ABSTRACT
The evaluation of musculoskeletal tumors requires a close interaction between the orthopedic oncologist, radiologist, and the pathologist. Successful outcome can be achieved in a considerable number of patients by following the appropriate diagnostic strategies and staging studies. The aim of this article is to outline the presentation, imaging, and staging of the primary and metastatic bone and soft tissue tumors. Some of the image-guided interventions for these tumors are also presented.
Keywords: Musculoskeletal, tumor, biopsy, staging, embolization
Musculoskeletal tumors are a rare and diverse group. Sarcomas of the bone and cartilage comprise only ∼0.5% of all malignancies in humans. Their incidence is considerably higher in children than adults. The incidence of soft tissue sarcomas is 3 to 4 times higher, and the majority of these cases are seen after the fifth decade. Benign bone and soft tissue tumors are 100 times more common than malignant tumors, with an overall incidence of ∼300 per 100,000 population.1 The incidence per year of breast, prostate, and lung cancers in the United States of America is nearly 180,000 to 200,000 each, reflecting the low incidence of primary bone and soft tissue tumors. As the survival of patients with carcinomas is gradually extending, presentation with bone metastases will also rise.
A general orthopedic surgeon or radiologist in the community may encounter only few cases of bone tumors per year. Patients with an unknown musculoskeletal tumor should be referred to a specialist center, because the cases are managed in close interaction between the orthopedic oncologist, radiologist, and the pathologist, and the outcomes are superior.2 This approach will often eliminate unnecessary diagnostic studies and inappropriate and inadequate biopsies, which may delay the diagnosis and adversely affect the outcome.
PRESENTATION
Malignant bone tumors are painful because as the bone architecture is destroyed, the weakening leads to microfractures, causing pain. In children, presentation with pathological fracture without prior symptoms often indicates a benign lesion (i.e., bone cyst) that has gradually weakened the cortex, resulting in a fatigue fracture. Same clinical picture in the elderly suggests invasion of bone by lytic metastasis or, in some cases, metabolic bone disease such as hyperparathyroidism. Malignant soft tissue tumors most often present as a painless enlarging mass and occasionally with pain due to pressure on surrounding tissues (e.g., nerves) or due to erosion of an adjacent bone.
Patients with spinal tumors most often present with axial pain and some with radicular pain (if the tumor extends to and compresses the nerve roots). Fewer present with cauda equina syndrome, a condition more often seen in metastatic disease and in rapidly growing primary tumors. Although a majority may present with axial or radicular pain, about a third present with a motor deficit. Only a small percent have bowel/bladder sphincter dysfunction.3
When evaluating a primary bone tumor, age of the patient is an important parameter. In young children (<5 years), bone lesion with a soft tissue mass are more likely osteomyelitis or Langerhans cell histiocytosis, rather than a primary malignant bone tumor. Less likely are Ewing's sarcoma, metastasis from neuroblastoma, or involvement by leukemia. The peak incidence of primary osteosarcoma occurs in the second decade of life, which corresponds to the maximal period of skeletal growth. In patients over 50, myeloma or metastases are much more likely, although chondrosarcoma and secondary osteosarcoma (due to osteonecrosis, radiation, etc.) are the primary bone malignancies. Most soft tissue sarcomas tend to occur in the older population, and rhabdomyosarcoma and synovial sarcoma tend to occur in children and adolescents.4
Pertinent information from the history includes night pain/rest pain and increase in the size of a mass.5 Night pain is less likely in a stress reaction/fracture. Similarly, recent or subacute trauma raises the suspicion of a hematoma or myositis ossificans. Other causes of bone lesions, such as metabolic disorders and avascular necrosis from steroid use, should be pursued in the history.
LABORATORY STUDIES
Routine screening tests such as a complete blood count and basic chemistry panel rarely help with the diagnosis of a musculoskeletal tumor. The erythrocyte sedimentation rate may be helpful to exclude infection. However, Ewing's sarcoma, Langerhans cell histiocytosis, leukemia, and lymphoma may also cause an elevated erythrocyte sedimentation rate.6 Tumors involving the bone and causing bone destruction may cause elevation of serum alkaline phosphatase. This can also be found due to bone injuries, recent surgery, and in Paget's disease of the bone. Elevated serum lactate dehydrogenase is sometimes found in large, high-grade tumors that are necrotic.
DIAGNOSTIC IMAGING
Despite more sophisticated imaging modalities, the diagnosis of many bone tumors can be made based on plain radiographs. Analysis of plain radiographs for periosteal reaction, junction of the lesion with the host bone, cortical disruption, matrix of the lesion and site, and the number of the lesion(s) in skeleton can further direct the diagnosis.
Most benign bone tumors arise in the metaphyses of long bones (e.g., nonossified fibroma, unicameral or aneurysmal bone cysts, and osteoblastoma). Osteosarcomas involve metaphyses of long bones, especially around the knee. The differential diagnoses of a lytic epiphyseal lesion in a child are chondroblastoma, clear cell chondrosarcoma, or infection. After the growth plate closure, it is most likely a giant cell tumor. Fibrous dysplasia and round cell tumors frequently present as diaphyseal lesions. Certain tumors have a predilection for certain sites, such as in the tibia for adamantinoma or chondromyxoid fibroma; sacrum for metastases, myeloma, chordoma, and giant cell tumor; posterior aspect of the distal femur for parosteal osteosarcoma. Within the axial skeleton, the vertebral body is a common site for metastases, hemangioma, and myeloma and the posterior elements involved by osteoid osteoma, osteoblastoma, and aneurysmal bone cyst.7
Polyostotic lesions in children could be due to osteochondromatosis, fibrous dysplasia, Langerhans cell histiocytosis, chronic multifocal osteomyelitis, Ollier and Maffucci diseases, lymphoma, and metastases. In older patients, myeloma and metastatic disease should be high on the list.
Plain radiographs are not as helpful in the diagnosis of soft tissue tumors as in bone tumors. Soft tissue calcifications, if smooth and round, can be suggestive of a hemangioma, whereas if coarse and sparse, could be found in the central necrotic areas of sarcomas.
Following the initial plain radiograph evaluation, further analysis can be done with computed tomography (CT) scans or magnetic resonance imaging (MRI), to delineate the extent of the tumor within the bone and also any extension into the soft tissue. The multiplanar imaging capabilities and superior anatomic resolution of MRI are very helpful in defining the size, contents, and relationship of the lesion to adjacent neurovascular structures, which are all necessary for planning the biopsy and tumor resection. MRI is highly reliable for planning limb salvage or an amputation, but the interaction between the orthopedic surgeon and the radiologist in reviewing the imaging studies is crucial prior to the procedure. An MRI of the whole length of the involved bone allows visualization of the entire length to detect skip metastases.4
Whole-body technetium-99m bone scan will help identify other sites of involvement, and hence help stage the disease, or even identify another location that may be an easier site for obtaining a piece of tissue for diagnostic purposes. However, its role for local staging and differentiation between a benign and malignant tumor is limited.
Angiography is used less frequently since MRI quality has improved significantly. Angiography is used in cases where the tumor is very vascular and may need preoperative embolization, and also in centers where limb perfusion with chemotherapy is still part of the treatment regimen.
STAGING
For staging a musculoskeletal neoplasm, the grade and site of the tumor and presence or absence of metastasis are considered. The grade of the tumor reflects biological aggressiveness, which is best conceptualized as “clinical” or “surgical.” Sometimes a malignant tumor with a more benign histological appearance maybe considered high-grade based on its clinical behavior.8 Grading of the tumors is classified as G0 (benign), G1 (low-grade malignant), or G2 (high-grade malignant). The local extension of the tumor is described as T0 (lesion is confined within its capsule), T1 (lesion has reactive zone instead of capsule but both the lesion and reactive zone are contained within a compartment), and T2 (lesion is outside of the compartment). Final consideration in staging is the absence (M0) or presence (M1) of the metastasis (Table 1).
Table 1.
MSTS Staging for Bone and Soft Tissue Sarcomas
| Stage (MSTS) | Grade | Location/Extension | Metastasis |
|---|---|---|---|
| MSTS, Musculoskeletal Tumor Society. | |||
| IA | Low (G1) | Intracompartmental (T1) | M0 |
| IB | Low (G1) | Extracompartmental (T2) | M0 |
| IIA | High (G2) | Intracompartmental (T1) | M0 |
| IIB | High (G2) | Extracompartmental (T2) | M0 |
| III | Low/high(G1–G2) | Intra/extra (T1–T2) | Regional/distant, M1 |
For benign bone and soft tissue tumors, the staging system commonly used is that of the Musculoskeletal Tumor Society (MSTS). Arabic numerals grading from 1 to 3 are used. The inactive lesions are graded as 1 (e.g., lipoma, nonossifying fibroma); active but slow-growing lesions are graded 2 (some aneurysmal bone cysts, unicameral bone cysts, etc.); and aggressive lesions such as a growing giant cell tumor or desmoid tumor as 3.
MSTS staging system for bone sarcomas is widely used. According to this system, stage I lesions are low-grade malignant tumors; stage II are high-grade tumors; and stage III are either low or high grade, but with metastatic disease. Within this system, tumors within a compartment are categorized as A, and those that extend outside the compartment as B.8
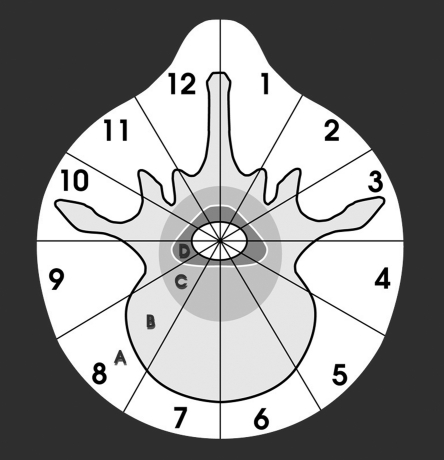
In the spine, the lesions are evaluated based on the location within the vertebra and thereby aiding in the surgical planning (Fig. 1).
Figure 1.
Surgical planning of a vertebral tumor (Weinstein-Boriani-Biagini diagram). The vertebra is divided into 12 segments (“clock face”) and concentrically layered sequentially from extraosseous “A” to epidural “D” and intraspinal “E.”
Soft tissue sarcomas are usually classified according to the American Joint Committee on Cancer system, which has comparable criteria with MSTS.9
BIOPSY
Different methods of biopsy are available, including needle biopsy (fine-needle aspiration or core biopsy with a larger needle) and open biopsy (incisional or excisional). Although open biopsy is still considered a gold standard, many centers have moved toward percutaneous biopsy. Percutaneous biopsy is less invasive, requires smaller dose of anesthetics and analgesics, causes minimal bleeding and biopsy tract contamination, and is a cost-effective method. However, it is less reliable in obtaining an adequate representative specimen for grading and further special studies. For heterogeneous tumors, very myxoid or cystic lesions and bone tumors without soft tissue component, a needle core may not give a high diagnostic yield. Biopsy tracts should be placed in areas that can be excised safely during definitive surgery, avoiding vital neurovascular structures, major tendons (e.g., patellar tendon), and joint spaces. It is valuable to discuss the biopsy route with the orthopedic oncologist responsible for the patient's future management. Biopsy should be performed under aseptic conditions, and prophylactic antibiotics may be considered in high-risk patients. Infection at the tumor site is a devastating complication that may interfere with the subsequent treatments such as chemo- or radiotherapy or limb-saving surgery.
IMAGE-GUIDED INTERVENTIONS
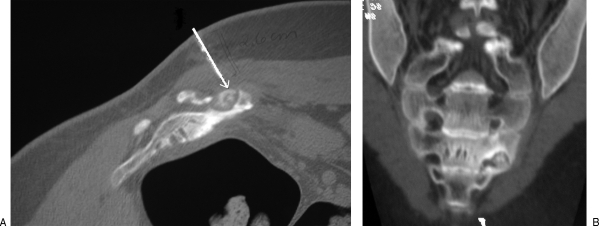
Minimally invasive interventions are reasonable alternatives to treat patients if conventional surgery may result in higher morbidity. A typical case is that of the osteoid osteoma, treated historically with wide resection to excise the small nidus that is not easily identified during surgery. However, such treatment is associated with a high risk of fracture and may require external or internal support and longer immobilization. Over the last decade, percutaneous CT-guided ablation became popular because of the high rate of success and a very low morbidity; it is used in most sites of the skeleton, except when in close proximity to neurovascular structures (Fig. 2).
Figure 2.
(A) Axial and (B) coronal images of an osteoid osteoma in the sacrum (left S3 foramina). Due to the location, adjacent to the nerve in the foramina, radiofrequency ablation was not recommended, and an open excision was performed.
Another candidate is the patient with metastatic disease, who cannot tolerate the conventional surgical methods because of immunocompromised status due to ongoing chemotherapy, poor nutrition, and comorbid medical conditions. Image-guided minimally invasive procedures are better tolerated by these patients, resulting in less soft tissue trauma, minimal blood loss, and shorter hospitalization. These methods rarely interfere with the adjuvant treatments, and their overall morbidity is considerably lower compared with conventional surgery.
Percutaneous vertebral augmentation with cement (vertebroplasty, kyphoplasty) is considered a valuable technique in the treatment of painful lesions of the spine, which may or may not have fractured. This technique is used for benign lesions (e.g., painful vertebral hemangiomas) or metastatic lesions (myeloma or metastatic adenocarcinoma).
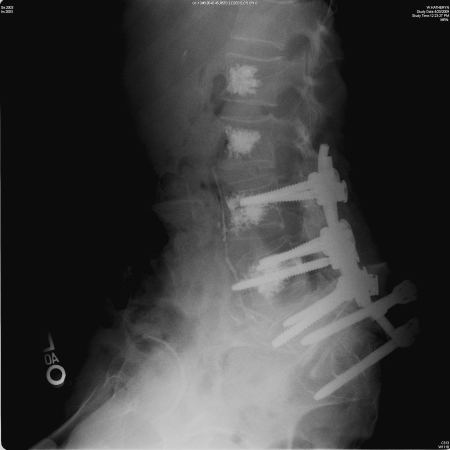
Cement augmentation can also be used after an instrumented spinal fusion, in cases of poor bone quality with a high risk of fixation failure (Fig. 3). Percutaneous cement augmentation can be done a few days after the primary fusion procedure by the interventional radiologist. Augmenting the adjacent levels to the fusion decreases the chance of a compression fracture, due to increased stresses transferred from the fixed segment.10,11
Figure 3.
Lumbosacral fusion for a painful compression fracture of S1 with neurological deficit, due to myeloma. Postoperative augmentation of the fixation with percutaneous vertebroplasty of the vertebra with implants, as well as a couple of levels above.
Percutaneous ablation of tumors has grown in use and technology over the last two decades. For musculoskeletal tumors, image-guided radiofrequency ablation (RFA) has been used to treat some benign and metastatic bone lesions. Osteoid osteoma, for example, can be successfully treated with RFA.12 The advantages of RFA over surgery are short procedure, possibility of outpatient treatment, favorable morbidity rate, and overall cost.13
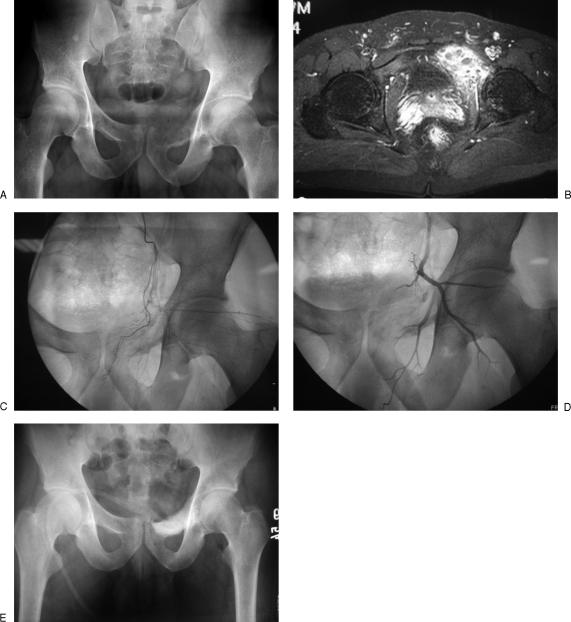
Part of the surgical planning for vascular tumors includes embolization of the lesion prior to the surgery. This method is especially useful for hypervascular tumors such as aneurysmal bone cyst, giant cell tumor, angiosarcoma, leiomyosarcoma, metastatic hypernephroma, thyroid cancer, among others. Preoperative arterial embolization improves surgical vision and allows safer and faster surgery by decreasing bleeding from the surgical site. These effects are particularly important for intralesional procedures in areas where tourniquet use is not possible (Fig. 4). Tumors supplied by a rich capillary network, such as metastatic melanoma or myeloma, respond poorly to embolization. To maximize the effects of the embolization, the planned surgery should be conducted within 24 to 48 hours.14
Figure 4.
(A) Aneurysmal bone cyst of the left superior pubic ramus. (B) Magnetic resonance imaging shows multiple blood-filled cavities and vascularity of the tumor. (C) Preembolization angiograms show increased vascularity around the lesion. (D,E) Patient was treated successfully with preoperative embolization, and then intralesional curettage, local adjuvant, and bone grafting.
Embolization is also used as a palliative treatment in painful bone metastasis when surgery cannot be performed because of the size or location of the tumor or the general status of the patient. Pain relief is possibly achieved by inhibiting and reducing tumor growth that decreases the pressure on adjacent neural structures. In patients with spinal cord compression, embolization may improve the neurological status of the patient.
Sequential embolization has also been described as a primary treatment alternative for hypervascular bone tumors, such as aneurysmal bone cyst and giant cell tumor. This method is recommended when the tumor is inoperable or surgery is technically difficult or may result in considerable morbidity (Fig. 5). In pelvic and spinal tumors, arterial occlusion has been reported to devascularize tumors, cause calcification of their margins, alleviate pain, and even result in complete growth arrest of the lesions.15,16
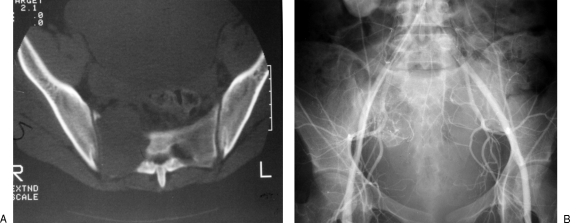
Figure 5.
(A) Axial computed tomography image showing a destructive lesion of the right ala, due to a giant cell tumor. (B) Angiogram showing increased vascularity to the tumor. The patient was treated with serial arterial embolization, with good results of symptom relief and halting tumor growth.
REFERENCES
- Rydholm A, Berg N O, Gullberg B, Thorngren K G, Persson B M. Epidemiology of soft-tissue sarcoma in the locomotor system. A retrospective population-based study of the inter-relationships between clinical and morphologic variables. Acta Pathol Microbiol Immunol Scand [A] 1984;92:363–374. [PubMed] [Google Scholar]
- Mankin H J, Mankin C J, Simon M A, Members of the Musculoskeletal Tumor Society The hazards of the biopsy, revisited. J Bone Joint Surg Am. 1996;78:656–663. doi: 10.2106/00004623-199605000-00004. [DOI] [PubMed] [Google Scholar]
- Constans J P, de Divitiis E, Donzelli R, Spaziante R, Meder J F, Haye C. Spinal metastases with neurological manifestations. Review of 600 cases. J Neurosurg. 1983;59:111–118. doi: 10.3171/jns.1983.59.1.0111. [DOI] [PubMed] [Google Scholar]
- Donthineni R, Ofluoglu O. In: Joseph T. Ferrucci, et al, editor. Taverras & Ferruci's Radiology. Philadelphia: Lippincott Williams & Wilkins; 2004. Orthopaedic evaluation of the patient with suspected musculoskeletal tumor. pp. 1–6.
- Skrzynski M C, Biermann J S, Montag A, Simon M A. Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 1996;78:644–649. doi: 10.2106/00004623-199605000-00002. [DOI] [PubMed] [Google Scholar]
- Letson G D, Greenfield G B, Heinrich S D. Evaluation of the child with a bone or soft-tissue neoplasm. Orthop Clin North Am. 1996;27:431–451. [PubMed] [Google Scholar]
- Greenspan A, Remagen W. Differential Diagnosis of Tumors and Tumor-Like Lesions of Bones and Joints. 1st ed. Philadelphia: Lippincott-Raven; 1997. pp. 1–25.
- Wolf R E, Enneking W F. The staging and surgery of musculoskeletal neoplasms. Orthop Clin North Am. 1996;27:473–481. [PubMed] [Google Scholar]
- Greene FL, Page DL, Fleming FD, et al, editor. American Joint Committee on Cancer: Cancer Staging Manual. 6th ed. New York: Springer; 2002. pp. 221–226.
- Lattig F. Bone cement augmentation in the prevention of adjacent segment failure after multilevel adult deformity fusion. J Spinal Disord Tech. 2009;22:439–443. doi: 10.1097/BSD.0b013e31818d6493. [DOI] [PubMed] [Google Scholar]
- Becker S, Chavanne A, Spitaler R, et al. Assessment of different screw augmentation techniques and screw designs in osteoporotic spines. Eur Spine J. 2008;17:1462–1469. doi: 10.1007/s00586-008-0769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal D I. Percutaneous radiofrequency treatment of osteoid osteomas. Semin Musculoskelet Radiol. 1997;1:265–272. doi: 10.1055/s-2008-1080147. [DOI] [PubMed] [Google Scholar]
- Lindner N J, Ozaki T, Roedl R, Gosheger G, Winkelmann W, Wörtler K. Percutaneous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg Br. 2001;83:391–396. doi: 10.1302/0301-620x.83b3.11679. [DOI] [PubMed] [Google Scholar]
- Sabharwal T, Salter R, Adam A, Gangi A. Image-guided therapies in orthopedic oncology. Orthop Clin North Am. 2006;37:105–112. doi: 10.1016/j.ocl.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Boriani S, De Iure F, Campanacci L, et al. Aneurysmal bone cyst of the mobile spine: report on 41 cases. Spine (Phila Pa 1976) 2001;26:27–35. doi: 10.1097/00007632-200101010-00007. [DOI] [PubMed] [Google Scholar]
- Lackman R D, Khoury L D, Esmail A, Donthineni-Rao R. The treatment of sacral giant-cell tumours by serial arterial embolisation. J Bone Joint Surg Br. 2002;84:873–877. doi: 10.1302/0301-620x.84b6.13178. [DOI] [PubMed] [Google Scholar]