, respectively. Type IV lesions are composed of essentially ectatic dysplastic veins.50,51,52 Type III and IV lesions have a much high risk of sequelae such as secondary phlebitis due to efflux of sclerosant from the lesion during sclerotherapy50,51 (Fig. 10).
Figure 10.
Venous malformation (VM) morphology on phlebography. (A) Type I VM exhibiting negligible venous outflow. (B) Type II VM revealing normal-sized venous outflow (arrows). (C) Type III VM with rapid efflux of contrast via enlarged venous channels (arrowheads). (D) Type IV VM anatomy consisting almost entirely of enlarged venous spaces.
Once anatomic considerations have been addressed, sclerosant can be administered intraluminally into the VM. The most common currently used sclerosants in the literature at present are ethanol (dehydrated alcohol, injection USP, 100% v/v; Sandoz Canada, Inc., Boucherville, Quebec, Canada), sodium tetradecyl sulfate (Thromboject 1% and 3%, Omega, Montreal, Quebec, Canada), and polidocanol (Aethoxysclerol, Kreussler, Weisbaden, Germany). Ethanol is very potent, inexpensive, and a readily available sclerosant that exerts its endothelial-cidal effect by causing the endothelial monolayer to dehydrate, precipitate, slough, and denude.49,62,63,64 This, among other effects, results in a permanent vascular closure.49,64 However, because of this agent's high toxicity, it must be used with extreme caution to avoid collateral nontarget necrosis of adjacent tissues that may occur in the setting of overinjection, localized extravasation, or reflux either within the soft tissues or skin.64,65 Additionally, exit of ethanol into the normal deep venous outflow can result in clinically significant thrombophlebitis or more distant effects of intoxication, hemoglobinuria, pulmonary artery hypertension, pulmonary embolus, bronchospasm, hyperthermia, cardiopulmonary collapse, and death.48,56,62,64,65,66,67,68,69,70 For these reasons, care should be taken to not exceed a per-procedure administered dose of greater than 1 mL/kg.49
Sodium tetradecyl sulfate (STS; Thromboject 1 and 3%, Omega) is an ionic surfactant that has a soapy consistency and similarly causes intimal necrosis and ultimate vascular closure, which, however, may not be as permanent as ethanol.12,49,55,58,71 Although not without some risk of systemic toxicity, STS likely offers a less toxic alternative to ethanol in the treatment of VMs with lower rates of skin necrosis, nerve injury, and systemic complications and has the added benefit of being able to form a relatively stable foam.55,56,58,71
Polidocanol (Aethoxysclerol, Kreussler) is a detergent sclerosant available in the treatment of VMs that has the added benefit of having a local anesthetic effect.59,72,73 Available in 0.25 to 4% concentrations, this agent may exert a lesser endothelial-cidal effect; however, the combination of good efficacy, ability to foam, and low local and systemic complication rates may make this the agent of choice.53,54,59,61,73,74,75,76,77
There are now increasing reports in the literature describing excellent results with the use of bleomycin A5 (Pingyangmycin) in the treatment of VMs.78,79,80,81,82,83 This agent has always carried with it its long-feared chemotherapy-associated complication of pulmonary fibrosis. However, with reduced dosing first established in LMs (e.g., less than 1 mg/kg per session, more than 2 weeks between sessions, and no more than 5 mg/kg lifetime), this complication has not yet been reproducibly identified.83,84,85,86,87 Other currently used or historically significant sclerosant agents in the treatment of VMs include ethanolamine oleate,65,88,89,90 sodium morrhuate,56 and alcoholic solution of zein (Ethibloc, Ethicon, Norderstedt, Germany),50,91,92,93 to name a few.
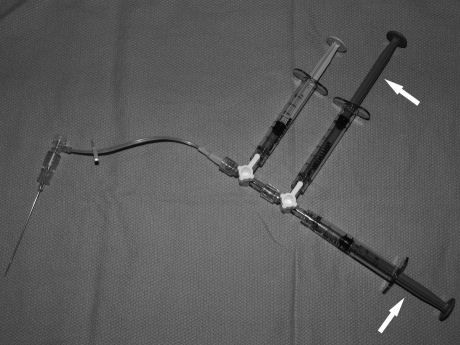
Immediately prior to injecting the sclerosant, some advocate aspiration and/or manual compression of the lesion to reduce blood/contrast volume thereby maximizing sclerosant concentration and effect; however, this is not always possible.56,94 To introduce the chosen sclerosant, the saline syringe should be removed, the agent should be loaded into two low-volume syringes attached by way of a second three-stopcock assembly, which is then in turn attached to the remaining open port of the first three-way stopcock (Fig. 11). The sclerosant should be carefully injected by hand at an appropriate rate and pressure to displace the contrast residing in the VM from the previous phlebogram2,16 (Fig. 12). This can be performed under “road map” technique or can be performed at a 1 frame-per-second or less low-dose digital subtraction technique. Care must be taken to observe for evidence of extravasation, nontarget migration of the sclerosant, and venous efflux into the deep circulation.56 Additionally, one must continuously observe for signs of skin blanching, “duskiness,” or induration that may portend skin necrosis.2,16 If any of the above are observed, injection must immediately cease. As locally injected venous channels occlude, an appropriately increased resistance to injection will be observed. Based on the known magnetic resonance distribution of the lesion, one can consider additional sites of puncture within lesion.55,58,95 To avoid untoward elevation of intralesional pressure leading to nontarget phenomena or overly rapid sclerosant efflux into the deep venous circulation, Puig and colleagues described placing a second needle to vent and direct sclerosant in a more controlled and desirable fashion96 (Fig. 13).
Figure 11.
Sclerotherapy apparatus. After removal of the earlier phlebographic saline syringe, dual syringes containing sclerotherapy agent (arrows) are attached via a second stopcock to the first stopcock.
Figure 12.
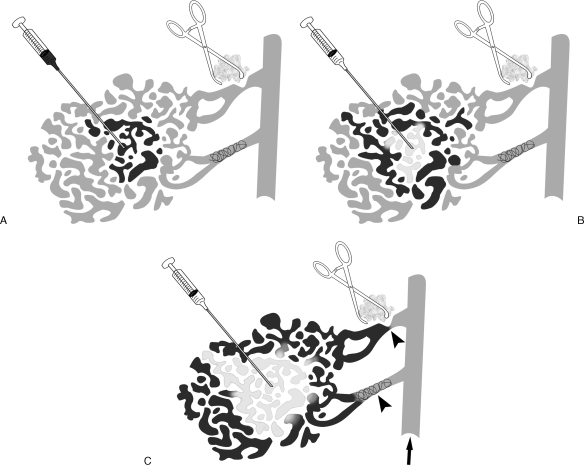
Delivery of sclerosant under radiographic guidance. (A) Contrast injection (black) confirms intraluminal needle position within the central portion of venous malformation. (B) Initial sclerosant administration (white) displaces contrast more peripherally within the lesion. (C) Sclerosant administration is continued to displace contrast to the periphery of the lesion, observing for signs of untoward sclerosant efflux to the deep venous system (arrow). To control efflux from the lesion, focal compression, tourniquets, and/or intraluminal outflow occlusive techniques may be required (arrowheads).
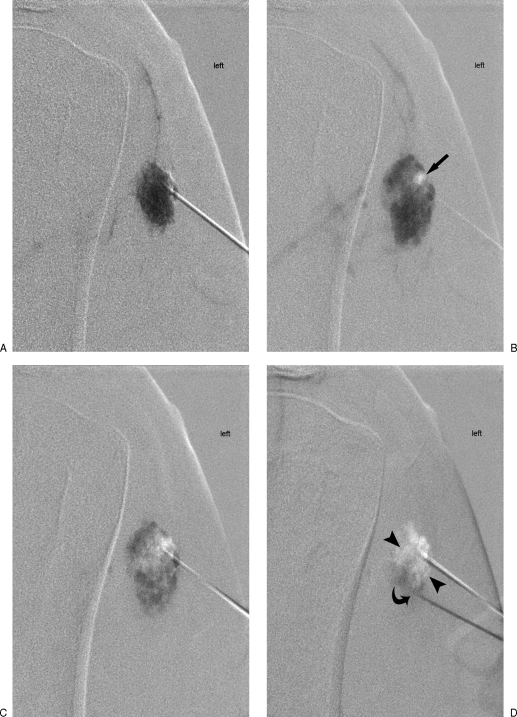
Figure 13.
Venous malformation (VM) sclerotherapy using road map and Puig technique.96 (A) Initial contrast (dark) single-needle injection under road map technique begins to opacify a type II deltoid VM. (B) Further contrast injection more fully opacifies the lesion and is followed by radiolucent ethanol, which centrally displaces contrast (arrow). (C) Further permeation of sclerosant through the malformation is limited secondary to increased pressure and distention within the lesion. (D) Placement of second needle more caudally vents and decompresses the lesion (arrowheads) and allows further sclerosant to be delivered as contrast exits out the second needle inferiorly (curved arrow).
To improve visualization of sclerosant, one can introduce STS that has been diluted with contrast in a 2:1 ratio. Ethanol can precipitate with some contrast agents and, as such, should not be injected. However, ethanol can be mixed with ethiodized oil (Lipiodol Ultra Fluid, Guerbert Group, Roissy CdG Cedex, France) in a 9:1 ratio to improve visualization. This has the added benefit of increasing the viscosity of the agent, thereby improving dwell time within the lesion and resultant sclerosant effect (Fig. 14).
Figure 14.
Venous malformation (VM) sclerotherapy using opacified sclerosant and digital subtraction angiography technique. (A) DSA mask obtained after opacification of a large dysmorphic type II to III VM reveals initial radiolucent sclerosant (arrow) displacing contrast. (B) Addition of ethiodized oil to ethanol allows better opacification of agent within the periphery of lesion (arrowheads) and increased viscosity and therefore “dwell-time” of agent.
Another common method of increasing viscosity/dwell time of sclerosant within the lesion is through the creation of a sclerosant foam.97,98 The introduced foam displaces blood and slows agent transit through the lesion, resulting in improved adherence and concentration presented to the endothelium and thereby a sclerosant effect.98,99,100 Although commercially available for specific applications, sclerosant foams may also be easily created in the case room by rapidly passing sclerosant and a gas through a partially closed three-way stopcock between two syringes.98 Various combinations of air or carbon dioxide and STS or polidocanol have been used to form durable foams that can be followed fluoroscopically and sonographically during injection.53,55,59,73,76,98
Major limitations to sclerotherapy into type II and particularly type III and IV VMs due to premature efflux into the deed venous system can be overcome by several techniques to limit outflow during sclerosant delivery. Most simply, pneumatic cuffs or tourniquets can be applied downstream of the venous outflow to help contain sclerosant within the lesion to exert its endothelial-cidal effect.21 Additionally, focal compression with a device as simple as a clamp can be used under fluoroscopy to occlude or reduce dominant venous outflow.58 Finally, if anatomy and venous drainage is limiting and complex, direct percutaneous access can be achieved, and with the aid of local compression, the communicating venous outflow between the malformations and systemic circulation can be selectively embolized/sclerosed84 (Fig. 12).
Real-time MRI techniques have been developed for sclerosant delivery but are beyond the scope of this discussion.90,101,102
The procedure is ended when sufficient amount of the lesion is treated, when the maximum allowable sclerosant dose is reached, or if a significant complication occurs.2 Puncture needles can be left in place for several minutes. The skin should be inspected; if the patient is awake, a neurovascular assessment should be performed. If there is concern regarding deep venous sclerosant injury, deep venous irrigation with normal saline via peripheral intravenous line may be useful.2 Compression dressings may augment wall apposition and healing within the lesion, and limb elevation may be indicated for some lesions.55,58,61,73,103,104 Prior to leaving the case room, the skin should be inspected, and when the patient is awake, a neurovascular assessment should be performed and documented.
TECHNIQUE OF SCLEROTHERAPY FOR LYMPHATIC MALFORMATIONS
LM sclerotherapeutic technique is reserved essentially for macrocystic lesions that meet the previously described clinical criteria; however, some success has been observed with microcystic lesions in selected cases.105 Similar to VMs, the patient is placed under conscious sedation or general anesthesia, based on age or lesion location, and the patient is draped and prepped. The dominant symptomatic spaces of an LM are identified and entered under real-time ultrasound guidance, and the fluid is aspirated.106,107,108,109 Although the needle is often sufficient, a pigtail or small infusion catheter can be inserted, and contrast can be injected to further define the lesion, taking note of the volume and distribution of contrast.110,111,112,113 The lesion can then be injected with sclerosant (Fig. 15).
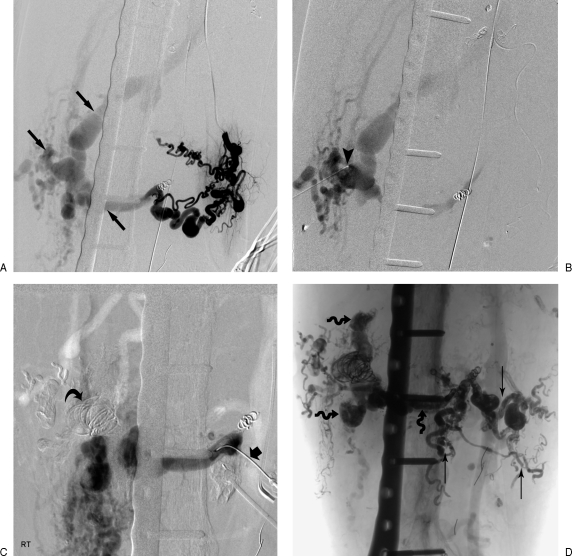
Figure 15.
Sclerotherapy of lymphatic malformations (LMs). (A) Mixed LM with a more cranial macrocystic region (arrow) and a more caudal microcystic region (arrowhead). (B) Cannulation and contrast opacification of the cranial macrocystic LM components identified in (A) prior to OK-432 sclerotherapy.
Until relatively recently, the armamentarium of sclerosants used to treat LMs were very similar those of VMs. However, three other agents have become the mainstay of current percutaneous management for LMs due to their significantly improved patient tolerance and complication profile while maintaining equal or better therapeutic results. First used in 1987 and now most commonly used, OK-432 (Picibanil, Chugai Pharmaceuticals, Tokyo, Japan) is a lyophilized powder of Streptococcus pyogenes (group A, type 3, Su strain) incubated with benzylpenicillin, which induces apoptosis of lymphatic endothelium/local cellular inflammatory reaction.107,109,112,114,115,116,117,118,119,120,121 The agent is to be mixed 0.1 mg/10 mL normal saline concentration before injection and administered to a maximum of 0.2 to 0.3 mg per session with sessions spaced 1 to 2 months apart.107,121 Bleomycin was first used in 1977 and has since been modified and used extensively by inciting a nonspecific inflammatory reaction resulting in cyst closure.79,83,85,87,105,122,123,124,125,126,127,128,129 As described earlier, to avoid the theoretical risk of pulmonary fibrosis, traditionally bleomycin hydrochloride was mixed 1 to 2 mg/mL normal saline and introduced into the lesion with no more than 1 mg/kg per session, more than 2 weeks between sessions, and no greater than 5 to 6 mg/kg lifetime dose.83,85,86 Doxycycline is increasingly being found as effective agent in the treatment of LMs and has been used as a specific treatment for microcystic components.111,130,131,132 Combinations of ethanol and/or STS are also being used to treat catheterized macrocystic lesions that are subsequently placed to suction for several days with excellent results and low complication rates.131
Irrespective of the agent injected, the site is inspected for untoward induration, swelling, or discoloration, and the patient's neurovascular status is evaluated and documented before leaving the case room.
TECHNIQUE OF EMBOLOSCLEROTHERAPY IN AVMS
Treatment of an AVM should be based on the criteria set out by Lee and Schobinger at the 8th ISSVA in Amsterdam29 and by Lee.4 Cardiology consultation with echocardiography and shunt calculations may allow assessment of impact of shunting on the cardiovascular system and establish a pretherapeutic baseline. CTA correlation is recommended to define the architecture, tissue involvement, and size and orientation of feeding and draining vessels to assist in preprocedural planning.44 In the case room, the patient is more often placed under general anesthesia, and a Swan catheter is placed to monitor pulmonary arterial pressures.49,133 Drape and prep should allow for bifemoral arterial and venous access and should also include a generous area of skin prep over the region of the AVM proper. Conventional transcatheter arteriography should be performed either on a separate day or immediately preceding anticipated percutaneous embolosclerotherapy for procedural mapping of the architecture of the lesion.16 According to the AVM classification of Do and Houdart, the type of AVM can influence technique and probability of therapeutic success.134,135 Type I lesions (arteriovenous) have no more than three arteries shunting into a single vein. Type II lesions (arteriolovenous) have multiple arterioles entering a single vein. Type IIIa and IIIb lesions (arteriolovenulous) occur at the intersection of multiple arterioles and draining veins that are nondilated or dilated, respectively.
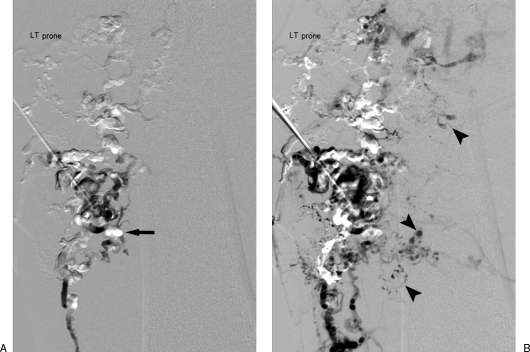
The successful percutaneous treatment of an AVM is based on delivering a focused sclerosant endothelial-cidal effect directly to the nidus (Fig. 16). Only eradication of the nidus offers the possibility of cure.136 Transarterial deposition of coils or nonsclerosing liquid embolics can produce an immediately gratifying abrupt flow reduction within AVMs. However, when used solely without the addition of a sclerosant, the nidus will often be stimulated to rapidly collateralize and recanalize, making further percutaneous management more difficult.16,48,49,136,137,138,139,140
Figure 16.
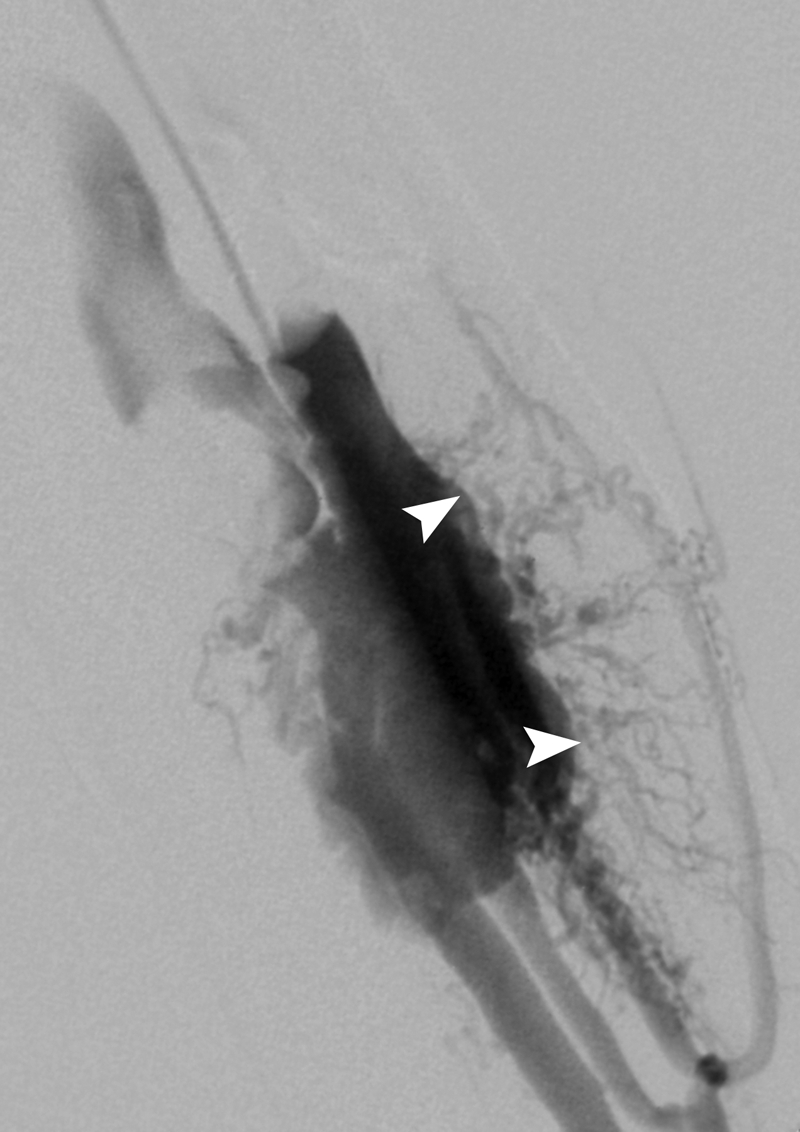
The arteriovenous malformation (AVM) nidus. Retrograde venous balloon-occlusion contrast injection reveals the AVM nidus transition point between the arteriolar and venular circulation (arrowheads). This site is the target of any embolosclerotherapy procedure.
The high-flow physiology of the nidus acting as a low-pressure cell recruiting regional arterial inflow and shunting into the venous system presents a unique challenge in the delivery of a given endothelial-cidal agent.29,63 Any given agent will become diluted, and rapid transit through the lesion will lead to shortened dwell time.63 Sclerosant agents such as ethanol administered arterially can flow into occult adjacent normal vessels and lead to nontarget embolization with catastrophic consequences.48
To minimize these factors and maximize effect on the nidus, a combination of techniques must be employed that involves both nidal-specific access and flow reduction techniques.16 Nidal-specific access allows maximal concentration and/or accuracy of agent delivered to the nidus and can be achieved in three ways. A coaxial microcatheter system can be advanced in as close proximity to the nidus as possible before delivering the agent.48,49,137,141 Because this can be complex and time-consuming, retrograde transvenous approaches to the nidus for agent delivery can be performed either via transfemoral, transjugular, or adjacent retrograde transvenous approach.133,137,142,143 Most simply, direct transcutaneous puncture of the nidus can be performed under combined ultrasound and fluoroscopic guidance to deliver the agent.48,49,133,139,142,144,145,146
Flow reduction techniques can be incorporated to slow flow within the lesion and allow agents to exert their maximal effect and minimize the risk of passage into the systemic venous circulation. This can be achieved temporarily using appropriately sized transarterial and/or transvenous balloons that are placed coaxially or via separate vascular puncture.48,49,136 They are inflated during agent delivery and are deflated once the particular maneuver is completed. Permanent arterial flow reduction can be achieved by way of transarterial occlusion of portions of the nidal inflow with either coils or liquid embolics such as glues to assist and render a nidus more vulnerable to the effects of a sclerosant such as ethanol by increasing dwell time.16 In most cases, these materials are not a sole replacement for diligent use of sclerosants such as ethanol in AVM therapy. However, permanent transvenous occlusion with either coils and/or glues with the addition of a sclerosant may offer the most definitive and permanent AVM therapy to date133,142 (Fig. 17).
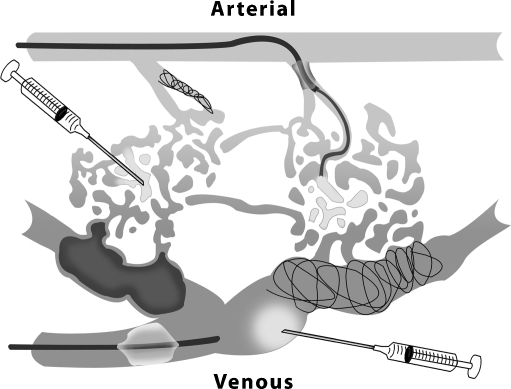
Figure 17.
Nidal-specific and flow-reduction techniques in the treatment of arteriovenous malformations. Sclerosant/embolic can be delivered transarterially via microcatheter, by direct puncture of the arteriolar nidus (left syringe), and/or by direct puncture of the venous nidus (lower right syringe). The venous nidus can also be selected directly or transvenously for delivery of coils or liquid embolics, which may be definitive. Flow reduction can be achieved transiently with the inflation of arterial or venous balloons or permanently with arterials coils to increase the effectiveness of sclerosant delivery.
The liquid embolics used in AVM management worldwide include N-butyl-2-cyanoacrylate (Histoacryl; B. Braun, Melsungen, Germany) and N-butyl cyanoacrylate (Trufill n-BCA Liquid Embolic System, Cordis Neurovascular Inc., Johnson and Johnson, Miami Lakes, FL), which are adhesive glues that polymerize when exposed to blood, inciting an acute inflammatory granulomatous reaction.147 Agents such as Histoacryl are mixed with ethiodized oil for radiopacity in a 1:1 to 1:5 ratio to adjust desired time to polymerization based on the local milieu into which they are injected. Ethylene vinyl alcohol copolymer (Onyx Liquid Embolic System; Micro Therapeutics, Inc., Irvine, CA) is nonadhesive and is mixed with tantalum powder for radiopacity. It can be administered slowly in a controlled fashion as the material fills and conforms to the desired vascular structure resulting in a spongelike mass.148,149,150
Once the above techniques have been appropriately employed, an immediately presclerotherapy contrast injection should demonstrate the desired flow within an exclusively abnormal AVM vascular territory devoid of normal vascularity. Ethanol can then be safely administered in 0.5 to 1 mL aliquots to reproduce the volume and flow prior to the contrast injection.48,133 The region is observed for 5 to 10 minutes, and the process is repeated until desired stasis is achieved. This process is repeated throughout the lesion in conjunction with flow reduction techniques, most preferably with permanent venous occlusion133 (Figs. 18 and 19).
Figure 18.
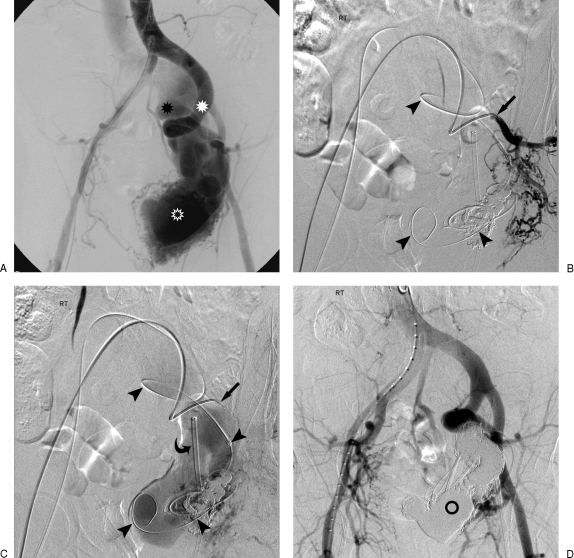
Combined transarterial and transvenous approaches to arteriovenous malformation (AVM) treatment. (A) Large left pelvic AVM with dilated hypertrophied arterial feeders (white asterisk) leading to very large nidus (hollow asterisk) and then onto massively dilated internal iliac venous drainage (black asterisk). Early phase (B) and late phase (C) internal iliac angiogram of AVM through transarterial coaxial catheter system (arrows) for diagnostic arterial monitoring and access for subselective embolosclerotherapy. A second coaxial retrograde venous catheter (arrowheads) lies within the massively dilated venous nidus. A third retrograde venous sheath lies near the entrance to the left internal iliac vein (curved arrow) for future iliac occlusion. (D) Final diagnostic angiogram after deposition of multiple coils, glue, transarterial and transvenous ethanol revealing complete elimination of the nidus and no evidence of arteriovenous shunt (hollow circle).
Figure 19.
Direct nidal-specific access. (A) One of many selective transarterial injections reveals complex arterial anatomy ending in a common distant nidal region (arrows). (B) Direct needle puncture of lateral nidal region (arrowhead). (C) Deposition of coils (curved arrow) and ethanol followed by second direct puncture (thick arrow). (D) Later deposition of ethanol and glue transvenously (spiral arrows) and transarterially (thin arrows) resulting in regional stasis.
The procedure is ended when a desired territory has been treated to stasis. Hemostasis should be achieved, and skin evaluation and neurovascular assessment should be performed prior to the patient leaving the case room.
INPATIENT POSTPROCEDURAL MANAGEMENT OF VASCULAR MALFORMATIONS
For all percutaneous vascular malformations procedures, postprocedural orders should include frequent-interval skin and neurovascular assessments of the involved regions over several hours. The patient should be encouraged to resume activity as tolerated as soon as possible. Postprocedural investigations may include complete blood count and urinalysis to document for hemoglobinuria. If there are concerns regarding sclerosant entering the deep venous system, Doppler assessment the same day or the following day may be helpful to rule out deep venous thrombosis.2,16 Based on the anticipated level of expected pain postprocedure, analgesia may range from oral acetaminophen to oxycodone or intravenous morphine.55,101 Expected postprocedural swelling can be managed with ketorolac or ibuprofen; however, steroids can be routinely used when treatment is near vital structures or on the setting of some AVMs.55,58,133 Low-molecular-weight heparin should be instituted if percutaneous treatment is performed in conjunction with surgery.13,19 If the treated lesion was intramuscular or near a joint, arrangements for outpatient physiotherapy are recommended to avoid contracture.2,5 Barring complications, patients can be discharged the same day or the next day with appropriate analgesic/anti-inflammatory prescription, and they should have their next encounter with the radiology service arranged before discharge.
COMPLICATIONS OF PERCUTANEOUS THERAPY FOR VASCULAR MALFORMATIONS
Complication type and severity vary greatly between malformation type and the method of intervention. Minor complications of VM sclerotherapy include swelling, pain, erythema, and tenderness along with skin complications that can run the spectrum of blistering, hyperpigmentation, ulceration to necrosis. Major complications include temporary or permanent nerve injury or paralysis, muscular contracture or compartment syndrome, thrombophlebitis or deep venous thrombosis, pulmonary embolism, hemolysis, renal toxicity, anaphylaxis, bradycardia, and cardiovascular collapse.21,36,55,151,152 An appearance of increased skin involvement or absence of muscular involvement on MRI increases the risk of skin complications, and increased intramuscular involvement is predictive of posttherapy contractures.36,151 Type I and II lesions are predictive of complication-free sclerotherapy.51 Ethanol is associated with the highest rates of complications, with the largest and very representative series within the ethanol literature reporting a combined major and minor complication rate of 12% of sessions and 28% of patients.2,16,46,54,55,95,103,151,152,153,154 The per-session complications included all skin phenomena (8.8%), major skin necrosis (1.5%), transient nerve impairment (0.8%), and permanent nerve injury (0.5%), deep veinous thrombosis (1.25%), and pulmonary embolism (0.25%); 1.1% of patients experienced muscular contracture. Complication rates from the use of STS are significantly lower, with multiple series reporting only minor complications ranging from 0 to 14% of patients and one study reporting total major complications of under 2% of patients.55,58,71,104 Polidocanol and bleomycin A5 have a similarly low complication rates, primarily causing expected levels of local erythema and swelling with low single-digit session rates of skin necrosis and nerve injury.53,54,59,61,73,79,81,83,84,85,155,156 No major systemic complications are reported except for rare bradyarrhythmias and reversible cardiac arrest with polidocanol.54,75
With the more common use of OK-432, bleomycin, and doxycycline, complication rates in the treatment of LMs remain very low. Aside from airway impairment posttherapy for lesions involving the neck, major complication rates of nearly 0% are seen with these agents, with only sporadic reports of occasional ulceration or local cellulitis posttherapy.79,83,84,85,87,105,107,109,112,115,117,118,123,126,130,131,157
AVM embolosclerotherapy has the same constellation of major and minor complications as sclerotherapy of VMs; however, complications occur more frequently and have an increased likelihood of being major. Minor complications occur in 6 to 17% of session and up to 45% of patients and major complications occur in 2 to 10% of sessions and up to 25% of patients.48,63,133,134,136,139,142
OUTPATIENT POSTPROCEDURAL MANAGEMENT AND DECISION MAKING
Sufficient time must pass between sclerotherapy and sessions to allow postprocedural pain and swelling to resolve and to allow assessment of new baseline level of symptoms. For VMs, repeat treatments are based on persistence of symptoms and are usually repeated every 3 weeks to 3 months.21,55,103 LMs are usually monitored sonographically, and persisting lesions are treated every 1 to 2 months.105,107,121,131 Because inflammatory change can be slow to resolve and full involution can be gradual, follow-up MRI evaluation not earlier than 3 months and preferably at 6 months postprocedure is recommended for all patients.2,16,21 Successfully treated regions will demonstrate decreased volume and decreased T1 and T2 signal intensity and decreased enhancement. Therapy is concluded at the point the patient symptoms have sufficiently resolved that they no longer meet criteria for therapy.
If necessary, the interval of repeat AVM treatment is based on the persistence of physical findings, or Doppler, CTA, or MR findings and should be scheduled as soon as is practical based on patient tolerance. This is aimed at not allowing “ground to be lost” by interval neovascular recruitment by the persisting nidus. Therapy should be concluded when the nidus is obliterated and the patient is no longer symptomatic.
Even if successfully treated clinically, low-flow vascular malformations can recur and patients should be counseled to contact the radiology service with concerns. In the case of high-flow lesions, multiyear increasing-interval sonographic assessment is suggested to monitor for possible recurrence.
CLINICAL OUTCOMES OF PERCUTANEOUS THERAPY FOR VASCULAR MALFORMATIONS
In a recent retrospective study grouping all peripheral vascular malformations, initial complete or partial symptomatic relief was achieved in 58% of patients; however, this gradually decreased to 42% on 3- to 5-year follow-up.158 In the treatment of VMs, no one agent or technique has yet proven itself to be superior; however, various morphological and clinical observations have been correlated with outcome.2,16 Cavitary and dysmorphic lesions have been correlated with better outcomes compared with spongy lesions.21 MR grading of VM size and infiltration predicts 71% clinical success in grade 1 lesions and 0% in grade 3 lesions.153 Significant swelling and longer recovery have been correlated with better therapeutic effect.60 Clinical improvement or good to excellent results have been reported with sclerotherapy of VMs using ethanol (53 to 100%),46,60,95,103,152,153 polidocanol (78 to 100%),53,54,59,73,159 ethanolamine oleate (88 to 100%),65,89,90 and STS alone or in conjunction with other agents (67 to 86%),55,58,71,104 Bleomycin A5 alone resulted in clinical success in 52 to 100% of cases, and when used in combination with other agents, clinical success was achieved in 63 to 100% of cases.78,80,81,82,83,84
Current multisession sclerotherapeutic treatment of LMs now yields excellent results. Among all LM types, good to excellent results are achieved using OK-432 or bleomycin in 58 to 93% and 70 to 88% of patients, respectively.79,83,85,87,105,107,109,112,114,115,117,118,121,126,160 As expected, clinical success is greater in macrocystic (77 to 100%) versus microcystic subtypes (0 to 80%), with one prospective trial demonstrating 95% of successes being macrocystic and 80% of failures or intermediate responders being microcystic in nature. Doxycycline used alone or in combination with other ethanol/STS therapy appears to yield similarly very good results.111,131
The largest, most recent studies documenting routine combined transarterial, transvenous, and direct puncture ethanol techniques in the treatment of AVMs now find that significant clinical response rate has approached 68 to 100% with cure rates ranging from 32 to 88%.48,133,134,142 When stratified by morphology, the highest cure rates have been found in type II AVMs (85%), with the most common pure type IIIb lesion having a 33% cure rate and mixed types demonstrating intermediate response and no outright cures.134
REFERENCES
- Lee B B, Bergan J J. Advanced management of congenital vascular malformations: a multidisciplinary approach. Cardiovasc Surg. 2002;10:523–533. doi: 10.1016/s0967-2109(02)00072-8. [DOI] [PubMed] [Google Scholar]
- Legiehn G M, Heran M K. Venous malformations: classification, development, diagnosis, and interventional radiologic management. Radiol Clin North Am. 2008;46:545–597. doi: 10.1016/j.rcl.2008.02.008. vi. [DOI] [PubMed] [Google Scholar]
- Nagy M, Brodsky L. Multidisciplinary approach to management of hemangiomas and vascular malformations. Facial Plast Surg Clin North Am. 2001;9:551–559. [PubMed] [Google Scholar]
- Lee B B. New approaches to the treatment of congenital vascular malformations (CVMs)—a single centre experience. Eur J Vasc Endovasc Surg. 2005;30:184–197. doi: 10.1016/j.ejvs.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Donnelly L F, Adams D M, Bisset G S., III Vascular malformations and hemangiomas: a practical approach in a multidisciplinary clinic. AJR Am J Roentgenol. 2000;174:597–608. doi: 10.2214/ajr.174.3.1740597. [DOI] [PubMed] [Google Scholar]
- Mulliken J B, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- Mulliken J B, Zetter B R, Folkman J. In vitro characteristics of endothelium from hemangiomas and vascular malformations. Surgery. 1982;92:348–353. [PubMed] [Google Scholar]
- Jackson I T, Carreño R, Potparic Z, Hussain K. Hemangiomas, vascular malformations, and lymphovenous malformations: classification and methods of treatment. Plast Reconstr Surg. 1993;91:1216–1230. doi: 10.1097/00006534-199306000-00006. [DOI] [PubMed] [Google Scholar]
- Fishman S J, Mulliken J B. Hemangiomas and vascular malformations of infancy and childhood. Pediatr Clin North Am. 1993;40:1177–1200. doi: 10.1016/s0031-3955(16)38656-4. [DOI] [PubMed] [Google Scholar]
- Kilpatrick S E. Diagnostic Musculoskeletal Surgical Pathology: Clinicoradiologic and Cytologic Correlations. Philadelphia: Saunders; 2004.
- North P E, Mihm M C., Jr Histopathological diagnosis of infantile hemangiomas and vascular malformations. Facial Plast Surg Clin North Am. 2001;9:505–524. [PubMed] [Google Scholar]
- Hein K D, Mulliken J B, Kozakewich H P, Upton J, Burrows P E. Venous malformations of skeletal muscle. Plast Reconstr Surg. 2002;110:1625–1635. doi: 10.1097/01.PRS.0000033021.60657.74. [DOI] [PubMed] [Google Scholar]
- Mazoyer E, Enjolras O, Laurian C, Houdart E, Drouet L. Coagulation abnormalities associated with extensive venous malformations of the limbs: differentiation from Kasabach-Merritt syndrome. Clin Lab Haematol. 2002;24:243–251. doi: 10.1046/j.1365-2257.2002.00447.x. [DOI] [PubMed] [Google Scholar]
- Dompmartin A, Acher A, Thibon P, et al. Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol. 2008;144:873–877. doi: 10.1001/archderm.144.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clearkin K P, Enzinger F M. Intravascular papillary endothelial hyperplasia. Arch Pathol Lab Med. 1976;100:441–444. [PubMed] [Google Scholar]
- Legiehn G M, Heran M K. Classification, diagnosis, and interventional radiologic management of vascular malformations. Orthop Clin North Am. 2006;37:435–474. vii–viii. doi: 10.1016/j.ocl.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Boyd J B, Mulliken J B, Kaban L B, Upton J, III, Murray J E. Skeletal changes associated with vascular malformations. Plast Reconstr Surg. 1984;74:789–797. doi: 10.1097/00006534-198412000-00010. [DOI] [PubMed] [Google Scholar]
- Savader S J, Savader B L, Otero R R. Intraosseous arteriovenous malformations mimicking malignant disease. Acta Radiol. 1988;29:109–114. [PubMed] [Google Scholar]
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219–225. doi: 10.1016/s0190-9622(97)70284-6. [DOI] [PubMed] [Google Scholar]
- Garzon M C, Huang J T, Enjolras O, Frieden I J. Vascular malformations: Part I. J Am Acad Dermatol. 2007;56:353–370. quiz 371–374. doi: 10.1016/j.jaad.2006.05.069. [DOI] [PubMed] [Google Scholar]
- Dubois J, Soulez G, Oliva V L, Berthiaume M J, Lapierre C, Therasse E. Soft-tissue venous malformations in adult patients: imaging and therapeutic issues. Radiographics. 2001;21:1519–1531. doi: 10.1148/radiographics.21.6.g01nv031519. [DOI] [PubMed] [Google Scholar]
- Dubois J, Garel L. Imaging and therapeutic approach of hemangiomas and vascular malformations in the pediatric age group. Pediatr Radiol. 1999;29:879–893. doi: 10.1007/s002470050718. [DOI] [PubMed] [Google Scholar]
- Nicolaides A N, Breddin H K, Carpenter P, et al. European Genetics Foundation. Cardiovascular Disease Educational and Research Trust. International Union of Angiology. Mediterranean League on Thromboembolism Thrombophilia and venous thromboembolism. International consensus statement. Guidelines according to scientific evidence. Int Angiol. 2005;24:1–26. [PubMed] [Google Scholar]
- Abernethy L J. Classification and imaging of vascular malformations in children. Eur Radiol. 2003;13:2483–2497. doi: 10.1007/s00330-002-1773-8. [DOI] [PubMed] [Google Scholar]
- Alqahtani A, Nguyen L T, Flageole H, Shaw K, Laberge J M. 25 years' experience with lymphangiomas in children. J Pediatr Surg. 1999;34:1164–1168. doi: 10.1016/s0022-3468(99)90590-0. [DOI] [PubMed] [Google Scholar]
- Emery P J, Bailey C M, Evans J N. Cystic hygroma of the head and neck. A review of 37 cases. J Laryngol Otol. 1984;98:613–619. doi: 10.1017/s0022215100147176. [DOI] [PubMed] [Google Scholar]
- Buckmiller L M. Update on hemangiomas and vascular malformations. Curr Opin Otolaryngol Head Neck Surg. 2004;12:476–487. doi: 10.1097/01.moo.0000145946.67222.01. [DOI] [PubMed] [Google Scholar]
- Frieden I, Enjolras O, Esterly N. In: Schachner LA, Hansen RC, editor. Pediatric Dermatology. 3rd ed. New York: Mosby; 2003. Vascular birthmarks, other abnormalities of blood vessels and lymphatics.
- Kohout M P, Hansen M, Pribaz J J, Mulliken J B. Arteriovenous malformations of the head and neck: natural history and management. Plast Reconstr Surg. 1998;102:643–654. doi: 10.1097/00006534-199809030-00006. [DOI] [PubMed] [Google Scholar]
- Paltiel H J, Burrows P E, Kozakewich H P, Zurakowski D, Mulliken J B. Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology. 2000;214:747–754. doi: 10.1148/radiology.214.3.r00mr21747. [DOI] [PubMed] [Google Scholar]
- Trop I, Dubois J, Guibaud L, et al. Soft-tissue venous malformations in pediatric and young adult patients: diagnosis with Doppler US. Radiology. 1999;212:841–845. doi: 10.1148/radiology.212.3.r99au11841. [DOI] [PubMed] [Google Scholar]
- Sintzoff S A, Jr, Gillard I, Gansbeke D Van, Gevenois P A, Salmon I, Struyven J. Ultrasound evaluation of soft tissue tumors. J Belge Radiol. 1992;75:276–280. [PubMed] [Google Scholar]
- Konez O, Burrows P E. Magnetic resonance of vascular anomalies. Magn Reson Imaging Clin N Am. 2002;10:363–388. vii. doi: 10.1016/s1064-9689(01)00009-5. [DOI] [PubMed] [Google Scholar]
- Redondo P. [Vascular malformations (II). Diagnosis, pathology and treatment] Actas Dermosifiliogr. 2007;98:219–235. [PubMed] [Google Scholar]
- Moukaddam H, Pollak J, Haims A H. MRI characteristics and classification of peripheral vascular malformations and tumors. Skeletal Radiol. 2009;38:535–547. doi: 10.1007/s00256-008-0609-2. [DOI] [PubMed] [Google Scholar]
- Fayad L M, Fayad L, Hazirolan T, Bluemke D, Mitchell S. Vascular malformations in the extremities: emphasis on MR imaging features that guide treatment options. Skeletal Radiol. 2006;35:127–137. doi: 10.1007/s00256-005-0057-1. [DOI] [PubMed] [Google Scholar]
- Meyer J S, Hoffer F A, Barnes P D, Mulliken J B. Biological classification of soft-tissue vascular anomalies: MR correlation. AJR Am J Roentgenol. 1991;157:559–564. doi: 10.2214/ajr.157.3.1872245. [DOI] [PubMed] [Google Scholar]
- Vilanova J C, Barceló J, Smirniotopoulos J G, et al. Hemangioma from head to toe: MR imaging with pathologic correlation. Radiographics. 2004;24:367–385. doi: 10.1148/rg.242035079. [DOI] [PubMed] [Google Scholar]
- Ohgiya Y, Hashimoto T, Gokan T, et al. Dynamic MRI for distinguishing high-flow from low-flow peripheral vascular malformations. AJR Am J Roentgenol. 2005;185:1131–1137. doi: 10.2214/AJR.04.1508. [DOI] [PubMed] [Google Scholar]
- Rijswijk C S van, der Linden E van, der Woude H J van, Baalen J M van, Bloem J L. Value of dynamic contrast-enhanced MR imaging in diagnosing and classifying peripheral vascular malformations. AJR Am J Roentgenol. 2002;178:1181–1187. doi: 10.2214/ajr.178.5.1781181. [DOI] [PubMed] [Google Scholar]
- Kern S, Niemeyer C, Darge K, Merz C, Laubenberger J, Uhl M. Differentiation of vascular birthmarks by MR imaging. An investigation of hemangiomas, venous and lymphatic malformations. Acta Radiol. 2000;41:453–457. doi: 10.1080/028418500127345677. [DOI] [PubMed] [Google Scholar]
- Rak K M, Yakes W F, Ray R L, et al. MR imaging of symptomatic peripheral vascular malformations. AJR Am J Roentgenol. 1992;159:107–112. doi: 10.2214/ajr.159.1.1609682. [DOI] [PubMed] [Google Scholar]
- Dobson M J, Hartley R W, Ashleigh R, Watson Y, Hawnaur J M. MR angiography and MR imaging of symptomatic vascular malformations. Clin Radiol. 1997;52:595–602. doi: 10.1016/s0009-9260(97)80251-6. [DOI] [PubMed] [Google Scholar]
- Gulati M S, Paul S B, Batra A, Sarma D, Dadhwal V, Nath J. Uterine arteriovenous malformations: the role of intravenous “dual-phase” CT angiography. Clin Imaging. 2000;24:10–14. doi: 10.1016/s0899-7071(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Villavicencio J L. Primum non nocere: is it always true? The use of absolute ethanol in the management of congenital vascular malformations. J Vasc Surg. 2001;33:904–906. doi: 10.1067/mva.2001.112234. [DOI] [PubMed] [Google Scholar]
- Rautio R, Saarinen J, Laranne J, Salenius J P, Keski-Nisula L. Endovascular treatment of venous malformations in extremities: results of sclerotherapy and the quality of life after treatment. Acta Radiol. 2004;45:397–403. doi: 10.1080/02841850410004913. [DOI] [PubMed] [Google Scholar]
- Rinker B, Karp N S, Margiotta M, Blei F, Rosen R, Rofsky N M. The role of magnetic resonance imaging in the management of vascular malformations of the trunk and extremities. Plast Reconstr Surg. 2003;112:504–510. doi: 10.1097/01.PRS.0000070986.81430.B4. [DOI] [PubMed] [Google Scholar]
- Do Y S, Yakes W F, Shin S W, et al. Ethanol embolization of arteriovenous malformations: interim results. Radiology. 2005;235:674–682. doi: 10.1148/radiol.2352040449. [DOI] [PubMed] [Google Scholar]
- Yakes W F, Rossi P, Odink H. How I do it. Arteriovenous malformation management. Cardiovasc Intervent Radiol. 1996;19:65–71. doi: 10.1007/BF02563895. [DOI] [PubMed] [Google Scholar]
- Dubois J M, Sebag G H, De Prost Y, Teillac D, Chretien B, Brunelle F O. Soft-tissue venous malformations in children: percutaneous sclerotherapy with Ethibloc. Radiology. 1991;180:195–198. doi: 10.1148/radiology.180.1.2052693. [DOI] [PubMed] [Google Scholar]
- Puig S, Aref H, Chigot V, Bonin B, Brunelle F. Classification of venous malformations in children and implications for sclerotherapy. Pediatr Radiol. 2003;33:99–103. doi: 10.1007/s00247-002-0838-9. [DOI] [PubMed] [Google Scholar]
- Puig S, Casati B, Staudenherz A, Paya K. Vascular low-flow malformations in children: current concepts for classification, diagnosis and therapy. Eur J Radiol. 2005;53:35–45. doi: 10.1016/j.ejrad.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Cabrera J, Cabrera J J, Jr, Garcia-Olmedo M A, Redondo P. Treatment of venous malformations with sclerosant in microfoam form. Arch Dermatol. 2003;139:1409–1416. doi: 10.1001/archderm.139.11.1409. [DOI] [PubMed] [Google Scholar]
- Mimura H, Kanazawa S, Yasui K, et al. Percutaneous sclerotherapy for venous malformations using polidocanol under fluoroscopy. Acta Med Okayama. 2003;57:227–234. doi: 10.18926/AMO/51909. [DOI] [PubMed] [Google Scholar]
- Tan K T, Kirby J, Rajan D K, Hayeems E, Beecroft J R, Simons M E. Percutaneous sodium tetradecyl sulfate sclerotherapy for peripheral venous vascular malformations: a single-center experience. J Vasc Interv Radiol. 2007;18:343–351. doi: 10.1016/j.jvir.2006.12.735. [DOI] [PubMed] [Google Scholar]
- de Lorimier A A. Sclerotherapy for venous malformations. J Pediatr Surg. 1995;30:188–193. discussion 194. doi: 10.1016/0022-3468(95)90558-8. [DOI] [PubMed] [Google Scholar]
- Hyodoh H, Hori M, Akiba H, Tamakawa M, Hyodoh K, Hareyama M. Peripheral vascular malformations: imaging, treatment approaches, and therapeutic issues. Radiographics. 2005;25(25 Suppl 1):S159–S171. doi: 10.1148/rg.25si055509. [DOI] [PubMed] [Google Scholar]
- O'Donovan J C, Donaldson J S, Morello F P, Pensler J M, Vogelzang R L, Bauer B. Symptomatic hemangiomas and venous malformations in infants, children, and young adults: treatment with percutaneous injection of sodium tetradecyl sulfate. AJR Am J Roentgenol. 1997;169:723–729. doi: 10.2214/ajr.169.3.9275886. [DOI] [PubMed] [Google Scholar]
- Pascarella L, Bergan J J, Yamada C, Mekenas L. Venous angiomata: treatment with sclerosant foam. Ann Vasc Surg. 2005;19:457–464. doi: 10.1007/s10016-005-4656-z. [DOI] [PubMed] [Google Scholar]
- Donnelly L F, Bisset G S, III, Adams D M. Marked acute tissue swelling following percutaneous sclerosis of low-flow vascular malformations: a predictor of both prolonged recovery and therapeutic effect. Pediatr Radiol. 2000;30:415–419. doi: 10.1007/s002470050775. [DOI] [PubMed] [Google Scholar]
- Yamaki T, Nozaki M, Sasaki K. Color duplex-guided sclerotherapy for the treatment of venous malformations. Dermatol Surg. 2000;26:323–328. doi: 10.1046/j.1524-4725.2000.99248.x. [DOI] [PubMed] [Google Scholar]
- Shin B S, Do Y S, Lee B B, et al. Multistage ethanol sclerotherapy of soft-tissue arteriovenous malformations: effect on pulmonary arterial pressure. Radiology. 2005;235:1072–1077. doi: 10.1148/radiol.2353040903. [DOI] [PubMed] [Google Scholar]
- Yakes W F, Haas D K, Parker S H, et al. Symptomatic vascular malformations: ethanol embolotherapy. Radiology. 1989;170(3 Pt 2):1059–1066. doi: 10.1148/radiology.170.3.2916057. [DOI] [PubMed] [Google Scholar]
- Yakes W F, Krauth L, Ecklund J, et al. Ethanol endovascular management of brain arteriovenous malformations: initial results. Neurosurgery. 1997;40:1145–1152. discussion 1152–1154. doi: 10.1097/00006123-199706000-00005. [DOI] [PubMed] [Google Scholar]
- Choi Y H, Han M H, O-Ki K, Cha S H, Chang K H. Craniofacial cavernous venous malformations: percutaneous sclerotherapy with use of ethanolamine oleate. J Vasc Interv Radiol. 2002;13:475–482. doi: 10.1016/s1051-0443(07)61527-9. [DOI] [PubMed] [Google Scholar]
- Behnia R. Systemic effects of absolute alcohol embolization in a patient with a congenital arteriovenous malformation of the lower extremity. Anesth Analg. 1995;80:415–417. doi: 10.1097/00000539-199502000-00037. [DOI] [PubMed] [Google Scholar]
- Garel L, Mareschal J L, Gagnadoux M F, Pariente D, Guilbert M, Sauvegrain J. Fatal outcome after ethanol renal ablation in child with end-stage kidneys. AJR Am J Roentgenol. 1986;146:593–594. doi: 10.2214/ajr.146.3.593. [DOI] [PubMed] [Google Scholar]
- Mason K P, Michna E, Zurakowski D, Koka B V, Burrows P E. Serum ethanol levels in children and adults after ethanol embolization or sclerotherapy for vascular anomalies. Radiology. 2000;217:127–132. doi: 10.1148/radiology.217.1.r00se30127. [DOI] [PubMed] [Google Scholar]
- Stefanutto T B, Halbach V. Bronchospasm precipitated by ethanol injection in arteriovenous malformation. AJNR Am J Neuroradiol. 2003;24:2050–2051. [PMC free article] [PubMed] [Google Scholar]
- Yakes W F, Baker R. Cardiopulmonary collapse: a sequelae of ethanol embolotherapy. Chicago, IL: Paper presented at: 79th Scientific Assembly and Annual Meeting of the Radiological Society of North America; 1993.
- Siniluoto T M, Svendsen P A, Wikholm G M, Fogdestam I, Edström S. Percutaneous sclerotherapy of venous malformations of the head and neck using sodium tetradecyl sulphate (sotradecol) Scand J Plast Reconstr Surg Hand Surg. 1997;31:145–150. doi: 10.3109/02844319709085481. [DOI] [PubMed] [Google Scholar]
- Guex J J. Indications for the sclerosing agent polidocanol (aetoxisclerol dexo, aethoxisklerol kreussler) J Dermatol Surg Oncol. 1993;19:959–961. doi: 10.1111/j.1524-4725.1993.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Jain R, Bandhu S, Sawhney S, Mittal R. Sonographically guided percutaneous sclerosis using 1% polidocanol in the treatment of vascular malformations. J Clin Ultrasound. 2002;30:416–423. doi: 10.1002/jcu.10091. [DOI] [PubMed] [Google Scholar]
- Cacciola E, Giustolisi R, Musso R, et al. Activation of contact phase of blood coagulation can be induced by the sclerosing agent polidocanol: possible additional mechanism of adverse reaction during sclerotherapy. J Lab Clin Med. 1987;109:225–226. [PubMed] [Google Scholar]
- Marrocco-Trischitta M M, Guerrini P, Abeni D, Stillo F. Reversible cardiac arrest after polidocanol sclerotherapy of peripheral venous malformation. Dermatol Surg. 2002;28:153–155. doi: 10.1046/j.1524-4725.2002.00344.x. [DOI] [PubMed] [Google Scholar]
- Nitecki S, Bass A. Ultrasound-guided foam sclerotherapy in patients with Klippel-Trenaunay syndrome. Isr Med Assoc J. 2007;9:72–75. [PubMed] [Google Scholar]
- Suzuki N, Nakao A, Nonami T, Takagi H. Experimental study on the effects of sclerosants for esophageal varices on blood coagulation, fibrinolysis and systemic hemodynamics. Gastroenterol Jpn. 1992;27:309–316. doi: 10.1007/BF02777748. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu D, Wang Y, Zhang W, Zhao F. Clinical study of sclerotherapy of maxillofacial venous malformation using absolute ethanol and pingyangmycin. J Oral Maxillofac Surg. 2009;67:98–104. doi: 10.1016/j.joms.2008.06.053. [DOI] [PubMed] [Google Scholar]
- Muir T, Kirsten M, Fourie P, Dippenaar N, Ionescu G O. Intralesional bleomycin injection (IBI) treatment for haemangiomas and congenital vascular malformations. Pediatr Surg Int. 2004;19:766–773. doi: 10.1007/s00383-003-1058-6. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sun M, Hou R, et al. Preliminary study of fibrin glue combined with pingyangmycin for the treatment of venous malformations in the oral and maxillofacial region. J Oral Maxillofac Surg. 2008;66:2219–2225. doi: 10.1016/j.joms.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Zhi K, Wen Y, Li L, Ren W. The role of intralesional Pingyangmycin in the treatment of venous malformation of facial and maxillary region. Int J Pediatr Otorhinolaryngol. 2008;72:593–597. doi: 10.1016/j.ijporl.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Chen W L, Yang Z H, Bai Z B, Wang Y Y, Huang Z Q, Wang Y J. A pilot study on combination compartmentalisation and sclerotherapy for the treatment of massive venous malformations of the face and neck. J Plast Reconstr Aesthet Surg. 2008;61:1486–1492. doi: 10.1016/j.bjps.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Mathur N N, Rana I, Bothra R, Dhawan R, Kathuria G, Pradhan T. Bleomycin sclerotherapy in congenital lymphatic and vascular malformations of head and neck. Int J Pediatr Otorhinolaryngol. 2005;69:75–80. doi: 10.1016/j.ijporl.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Jin Y, Lin X, Li W, Hu X, Ma G, Wang W. Sclerotherapy after embolization of draining vein: a safe treatment method for venous malformations. J Vasc Surg. 2008;47:1292–1299. doi: 10.1016/j.jvs.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Sung M W, Chang S O, Choi J H, Kim J Y. Bleomycin sclerotherapy in patients with congenital lymphatic malformation in the head and neck. Am J Otolaryngol. 1995;16:236–241. doi: 10.1016/0196-0709(95)90149-3. [DOI] [PubMed] [Google Scholar]
- Bechard D E, Fairman R P, DeBlois G G, Via C T. Fatal pulmonary fibrosis from low-dose bleomycin therapy. South Med J. 1987;80:646–649. doi: 10.1097/00007611-198705000-00024. [DOI] [PubMed] [Google Scholar]
- Okada A, Kubota A, Fukuzawa M, Imura K, Kamata S. Injection of bleomycin as a primary therapy of cystic lymphangioma. J Pediatr Surg. 1992;27:440–443. doi: 10.1016/0022-3468(92)90331-z. [DOI] [PubMed] [Google Scholar]
- Gabal A M. Percutaneous technique for sclerotherapy of vertebral hemangioma compressing spinal cord. Cardiovasc Intervent Radiol. 2002;25:494–500. doi: 10.1007/s00270-002-1944-7. [DOI] [PubMed] [Google Scholar]
- Johann A C, Aguiar M C, do Carmo M A, Gomez R S, Castro W H, Mesquita R A. Sclerotherapy of benign oral vascular lesion with ethanolamine oleate: an open clinical trial with 30 lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:579–584. doi: 10.1016/j.tripleo.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Lewin J S, Merkle E M, Duerk J L, Tarr R W. Low-flow vascular malformations in the head and neck: safety and feasibility of MR imaging-guided percutaneous sclerotherapy—preliminary experience with 14 procedures in three patients. Radiology. 1999;211:566–570. doi: 10.1148/radiology.211.2.r99ma09566. [DOI] [PubMed] [Google Scholar]
- Górriz E, Carreira J M, Reyes R, et al. Intramuscular low flow vascular malformations: treatment by means of direct percutaneous embolization. Eur J Radiol. 1998;27:161–165. doi: 10.1016/s0720-048x(97)00045-4. [DOI] [PubMed] [Google Scholar]
- Kühne D, Helmke K. Embolization with “Ethibloc” of vascular tumors and arteriovenous malformations in the head and neck. Neuroradiology. 1982;23:253–258. doi: 10.1007/BF00339391. [DOI] [PubMed] [Google Scholar]
- Riche M C, Hadjean E, Tran-Ba-Huy P, Merland J J. The treatment of capillary-venous malformations using a new fibrosing agent. Plast Reconstr Surg. 1983;71:607–614. doi: 10.1097/00006534-198305000-00003. [DOI] [PubMed] [Google Scholar]
- Deveikis J P. Percutaneous ethanol sclerotherapy for vascular malformations in the head and neck. Arch Facial Plast Surg. 2005;7:322–325. doi: 10.1001/archfaci.7.5.322. [DOI] [PubMed] [Google Scholar]
- Suh J S, Shin K H, Na J B, Won J Y, Hahn S B. Venous malformations: sclerotherapy with a mixture of ethanol and lipiodol. Cardiovasc Intervent Radiol. 1997;20:268–273. doi: 10.1007/s002709900150. [DOI] [PubMed] [Google Scholar]
- Puig S, Aref H, Brunelle F. Double-needle sclerotherapy of lymphangiomas and venous angiomas in children: a simple technique to prevent complications. AJR Am J Roentgenol. 2003;180:1399–1401. doi: 10.2214/ajr.180.5.1801399. [DOI] [PubMed] [Google Scholar]
- Cabrera Garrido J R, Cabrera Garcia-Olmedo J R, Garcia-Olmedo Dominquez M A. Elargissement des limites de la sclerotherapie: nouveaux produits sclerosants. Phlebologie. 1997;50:351–353. [Google Scholar]
- Tessari L. Nouvelle technique d'obtention de la sclero-mousse. Phlebologie. 2000;53:129. [Google Scholar]
- Frullini A, Cavezzi A. Sclerosing foam in the treatment of varicose veins and telangiectases: history and analysis of safety and complications. Dermatol Surg. 2002;28:11–15. doi: 10.1046/j.1524-4725.2002.01182.x. [DOI] [PubMed] [Google Scholar]
- Hamel-Desnos C, Desnos P, Wollmann J C, Ouvry P, Mako S, Allaert F A. Evaluation of the efficacy of polidocanol in the form of foam compared with liquid form in sclerotherapy of the greater saphenous vein: initial results. Dermatol Surg. 2003;29:1170–1175. discussion 1175. doi: 10.1111/j.1524-4725.2003.29398.x. [DOI] [PubMed] [Google Scholar]
- Boll D T, Merkle E M, Lewin J S. Low-flow vascular malformations: MR-guided percutaneous sclerotherapy in qualitative and quantitative assessment of therapy and outcome. Radiology. 2004;233:376–384. doi: 10.1148/radiol.2332031213. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Masumoto T, Okubo T, et al. Hemangiomas in the face and extremities: MR-guided sclerotherapy—optimization with monitoring of signal intensity changes in vivo. Radiology. 2003;226:567–572. doi: 10.1148/radiol.2262012157. [DOI] [PubMed] [Google Scholar]
- Lee C H, Chen S G. Direct percutaneous ethanol instillation for treatment of venous malformation in the face and neck. Br J Plast Surg. 2005;58:1073–1078. doi: 10.1016/j.bjps.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Woods J E. Extended use of sodium tetradecyl sulfate in treatment of hemangiomas and other related conditions. Plast Reconstr Surg. 1987;79:542–549. doi: 10.1097/00006534-198704000-00005. [DOI] [PubMed] [Google Scholar]
- Bai Y, Jia J, Huang X X, Alsharif M J, Zhao J H, Zhao Y F. Sclerotherapy of microcystic lymphatic malformations in oral and facial regions. J Oral Maxillofac Surg. 2009;67:251–256. doi: 10.1016/j.joms.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Dubois J, Garel L, Abela A, Laberge L, Yazbeck S. Lymphangiomas in children: percutaneous sclerotherapy with an alcoholic solution of zein. Radiology. 1997;204:651–654. doi: 10.1148/radiology.204.3.9280239. [DOI] [PubMed] [Google Scholar]
- Giguère C M, Bauman N M, Sato Y, et al. Treatment of lymphangiomas with OK-432 (Picibanil) sclerotherapy: a prospective multi-institutional trial. Arch Otolaryngol Head Neck Surg. 2002;128:1137–1144. doi: 10.1001/archotol.128.10.1137. [DOI] [PubMed] [Google Scholar]
- Goyal M, Mishra N K, Sharma A, Gaikwad S B, Mohanty B K, Sharma A. Alcohol ablation of symptomatic vertebral hemangiomas. AJNR Am J Neuroradiol. 1999;20:1091–1096. [PMC free article] [PubMed] [Google Scholar]
- Laranne J, Keski-Nisula L, Rautio R, Rautiainen M, Airaksinen M. OK-432 (Picibanil) therapy for lymphangiomas in children. Eur Arch Otorhinolaryngol. 2002;259:274–278. doi: 10.1007/s00405-001-0438-6. [DOI] [PubMed] [Google Scholar]
- Herbreteau D, Riche M C, Enjolras O, et al. Percutaneous embolization with Ethibloc of lymphatic cystic malformations with a review of the experience in 70 patients. Int Angiol. 1993;12:34–39. [PubMed] [Google Scholar]
- Molitch H I, Unger E C, Witte C L, vanSonnenberg E. Percutaneous sclerotherapy of lymphangiomas. Radiology. 1995;194:343–347. doi: 10.1148/radiology.194.2.7529933. [DOI] [PubMed] [Google Scholar]
- Rautio R, Keski-Nisula L, Laranne J, Laasonen E. Treatment of lymphangiomas with OK-432 (Picibanil) Cardiovasc Intervent Radiol. 2003;26:31–36. doi: 10.1007/s00270-002-1980-3. [DOI] [PubMed] [Google Scholar]
- Shankar K R, Roche C J, Carty H M, Turnock R R. Cystic retroperitoneal lymphangioma: treatment by image-guided percutaneous catheter drainage and sclerotherapy. Eur Radiol. 2001;11:1021–1023. doi: 10.1007/s003300000669. [DOI] [PubMed] [Google Scholar]
- Ogita S, Tsuto T, Tokiwa K, Takahashi T. Intracystic injection of OK-432: a new sclerosing therapy for cystic hygroma in children. Br J Surg. 1987;74:690–691. doi: 10.1002/bjs.1800740812. [DOI] [PubMed] [Google Scholar]
- Sung M W, Lee D W, Kim D Y, et al. Sclerotherapy with picibanil (OK-432) for congenital lymphatic malformation in the head and neck. Laryngoscope. 2001;111:1430–1433. doi: 10.1097/00005537-200108000-00020. [DOI] [PubMed] [Google Scholar]
- Uchida K, Inoue M, Araki T, Miki C, Kusunoki M. Huge scrotal, flank, and retroperitoneal lymphangioma successfully treated by OK-432 sclerotherapy. Urology. 2002;60:1112. doi: 10.1016/s0090-4295(02)01957-x. [DOI] [PubMed] [Google Scholar]
- Luzzatto C, Midrio P, Tchaprassian Z, Guglielmi M. Sclerosing treatment of lymphangiomas with OK-432. Arch Dis Child. 2000;82:316–318. doi: 10.1136/adc.82.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greinwald J H, Jr, Burke D K, Sato Y, et al. Treatment of lymphangiomas in children: an update of Picibanil (OK-432) sclerotherapy. Otolaryngol Head Neck Surg. 1999;121:381–387. doi: 10.1016/S0194-5998(99)70225-1. [DOI] [PubMed] [Google Scholar]
- Smith R J, Burke D K, Sato Y, Poust R I, Kimura K, Bauman N M. OK-432 therapy for lymphangiomas. Arch Otolaryngol Head Neck Surg. 1996;122:1195–1199. doi: 10.1001/archotol.1996.01890230041009. [DOI] [PubMed] [Google Scholar]
- Mikhail M, Kennedy R, Cramer B, Smith T. Sclerosing of recurrent lymphangioma using OK-432. J Pediatr Surg. 1995;30:1159–1160. doi: 10.1016/0022-3468(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Ogita S, Tsuto T, Nakamura K, Deguchi E, Iwai N. OK-432 therapy in 64 patients with lymphangioma. J Pediatr Surg. 1994;29:784–785. doi: 10.1016/0022-3468(94)90370-0. [DOI] [PubMed] [Google Scholar]
- Duman L, Karnak I, Akinci D, Tanyel F C. Extensive cervical-mediastinal cystic lymphatic malformation treated with sclerotherapy in a child with Klippel-Trenaunay syndrome. J Pediatr Surg. 2006;41:e21–e24. doi: 10.1016/j.jpedsurg.2005.10.067. [DOI] [PubMed] [Google Scholar]
- Baskin D, Tander B, Bankaoğlu M. Local bleomycin injection in the treatment of lymphangioma. Eur J Pediatr Surg. 2005;15:383–386. doi: 10.1055/s-2005-872922. [DOI] [PubMed] [Google Scholar]
- Mabeta P, Ionescu G O, Muir T, et al. Bleomycin levels in patients undergoing intralesional bleomycin treatment for lymphatic malformation. Wellington, New Zealand: Paper presented at: 15th Congress of the International Society for the Study of Vascular Anomalies; 2004.
- Zhong P Q, Zhi F X, Li R, Xue J L, Shu G Y. Long-term results of intratumorous bleomycin-A5 injection for head and neck lymphangioma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:139–144. doi: 10.1016/s1079-2104(98)90115-9. [DOI] [PubMed] [Google Scholar]
- Orford J, Barker A, Thonell S, King P, Murphy J. Bleomycin therapy for cystic hygroma. J Pediatr Surg. 1995;30:1282–1287. doi: 10.1016/0022-3468(95)90485-9. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Inomata Y, Utsunomiya H, et al. Sclerosing therapy with bleomycin emulsion for lymphangioma in children. Pediatr Surg Int. 1990;5:270–273. [Google Scholar]
- Tanigawa N, Shimomatsuya T, Takahashi K, et al. Treatment of cystic hygroma and lymphangioma with the use of bleomycin fat emulsion. Cancer. 1987;60:741–749. doi: 10.1002/1097-0142(19870815)60:4<741::aid-cncr2820600406>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Yura J, Hashimoto T, Tsuruga N, Shibata K. Bleomycin treatment for cystic hygroma in children. Nippon Geka Hokan. 1977;46:607–614. [PubMed] [Google Scholar]
- Cordes B M, Seidel F G, Sulek M, Giannoni C M, Friedman E M. Doxycycline sclerotherapy as the primary treatment for head and neck lymphatic malformations. Otolaryngol Head Neck Surg. 2007;137:962–964. doi: 10.1016/j.otohns.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Shiels W E, II, Kenney B D, Caniano D A, Besner G E. Definitive percutaneous treatment of lymphatic malformations of the trunk and extremities. J Pediatr Surg. 2008;43:136–139. discussion 140. doi: 10.1016/j.jpedsurg.2007.09.049. [DOI] [PubMed] [Google Scholar]
- Wible B C, Mitchell S. Doxycycline sclerotherapy of an intraosseous femoral lymphatic malformation: case report and literature review. J Vasc Interv Radiol. 2009;20:660–663. doi: 10.1016/j.jvir.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Cho S K, Do Y S, Kim D I, et al. Peripheral arteriovenous malformations with a dominant outflow vein: results of ethanol embolization. Korean J Radiol. 2008;9:258–267. doi: 10.3348/kjr.2008.9.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Y S, Park K B, Cho S K. How do we treat arteriovenous malformations (tips and tricks)? Tech Vasc Interv Radiol. 2007;10:291–298. doi: 10.1053/j.tvir.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Houdart E, Gobin Y P, Casasco A, Aymard A, Herbreteau D, Merland J J. A proposed angiographic classification of intracranial arteriovenous fistulae and malformations. Neuroradiology. 1993;35:381–385. doi: 10.1007/BF00588376. [DOI] [PubMed] [Google Scholar]
- Lee B B, Do Y S, Yakes W, Kim D I, Mattassi R, Hyon W S. Management of arteriovenous malformations: a multidisciplinary approach. J Vasc Surg. 2004;39:590–600. doi: 10.1016/j.jvs.2003.10.048. [DOI] [PubMed] [Google Scholar]
- Jackson J E, Mansfield A O, Allison D J. Treatment of high-flow vascular malformations by venous embolization aided by flow occlusion techniques. Cardiovasc Intervent Radiol. 1996;19:323–328. doi: 10.1007/BF02570183. [DOI] [PubMed] [Google Scholar]
- Rao V R, Mandalam K R, Gupta A K, Kumar S, Joseph S. Dissolution of isobutyl 2-cyanoacrylate on long-term follow-up. AJNR Am J Neuroradiol. 1989;10:135–141. [PMC free article] [PubMed] [Google Scholar]
- Tan K T, Simons M E, Rajan D K, Terbrugge K. Peripheral high-flow arteriovenous vascular malformations: a single-center experience. J Vasc Interv Radiol. 2004;15:1071–1080. doi: 10.1097/01.RVI.0000133858.36101.B3. [DOI] [PubMed] [Google Scholar]
- White R I, Jr, Pollak J, Persing J, Henderson K J, Thomson J G, Burdge C M. Long-term outcome of embolotherapy and surgery for high-flow extremity arteriovenous malformations. J Vasc Interv Radiol. 2000;11:1285–1295. doi: 10.1016/s1051-0443(07)61302-5. [DOI] [PubMed] [Google Scholar]
- Weber J. Techniques and results of therapeutic catheter embolization of congenital vascular defects. Int Angiol. 1990;9:214–223. [PubMed] [Google Scholar]
- Bae S, Do Y S, Shin S W, et al. Ethanol embolotherapy of pelvic arteriovenous malformations: an initial experience. Korean J Radiol. 2008;9:148–154. doi: 10.3348/kjr.2008.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuzaki K, Yamashita Y, Utsunomiya D, et al. Balloon-occluded retrograde transvenous embolization of a pelvic arteriovenous malformation. Cardiovasc Intervent Radiol. 1999;22:518–520. doi: 10.1007/s002709900443. [DOI] [PubMed] [Google Scholar]
- Doppman J L, Pevsner P H. Embolization of arteriovenous malformations by direct percutaneous puncture. AJR Am J Roentgenol. 1983;140:773–778. doi: 10.2214/ajr.140.4.773. [DOI] [PubMed] [Google Scholar]
- Gomes A S. Embolization therapy of congenital arteriovenous malformations: use of alternate approaches. Radiology. 1994;190:191–198. doi: 10.1148/radiology.190.1.8259403. [DOI] [PubMed] [Google Scholar]
- Hyodoh H, Fujita A, Hyodoh K, Furuse M, Kamisawa O, Hareyama M. High-flow arteriovenous malformation of the lower extremity: ethanolamine oleate sclerotherapy. Cardiovasc Intervent Radiol. 2001;24:348–351. doi: 10.1007/s00270-001-0026-6. [DOI] [PubMed] [Google Scholar]
- Pollak J S, White R I., Jr The use of cyanoacrylate adhesives in peripheral embolization. J Vasc Interv Radiol. 2001;12:907–913. doi: 10.1016/s1051-0443(07)61568-1. [DOI] [PubMed] [Google Scholar]
- Castaneda F, Goodwin S C, Swischuk J L, et al. Treatment of pelvic arteriovenous malformations with ethylene vinyl alcohol copolymer (Onyx) J Vasc Interv Radiol. 2002;13:513–516. doi: 10.1016/s1051-0443(07)61532-2. [DOI] [PubMed] [Google Scholar]
- Numan F, Omeroglu A, Kara B, Cantaşdemir M, Adaletli I, Kantarci F. Embolization of peripheral vascular malformations with ethylene vinyl alcohol copolymer (Onyx) J Vasc Interv Radiol. 2004;15:939–946. doi: 10.1097/01.RVI.0000130862.23109.52. [DOI] [PubMed] [Google Scholar]
- Taki W, Yonekawa Y, Iwata H, Uno A, Yamashita K, Amemiya H. A new liquid material for embolization of arteriovenous malformations. AJNR Am J Neuroradiol. 1990;11:163–168. [PMC free article] [PubMed] [Google Scholar]
- Fayad L M, Hazirolan T, Carrino J A, Bluemke D A, Mitchell S. Venous malformations: MR imaging features that predict skin burns after percutaneous alcohol embolization procedures. Skeletal Radiol. 2008;37:895–901. doi: 10.1007/s00256-008-0534-4. [DOI] [PubMed] [Google Scholar]
- Lee B B, Do Y S, Byun H S, Choo I W, Kim D I, Huh S H. Advanced management of venous malformation with ethanol sclerotherapy: mid-term results. J Vasc Surg. 2003;37:533–538. doi: 10.1067/mva.2003.91. [DOI] [PubMed] [Google Scholar]
- Goyal M, Causer P A, Armstrong D. Venous vascular malformations in pediatric patients: comparison of results of alcohol sclerotherapy with proposed MR imaging classification. Radiology. 2002;223:639–644. doi: 10.1148/radiol.2233010025. [DOI] [PubMed] [Google Scholar]
- Lee B B, Kim D I, Huh S, et al. New experiences with absolute ethanol sclerotherapy in the management of a complex form of congenital venous malformation. J Vasc Surg. 2001;33:764–772. doi: 10.1067/mva.2001.112209. [DOI] [PubMed] [Google Scholar]
- Coetzee P F, Ionescu G O, Fourie P, et al. Results of intralesional bleomycin injection treatment for congenital vascular anomalies. Wellington, New Zealand: Paper presented at: 15th Congress of the International Society for the Study of Vascular Anomalies; 2004.
- Kim K H, Sung M W, Roh J L, Han M H. Sclerotherapy for congenital lesions in the head and neck. Otolaryngol Head Neck Surg. 2004;131:307–316. doi: 10.1016/j.otohns.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Yura J, Hashimoto T, Tsuruga N, Shibata K. Bleomycin treatment for cystic hygroma in children. Nippon Geka Hokan. 1977;46:607–614. [PubMed] [Google Scholar]
- der Linden E van, Pattynama P M, Heeres B C, de Jong S C, Hop W C, Kroft L J. Long-term patient satisfaction after percutaneous treatment of peripheral vascular malformations. Radiology. 2009;251:926–932. doi: 10.1148/radiol.2513081579. [DOI] [PubMed] [Google Scholar]
- Yamaki T, Nozaki M, Sasaki K. Color duplex-guided sclerotherapy for the treatment of venous malformations. Dermatol Surg. 2000;26:323–328. doi: 10.1046/j.1524-4725.2000.99248.x. [DOI] [PubMed] [Google Scholar]
- Sanlialp I, Karnak I, Tanyel F C, Senocak M E, Büyükpamukçu N. Sclerotherapy for lymphangioma in children. Int J Pediatr Otorhinolaryngol. 2003;67:795–800. doi: 10.1016/s0165-5876(03)00123-x. [DOI] [PubMed] [Google Scholar]
- Enjolras O. Classification and management of the various superficial vascular anomalies: hemangiomas and vascular malformations. J Dermatol. 1997;24:701–710. doi: 10.1111/j.1346-8138.1997.tb02522.x. [DOI] [PubMed] [Google Scholar]
- Vaidya S, Cooke D, Kogut M, Stratil P, Bittles M, Sidhu M. Imaging and percutaneous treatment of vascular anomalies. Semin Intervent Radiol. 2008;25:216–233. doi: 10.1055/s-0028-1085921. [DOI] [PMC free article] [PubMed] [Google Scholar]