ABSTRACT
Interventional radiology plays a major role in the management of bone tumors. Many different percutaneous techniques are available. Some aim to treat pain and consolidate a pathological bone (cementoplasty); others aim to ablate tumor or reduce its volume (sclerotherapy, thermal ablation). In this article, image-guided techniques of primary and secondary bone tumors with vertebroplasty, ethanol injection, radiofrequency ablation, laser photocoagulation, cryoablation, and radiofrequency ionization (coblation) will be reviewed. For each modality, the principles, the indications, and the results will be presented. The technical choice depends on the therapeutic intent—curative or palliative—and the need for consolidation, but also on the general status of the patient and the other therapeutic options. For the most complex cases, combined treatments can be required. However, the less disabling technique should always be considered first.
Keywords: Bone tumor, vertebroplasty, radiofrequency ablation, cryoablation, laser, coblation, sclerotherapy
During the past decades, the role of interventional radiology has rapidly increased thanks to the development of many different techniques. Vertebroplasty is now widely used for pain management and consolidation of vertebral metastases. Ethanol injection has also been reported for tumoral reduction and pain management of musculoskeletal tumors; if ethanol is still used in spinal vascular malformations such as aggressive hemangiomas, thermoablation techniques including radiofrequency ablation (RFA), laser ablation, and cryoablation are now preferred for the management of musculoskeletal tumors as they produce more controllable ablation. The detail of each technique including mechanism, the indications, and the results will be presented in this article.
CEMENTOPLASTY
Principle
Percutaneous injection of polymethylmethacrylate (PMMA) provides pain relief and bone strengthening in patients with malignant bone tumors.1 PMMA is highly resistant to compression forces but susceptible to torsion forces. Due to its mechanical properties, this cement is suitable for treatment of fractures involving weight-bearing bones, such as vertebral body and acetabulum, and in any bones subject to compression forces only (Fig. 1). In opposition, injection in long diaphyses does not achieve sufficient bone strengthening with possible fracture of the cement rod.
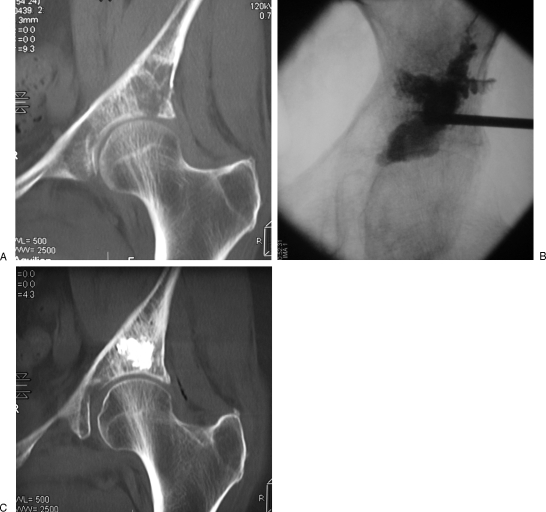
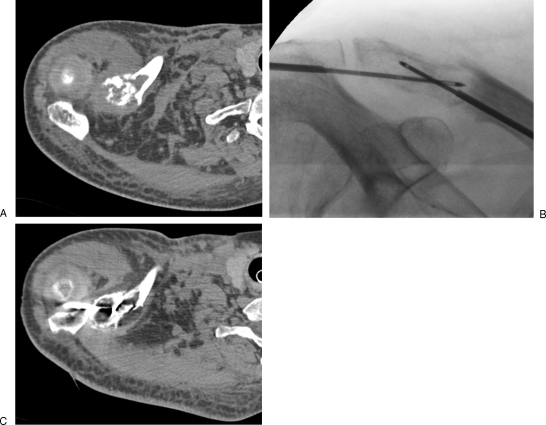
Figure 1.
Painful acetabular metastasis of lung cancer treated with cementoplasty. (A) Computed tomography (CT) scan with coronal reformation shows osteolytic tumor involving the acetabular roof, with risk of pathological fracture. (B, C) Fluoroscopic view and corresponding CT show the cement distribution. Cementoplasty achieves pain management and consolidation.
Indications
Painful osteolytic destruction of the vertebral body or other weight-bearing bones by metastases from cancers, multiple myeloma, and lymphomas is a source of pain and disability. Due to the multifocal nature of these lesions, surgical treatment is rarely undertaken.2 Radiation therapy does not provide consolidation nor relieve pain, and the therapeutic response is generally delayed. In opposition, cement injection allows fast consolidation resulting in effective pain relief. This procedure is performed in a palliative intent and does not stop tumor progression; thus, it should be considered as a complement, not a replacement, to other treatment modalities for cancer. When the bone tumor is associated with a painful paraosseous soft tissue invasion, cementoplasty can be combined with thermal ablation techniques such as RFA or cryoablation.3,4
For spinal metastases, cement injection (vertebroplasty) can be performed at multiple levels according to the multifocal nature of the disease process. However, vertebroplasty is specifically indicated for the management of local pain from metastatic disease and not for diffuse back pain with entire involvement of the spine. Cementoplasty has also been successfully used in extraspinal tumoral locations such acetabulum, femoral condyles, tibial ends, talus, or calcaneus. A detailed clinical examination with specific emphasis on neurological assessment must be performed in conjunction with the imaging findings to rule out other causes of pain.
Contraindications of cementoplasty include irreversible coagulation disorders and acute infection. Spinal tumors with large rupture of the posterior wall should be considered with caution as the risk of epidural cement leakage is increased. Vertebral metastases causing neurological symptoms or instability are generally contraindicated for percutaneous treatment; however, in some cases, vertebroplasty can be combined with laminectomy and surgical fixation to avoid a complex surgical anterior approach. Cement injection is difficult or even impossible in osteoblastic metastases; when pain is refractory to conventional treatments (pain killers, radiation therapy), thermal ablation techniques can be used to control pain by destroying the nerve endings at the bone-tumor interface.
Technique
Cementoplasty can be performed under general anesthesia or conscious sedation, depending on the general status of the patient, his positioning, and the estimated procedural time.4
Strict asepsis is mandatory. Intraprocedural intravenous antibiotic cover (cephazolin 1 mg) is recommended in immunocompromised patients, but there is no clear consensus on other patients. Local anesthesia of the whole pathway is performed with lidocaine 1% through a 22-gauge spinal needle. Under fluoroscopic guidance, a cementoplasty trocar (10 to 13 gauge) is inserted parallel to the spinal needle and introduced into the bone. For complex cases or if thermal ablation is combined, dual guidance with computed tomography (CT) and fluoroscopy allows precise needle placement, increases the operator comfort, and reduces the rate of complications. Because of the high radiation dose, CT fluoroscopy should not be used in routine practice.
Lumbar vertebroplasty is generally performed via a unilateral transpedicular oblique approach. At thoracic level, unilateral intercostopedicular route is preferred to avoid both the spinal canal and the pleura. At cervical level, the approach is anterolateral with a trajectory between the carotid artery laterally and the thyroid gland and the esophagus medially. For C1 and C2, a direct transoral approach is described; this is the most direct route to avoid the neural and vascular structures. Sacral puncture is commonly performed via a posterior approach when cementoplasty of the sacral wings is needed; an oblique posterolateral route through the iliac wing is sometimes necessary for cementoplasty of S1–S2 vertebral body consolidation.
For acetabular cementoplasty, the approach can be anterolateral through the iliac wing to avoid the femoral nerve or posterolateral, avoiding the sciatic nerve. For other bones, the proper pathway should be chosen, giving priority to the shortest and safest route, away from the neurovascular structures.
After controlling the proper position of the cementoplasty trocar, the stylet is removed and a fluoroscopic view is obtained and used as reference picture during cement injection, to watch for leakage. If needed, a bone biopsy can be performed coaxially at the same time. Venography is only performed in hypervascular tumors with active bleeding through the bone trocar; digital subtraction angiography is then necessary to analyze the venous drainage and to anticipate a likely route for cement leakage. In such cases, it is safer to wait until the cement is fairly thick before injecting it, to reduce the risk of intravasation.
Cement injection is slowly performed under continuous fluoroscopic control. The use of dedicated injection sets is recommended as it gives a better control of pressure and speed during injection; moreover, it reduces the radiation dose to the operator's hands.
Results
Percutaneous cementoplasty is an effective treatment for painful osteolytic bone metastases and myeloma.5,6 It treats pain and consolidates weight-bearing bone. If exothermic polymerization of PMMA can reach temperatures over 75°C, the cytotoxic effect is limited to 3 mm around the cement. Thus, the antitumoral effect of the cement is insufficient, and specific tumor therapy (i.e., chemotherapy, radiotherapy, or thermoablation) should be given in conjunction with cementoplasty for the tumor management where appropriate.7
Many authors report significant pain relief in 80 to 97% of cases after cementoplasty in cancer patients.2,5,6,8,9 Local pain is rapidly controlled and endures at long-term follow-up.
Patients are allowed to stand a few hours after cementoplasty. Overnight hospitalization is generally sufficient after percutaneous cementoplasty, but this may vary depending on the general status of the patient and the level of postprocedural analgesia.
In a series of 21 cancer patients treated with vertebroplasty, Alvarez et al showed significant and early improvement in the functional status of patients with spinal metastases.5 In this study, 77% of patients could walk again, and 81% were satisfied or very satisfied with the results.
In cases of bone tumor with painful extension to the surrounding soft tissues, improved results can be obtained by combining thermal ablation techniques and cementoplasty.10
Complications due to cementoplasty are mainly due to cement leakage. This incidence of leakage is higher in malignant disease compared with osteoporotic fractures but often remains asymptomatic.11 Cortical destruction, extension in the paraosseous soft tissues, and highly vascularized tumors are likely to increase the rate of complications.12 In case of cement leakage in contact with a nerve detected at the time of cementoplasty, active cooling with injection of saline close to the nerve should be performed during the polymerization phase to prevent radiculopathy caused by heating of the nerve root.13 Additional periradicular infiltration with steroids is sometimes needed. Venous intravasation of cement limited to the basivertebral vein is usually asymptomatic if the operator recognizes it early and immediately stops injection. However, failure to notice this can cause filling of the epidural or paravertebral veins, resulting in epidural cement leakage or pulmonary embolism.14 Minor disc leakage is common and remains of no consequence.
Infection secondary to cementoplasty occurs in less than 1% cases.15 To avoid this complication, careful preprocedural evaluation of the patients and strict asepsis are mandatory. Bleeding from the puncture site remains exceptional. Hypotension and arrhythmias have been reported during cement injection; they may be due to small amount of fat embolism secondary to bone marrow displacement. Anaphylactic reaction to PMMA resulting in death is exceptional but has been reported to the FDA.12
TUMOR ABLATION
Tumor ablation is defined as the direct application of chemical or physical therapies to a specific focal tumor to achieve its destruction. During the last decades, several percutaneous ablation techniques have emerged. Considering their success rate on renal, hepatic, and lung malignancies, many authors have proposed their use for palliative or curative treatment of bone and musculoskeletal tumors. Widely adopted image-guided ablation techniques include alcohol instillation and different thermal ablation methods like radiofrequency, microwave, cryoablation, laser photocoagulation, and low-temperature radiofrequency ionization (RFI). These different methods will be presented, highlighting their advantages and their specific indications.
General Indications of Percutaneous Musculoskeletal Ablation
The principal role of image-guided musculoskeletal tumor ablation lies in the palliative targeted minimally invasive ablation of painful metastases secondary to advanced cancer disease. Less frequently, percutaneous ablation can be considered in a curative intent either for benign bone tumors such as osteoid osteomas or for single metastasis in poor surgical candidates. In multidisciplinary oncology practice, the therapeutic intent has to be precisely defined considering the general status of the patient and the available therapeutic options.
Alcohol Ablation
Percutaneous ethanol injection is the simplest and cheapest method of percutaneous tumor ablation. Alcoholization causes tumor necrosis directly through cellular dehydration and indirectly through vascular thrombosis and tissue ischemia. A 22-gauge needle is inserted into the tumor and a mixture of iodinated contrast (25%) and lidocaine 1% (75%) is first injected to assess the diffusion extent and achieve local anesthesia. In the absence of intravasation or diffusion in contact with vulnerable structures, 3 to 30 mL of 96% ethanol is injected into the tumor. Ethanol injection in bone is very painful and requires neuroleptanalgesia. This technique has showed promising results for palliative management of painful bone tumors.16 However, the size and shape of the induced necrosis is poorly reproducible, due to the hardly predictable distribution of ethanol. One reason thermal ablation techniques are now preferred is that they provide much more predictable ablation volumes.
Ethanol injection is still used for the treatment of aggressive vertebral hemangiomas (AVH). AVH limited to the vertebral body are now widely treated with single vertebroplasty, but more complex AVH with paravertebral or epidural extension generally require combined therapies (Fig. 2): thrombosis and retraction of the AVH compartment is achieved with direct percutaneous intravertebral ethanol injection (sclerotherapy) via one or several 18-gauge spinal needles.17 Two weeks after sclerotherapy, magnetic resonance (MR) control with dynamic injection is obtained to assess to proper the obstruction of the AVH and subsequent vertebroplasty is performed to achieve consolidation.18 Laminectomy is only performed for major epidural extension with neurological deficit; however, prior percutaneous treatment with sclerotherapy and/or sclerotherapy highly reduces the risk of perioperative hemorrhage.19
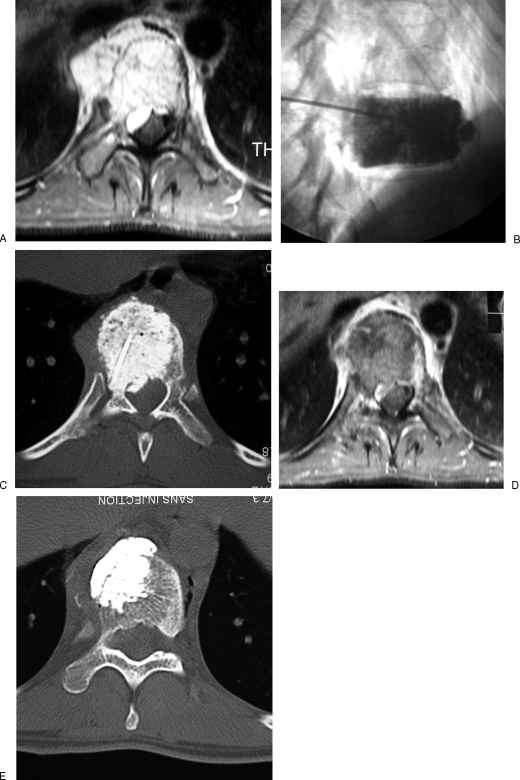
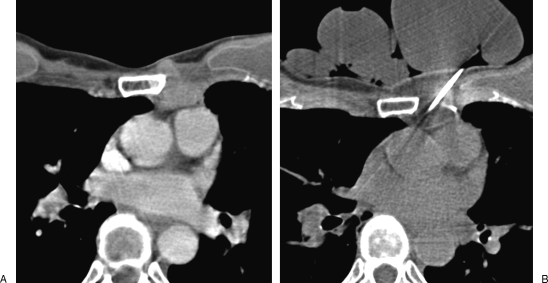
Figure 2.
Aggressive vertebral hemangioma of T10 vertebra treated with sclerotherapy and vertebroplasty. (A) Contrast-enhanced magnetic resonance (MR) image with fat saturation shows the aggressive hemangioma with paravertebral and epidural extension. (B, C) Sclerotherapy is performed with ethanol mixed with lipiodol. Note the optimal filling of the vertebra. (D) Contrast-enhanced MR image 2 weeks after sclerotherapy shows a large thrombosis of the hemangioma with shrinkage of the paravertebral and epidural components. (E) Vertebroplasty is then performed to prevent secondary collapse and achieve consolidation.
Laser Photocoagulation
Laser photocoagulation uses the absorbed energy of infrared light to produce heat and ablate the tumor. Light energy of 2.0 W will produce a spherical ablation volume of 1.6-cm diameter in bone. Higher powers result in charring and vaporization around the laser fiber tip. To ablate larger volumes, several bare fibers 2 cm apart spread through the lesion are necessary.20 This technique is fully MR compatible.
Because of the small ablated volume produced by a bare tip fiber, laser is now only used for small tumors or, exceptionally, if metallic implants contraindicate the use of RFA. Osteoid osteomas, which are by definition less than 1 cm in diameter, are one of the best indications for laser ablation in bone. The laser fiber is inserted into the nidus under CT guidance. If the osteoid osteoma is surrounded by thick cortical bone, a drill needle is used and the fiber is inserted coaxially. For subperiosteal tumors, an 18-gauge spinal needle is used to protect the optical fiber (Fig. 3). Once in position, the ablation is performed with continuous 2-W laser power for 6 to 10 minutes depending on the nidus size. The success rate of laser ablation of osteoid osteomas is excellent and similar to RFA.21,22 Minimally invasive percutaneous thermal treatment is now the gold standard for osteoid osteomas and has replaced surgical approach. Successful treatment in a single session is achieved in 95% cases. Intra-articular location of the nidus seems to be associated with a higher risk of recurrence.23 This painful benign tumor can only be treated under deep anesthesia (general, spinal anesthesia, or regional block). Postprocedural pain is systematically controlled with nonsteroidal anti-inflammatory drugs and analgesics.
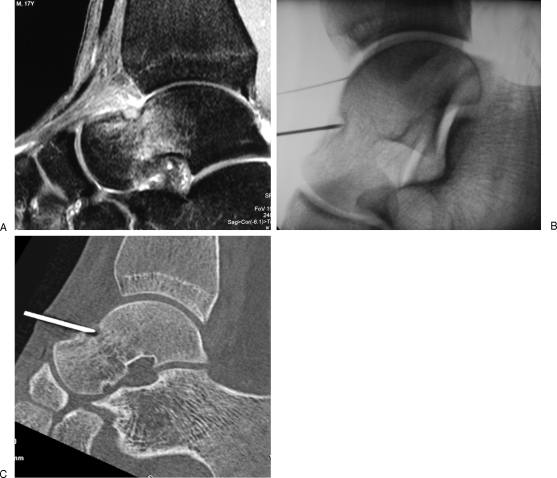
Figure 3.
Osteoid osteoma of the talus treated with laser ablation. (A) Sagittal T2-weighted Short Tau Inversion Recovery (STIR) magnetic resonance image shows a subperiosteal osteoid osteoma of the neck of the talus, with inflammatory reaction in the adjacent cancellous bone and in the soft tissues. (B, C) Fluoroscopic view and corresponding reformatted computed tomography show an 18-gauge spinal needle inside the nidus. The laser fiber is inserted coaxially for ablation. Note the intra-articular injection of saline via a 22-gauge needle to achieve thermal protection of the joint (B).
Radiofrequency Ablation
Radiofrequency ablation (RFA) is one of the most promising thermal techniques for the treatment of localized tumors. It was first applied in the early 1990s for the treatment of hepatic tumors. RFA applicators with straight or expandable electrodes are introduced percutaneously into the target tumor. Then, a high-frequency, alternating current (450 to 600 kHz) is delivered through the lesion, which causes agitation of the tissue ionic molecules, which in turn produces frictional heat. The electrical loop is closed with grounding pads attached at the thighs. The thermal effect depends on the electrical-conducting properties of the tissues treated. Local tissue temperatures between 60 and 100°C produce protein denaturation, immediate cell death, and coagulative necrosis of the tumor. Temperatures above 100°C induce vaporization and carbonization of the tissue adjacent to the electrode, which degrade the electrical conductance and result in suboptimal treatment effect. The efficacy of RFA may be limited by adjacent high-flow vessels that act as a cooling circuitry (“heat sink effect”). Nevertheless, RFA has rapidly taken over from alcohol ablation as it produces more controllable tumor ablation.
The use of RFA in bone tumors is mainly palliative but in some cases can also be curative. For palliative treatment of painful bone metastases refractory to conventional therapies, the principal aim of RFA is to ablate the bone-tumor interface, where the primary source of pain is located. Less often, RFA can be used in a curative intent for patients with single metastasis limited in size. Of note, palliative ablation of painful sclerotic bone metastases is possible with reduced power to avoid early impedance increase.24 Newly developed bipolar radiofrequency electrodes are of special interest in spinal and paraspinal applications; in bipolar arrays, the current flows from the tip of one electrode to the other and no grounding pad is used; thus, heat generated is mainly limited between the electrode tips, which allows faster and better controlled ablation with improved protection of the surrounding tissues.10 Multipolar radiofrequency devices are now available; several electrodes can be alternately activated to rapidly ablate larger volumes.
RFA is also an alternative efficient technique to treat osteoid osteomas; in that case, the ablation is performed with a 1-cm active tip electrode, with low power (∼5 W) to maintain the temperature at 90°C for 6 minutes inside the nidus.
For osteoid osteomas, the success rate of bone RFA is similar to laser (>85%).21 For painful bone metastases, significant and rapid pain relief is achieved in more than 75% cases with substantial decrease of medication. However, this technique is considered for patients with focal pain and limited number of metastases, but is not adapted for diffuse bone pain.
For tumors in close proximity to neurological structures or other vulnerable organs, thermal protection techniques are required (Fig. 4). They are based on continuous thermal monitoring of the vulnerable structure with thermosensors and on organ displacement or insulation using hydrodissection, CO2 insufflation, or balloon interposition.25 Large ablation volumes are generally avoided near the spine to avoid nerve injury. Experimental in vivo and ex vivo studies have provided valuable insights into the distribution of radiofrequency-generated heat in the spinal canal26,27; there is now firm evidence that vertebral posterior cortical defects expose the nerve roots and the spinal cord to excessive heat, producing local temperatures above 45°C. In the case of spinal ablation, the presence of an intact cortex to insulate the canal cannot be overstressed, and continuous thermal monitoring of the neural structures with thermosensors is highly recommended.28
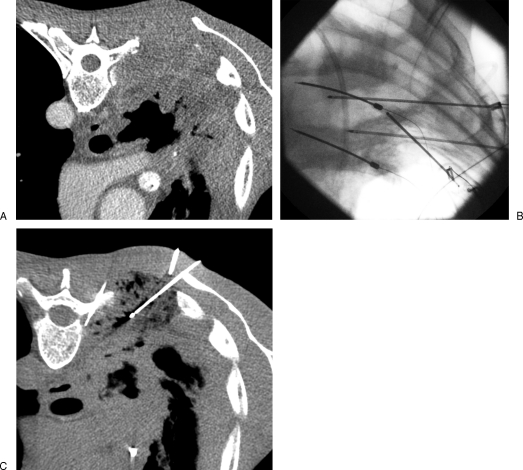
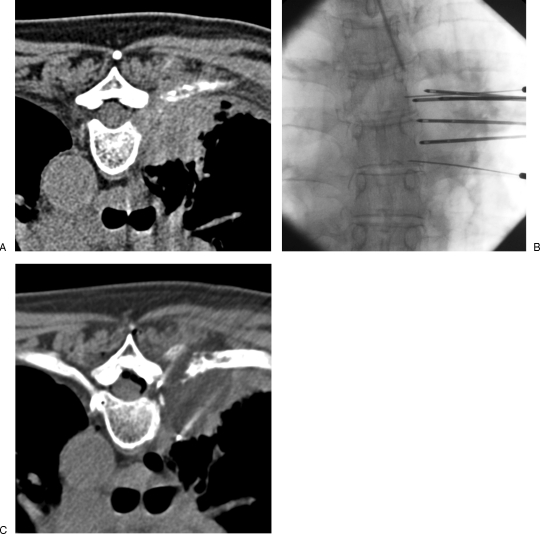
Figure 4.
Painful thoracic wall invasion treated with radiofrequency ablation. (A) Contrast-enhanced computed tomography (CT) scan shows a large tumoral infiltration of the posterior thoracic wall with rib destruction causing excruciating pain. (B, C) Fluoroscopic view and corresponding CT show three radiofrequency electrodes inserted into the tumor focusing on the pleural invasion and the rib osteolysis. Thermal control of the spinal canal is achieved by positioning thermosensors into the two adjacent foramina and continuous cooling with dextrose injection to maintain the foraminal temperature below 45°C.
Microwave Ablation
Microwave ablation is an emerging technology that depends on the application of an electromagnetic wave (around 900 MHz) through an antenna directly inserted into the target tumor. Electromagnetic waves cause agitation of ionic molecules and frictional heat, which result in tissue coagulative necrosis. In opposition to RFA, no grounding pads are necessary; moreover, it operates independently of any electrical current convection, and thus is less influenced by tissue impedance variabilities and heat sink phenomena. Three antennas can be activated synergically to produce fast ablation of large tumors. Microwave ablation can achieve higher intratumoral temperatures and larger ablation zones, faster than RFA. This technique raises interest for percutaneous treatment of large hepatic and lung tumors. If the use of microwave ablation has been reported to help surgical resection of osteosarcomas,29 no data are available about its percutaneous use in musculoskeletal tumors.
Cryoablation
Cryoablation is the application of extreme cold to destroy tumors. This technique has gained new interest since the development of miniaturized 17-gauge gas-driven cryoprobes that can be used percutaneously under CT or MR monitoring. Rapid expansion of high-pressure argon gas delivered through the cryoprobe produces sudden drop of temperature below −183°C by the Joule-Thompson phenomenon; in opposition, fast decompression of helium gas raises the temperature to +33°C, which allows active thawing. Cellular necrosis is systematically achieved with temperatures below −40°C. To avoid uncertain cell death with temperatures between 0 to −20°C, repeated freeze-thaw freeze cycles are needed. Indeed, ice crystals are mainly extracellular during the first freezing phase; during thawing phase, water diffuses into the intracellular compartment due to osmotic gradients, and the second freezing phase achieves intracellular ice crystals, leading to membrane rupture and cell death. The longer the duration of the thawing phase, the greater the cell damage it causes. Repetition of the treatment cycle is associated with more extensive and more certain tissue destruction.30 In addition to this direct cell injury, intravascular ice crystals create microthrombi and cause indirect ischemia.31 Of note, cryoablation efficacy may be compromised by tissue thawing form nearby high-flow vascular structures resulting in cool sink effect.
The major advantage of cryoablation compared with RFA is the precise visual control of the aggregated ice ball with both CT and MR.32 Multiplanar reformatting is used intermittently to monitor the extension of the ice ball, to check the proper covering of the tumor, and to control the safety gap with adjacent vulnerable tissues. Different types of cryoprobes are available, resulting in different volumes and shapes of ice ball (Figs. 5 and 6). Up to 25 cryoprobes can be activated simultaneously. For curative cryoablation, the margins of the ice ball should extend 3 to 5 mm beyond the tumor margins. In addition, cryoablation has intrinsic anesthetic properties that allow performing the procedure under mild sedation, or even local anesthetic.33 Reduction of peri- and immediate postprocedural pain with cryoablation is a major benefit in comparison to RFA. Cryoablation is also effective in sclerotic bone lesions; however, the visual control of the ice ball is poor in that situation. In opposition to RFA, surgical metallic fixation in contact with the tumor is not contraindicated for cryoablation. Safety of all thermal and cryoablation methods may be enhanced with the use of appropriate thermosensors and insulation techniques, particularly CO2 insufflation (Fig. 7).25 Hydrodissection, which is largely used as thermal protection with RFA, is not suitable with cryoablation.
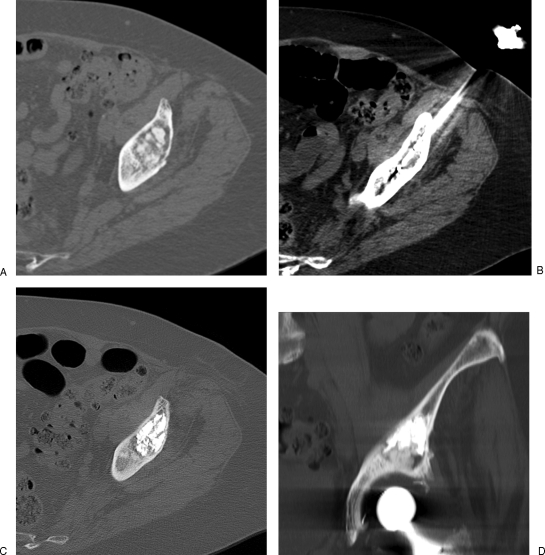
Figure 5.
Painful metastasis of the clavicle treated with cryoablation. (A) Computed tomography (CT) scan shows the osteolytic lesion with pathological fracture. (B, C) Two crossed cryoprobes are inserted coaxially through bone trocars to adapt the shape of the ice ball to the shape of the tumor. The hypodense ice ball is precisely monitored with CT (C). No further consolidation is required in this case.
Figure 6.
Painful parasternal metastasis of breast cancer treated with cryoablation. (A) Computed tomography scan shows the tumor in close contact with the mediastinum and the skin. (B) Crossed cryoprobes are inserted to achieve optimal covering of the tumor with the ice ball. Skin frostbite is avoided by applying continuously sterile gloves filled with warm saline.
Figure 7.
Painful paravertebral metastasis treated with cryoablation. (A) Computed tomography (CT) scan shows a large paravertebral mass invading the pleura, the rib, and the adjacent foramen. (B, C) Four cryoprobes are inserted into the tumor to achieve optimal tumoral covering with the ice ball. For thermal protection of the spinal cord, thermosensors are inserted into the foramina and insulation of the spinal canal is achieved with epidural CO2 dissection. Note the precise control of the ice ball margins and the gaseous gap with CT guidance. No further consolidation is required as the vertebral body is not involved.
Recent studies report the interest of cryoablation for the palliative treatment of bone metastases, with very promising results and few complications.34,35 If subsequent cementoplasty has to be performed for bone consolidation, complete thawing of the ice ball is needed and the tissue temperature should exceed +20°C before injecting cement (Fig. 8).
Figure 8.
Single acetabular metastasis of thyroid cancer treated with combined cryoablation and cementoplasty. (A) Computed tomography scan shows the acetabular osteolysis growing over a prior hip prosthesis. (B) Considering the single tumoral location, cryoablation is performed with a curative intent. Cryoprobes are inserted coaxially through bone trocars. Note the ice ball margins extending through the cortical bone into the iliac muscle. (C, D) After complete thawing of the ice ball, additional consolidation is needed and is performed in the same session with cementoplasty.
Radiofrequency Ionization
RFI is a low-temperature bipolar technique producing a plasma field at the tip of the electrode.36 The high-energy ionized plasma breaks the intramolecular bonds causing cavitation inside the tissues and subsequent decompression. This process is achieved at relatively low temperatures (40 to 70°C), compared with conventional thermal ablation radiofrequency devices. The ionization process consumes most of the heat, and no electrical current passes directly through the target tissue. The result is volumetric removal of the tumor with minimal damage to surrounding tissues. This technique is widely used in ear, nose, and throat surgery, cardiac surgery, arthroplasty, and spine nucleotomy. It has been recently introduced for bone tumor decompression. A dedicated 16-gauge bipolar electrode is inserted into the tumor coaxially through a bone trocar. A side-arm catheter is connected to the electrode to slowly inject saline solution for activation of the plasma field. The bent tip of the electrode allows digging several channels inside the tumor by rotating the electrode on its axis. These maneuvers are monitored under fluoroscopic guidance. If there is any risk of neurological damage, a combination of CT and fluoroscopic guidance is mandatory.
The best candidates for this technique are nonsurgical painful spinal tumors with intracanalar bulge, or rupture of the vertebral posterior wall with risk of tumor retropulsion after vertebroplasty (Fig. 9). RFI produces intratumoral cavitation with pressure reduction. After cavitation of spinal lesions, vertebral consolidation is achieved with subsequent cement injection. Spinal cavitation is advocated by some authors before vertebroplasty to reduce the risk of cement leakage37; unlike other intravertebral expandable systems that increase the intratumoral pressure, cavitation creates a void without increasing the pressure or moving the tumor.
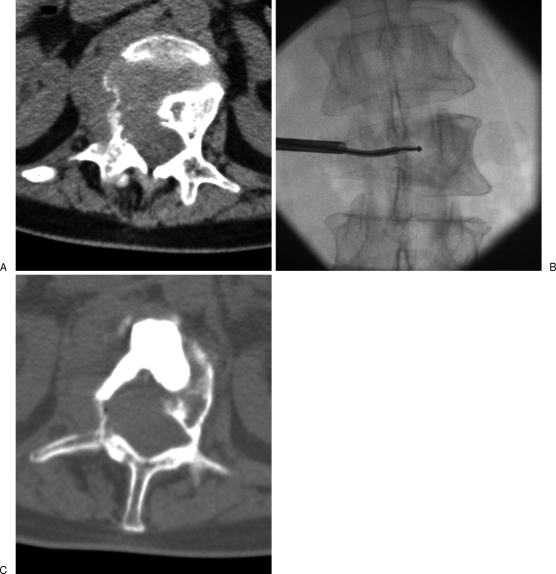
Figure 9.
Nonsurgical patient with painful lumbar metastasis bulging toward the spinal canal, treated with radiofrequency ionization and vertebroplasty. (A) Computed tomography scan shows a large osteolytic vertebral tumor with pathological fracture and foraminal and epidural extension. (B) Radiofrequency ionization is performed to create intratumoral cavitation and decompression, considering the intracanalar extension of the tumor. (C) Further consolidation is needed and achieved with vertebroplasty during the same session. Note the cement distribution in the created intratumoral cavitation.
Treatment Strategy
Management of patients with musculoskeletal tumors requires consideration of many factors: histology of the tumor, with differentiation of benign and malignant tumors; careful evaluation of the patient's general condition; an understanding of the disease process; a working knowledge of available treatment options; a clear definition of the therapeutic goal, curative or palliative; an appreciation of the degree of bone destruction (need for consolidation).
Curative treatment is considered for benign lesions such as osteoid osteomas or osteoblastomas. In such cases, the tumor is treated in a single session with a thermal ablation technique. Considering the small volume of the ablated zone, additional consolidation is not necessary. Less often, malignant nonsurgical patients with single or few slow-growing metastases can be treated with percutaneous image-guided ablation techniques, generally RFA or cryoablation. Thermal ablation is able to destroy the tumor but further weakens the involved bone; if weight-bearing bone is involved and there is a risk of pathological fracture, consolidation with cementoplasty or surgery is needed. However, cementoplasty is limited to flat bones subject to compression forces.
For palliative treatment of painful bone tumors, the therapeutic goal is no longer complete ablation but one or more of the following: tumor reduction, pain management, bone consolidation, or in some cases, tumor decompression. Although small metastases may be completely eradicated, the primary goal of symptomatic pain relief is to ablate the interface between the tumor and the pain-sensitive periosteum. If RFA is the most commonly used percutaneous ablation technique, cryoablation, which has similar indications, offers two major advantages in musculoskeletal pathology: peri- and postprocedural pain is highly reduced with low temperatures and the precise visual monitoring of the ice ball with CT or MR reduces the risk of thermal damage to surrounding vulnerable tissues, particularly neural structures. Cementoplasty is a cost-effective technique that achieves fast consolidation of bone tumors involving weight-bearing flat bones, particularly vertebra and acetabulum.
A good understanding of each available technique is mandatory to obtain the best results. Combination of different techniques is required for the most complex cases, but the less disabling technique should always be considered first. Finally, an excellent knowledge of thermal protection techniques is crucial to prevent complications, particularly when performing thermoablation close to neural structures.
REFERENCES
- Gangi A, Dietemann J L, Schultz A, Mortazavi R, Jeung M Y, Roy C. Interventional radiologic procedures with CT guidance in cancer pain management. Radiographics. 1996;16:1289–1304. discussion 1304–1306. doi: 10.1148/radiographics.16.6.8946536. [DOI] [PubMed] [Google Scholar]
- Cotten A, Dewatre F, Cortet B, et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200:525–530. doi: 10.1148/radiology.200.2.8685351. [DOI] [PubMed] [Google Scholar]
- Gangi A, Basile A, Basille A, et al. Radiofrequency and laser ablation of spinal lesions. Semin Ultrasound CT MR. 2005;26:89–97. doi: 10.1053/j.sult.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Mathis J M, Wong W. Percutaneous vertebroplasty: technical considerations. J Vasc Interv Radiol. 2003;14:953–960. doi: 10.1097/01.rvi.0000083255.29749.a8. [DOI] [PubMed] [Google Scholar]
- Alvarez L, Pérez-Higueras A, Quiñones D, Calvo E, Rossi R E. Vertebroplasty in the treatment of vertebral tumors: postprocedural outcome and quality of life. Eur Spine J. 2003;12:356–360. doi: 10.1007/s00586-003-0525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimony J S, Gilula L A, Zeller A J, Brown D B. Percutaneous vertebroplasty for malignant compression fractures with epidural involvement. Radiology. 2004;232:846–853. doi: 10.1148/radiol.2323030353. [DOI] [PubMed] [Google Scholar]
- Aebli N, Goss B G, Thorpe P, Williams R, Krebs J. In vivo temperature profile of intervertebral discs and vertebral endplates during vertebroplasty: an experimental study in sheep. Spine (Phila Pa 1976) 2006;31:1674–1678. discussion 1679. doi: 10.1097/01.brs.0000224193.52587.d8. [DOI] [PubMed] [Google Scholar]
- Weill A, Chiras J, Simon J M, Rose M, Sola-Martinez T, Enkaoua E. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199:241–247. doi: 10.1148/radiology.199.1.8633152. [DOI] [PubMed] [Google Scholar]
- Yamada K, Matsumoto Y, Kita M, Yamamoto K, Kobayashi T, Takanaka T. Long-term pain relief effects in four patients undergoing percutaneous vertebroplasty for metastatic vertebral tumor. J Anesth. 2004;18:292–295. doi: 10.1007/s00540-004-0252-6. [DOI] [PubMed] [Google Scholar]
- Buy X, Basile A, Bierry G, Cupelli J, Gangi A. Saline-infused bipolar radiofrequency ablation of high-risk spinal and paraspinal neoplasms. AJR Am J Roentgenol. 2006;186(5 Suppl):S322–S326. doi: 10.2214/AJR.05.0265. [DOI] [PubMed] [Google Scholar]
- Laredo J D, Hamze B. Complications of percutaneous vertebroplasty and their prevention. Skeletal Radiol. 2004;33:493–505. doi: 10.1007/s00256-004-0776-8. [DOI] [PubMed] [Google Scholar]
- Nussbaum D A, Gailloud P, Murphy K. A review of complications associated with vertebroplasty and kyphoplasty as reported to the Food and Drug Administration medical device related web site. J Vasc Interv Radiol. 2004;15:1185–1192. doi: 10.1097/01.RVI.0000144757.14780.E0. [DOI] [PubMed] [Google Scholar]
- Kelekis A D, Martin J B, Somon T, Wetzel S G, Dietrich P Y, Ruefenacht D A. Radicular pain after vertebroplasty: compression or irritation of the nerve root? Initial experience with the “cooling system.”. Spine (Phila Pa 1976) 2003;28:E265–E269. doi: 10.1097/00007632-200307150-00027. [DOI] [PubMed] [Google Scholar]
- Baumann C, Fuchs H, Kiwit J, Westphalen K, Hierholzer J. Complications in percutaneous vertebroplasty associated with puncture or cement leakage. Cardiovasc Intervent Radiol. 2007;30:161–168. doi: 10.1007/s00270-006-0133-5. [DOI] [PubMed] [Google Scholar]
- Vats H S, McKiernan F E. Infected vertebroplasty: case report and review of literature. Spine (Phila Pa 1976) 2006;31:E859–E862. doi: 10.1097/01.brs.0000240665.56414.88. [DOI] [PubMed] [Google Scholar]
- Gangi A, Kastler B, Klinkert A, Dietemann J L. Injection of alcohol into bone metastases under CT guidance. J Comput Assist Tomogr. 1994;18:932–935. doi: 10.1097/00004728-199411000-00016. [DOI] [PubMed] [Google Scholar]
- Gabal A M. Percutaneous technique for sclerotherapy of vertebral hemangioma compressing spinal cord. Cardiovasc Intervent Radiol. 2002;25:494–500. doi: 10.1007/s00270-002-1944-7. [DOI] [PubMed] [Google Scholar]
- Doppman J L, Oldfield E H, Heiss J D. Symptomatic vertebral hemangiomas: treatment by means of direct intralesional injection of ethanol. Radiology. 2000;214:341–348. doi: 10.1148/radiology.214.2.r00fe46341. [DOI] [PubMed] [Google Scholar]
- Ide C, Gangi A, Rimmelin A, et al. Vertebral haemangiomas with spinal cord compression: the place of preoperative percutaneous vertebroplasty with methyl methacrylate. Neuroradiology. 1996;38:585–589. doi: 10.1007/BF00626105. [DOI] [PubMed] [Google Scholar]
- Gangi A, Gasser B, De Unamuno S, et al. New trends in interstitial laser photocoagulation of bones. Semin Musculoskelet Radiol. 1997;1:331–338. doi: 10.1055/s-2008-1080157. [DOI] [PubMed] [Google Scholar]
- Rosenthal D I, Hornicek F J, Torriani M, Gebhardt M C, Mankin H J. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229:171–175. doi: 10.1148/radiol.2291021053. [DOI] [PubMed] [Google Scholar]
- Witt J D, Hall-Craggs M A, Ripley P, Cobb J P, Bown S G. Interstitial laser photocoagulation for the treatment of osteoid osteoma. J Bone Joint Surg Br. 2000;82:1125–1128. doi: 10.1302/0301-620x.82b8.11307. [DOI] [PubMed] [Google Scholar]
- Gangi A, Alizadeh H, Wong L, Buy X, Dietemann J L, Roy C. Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology. 2007;242:293–301. doi: 10.1148/radiol.2421041404. [DOI] [PubMed] [Google Scholar]
- Moser T, Cohen-Solal J, Bréville P, Buy X, Gangi A. [Pain assessment and interventional spine radiology] J Radiol. 2008;89:1901–1906. doi: 10.1016/s0221-0363(08)74785-1. [DOI] [PubMed] [Google Scholar]
- Buy X, Tok C H, Szwarc D, Bierry G, Gangi A. Thermal protection during percutaneous thermal ablation procedures: interest of carbon dioxide dissection and temperature monitoring. Cardiovasc Intervent Radiol. 2009;32:529–534. doi: 10.1007/s00270-009-9524-8. [DOI] [PubMed] [Google Scholar]
- Dupuy D E, Hong R, Oliver B, Goldberg S N. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. AJR Am J Roentgenol. 2000;175:1263–1266. doi: 10.2214/ajr.175.5.1751263. [DOI] [PubMed] [Google Scholar]
- Adachi A, Kaminou T, Ogawa T, et al. Heat distribution in the spinal canal during radiofrequency ablation for vertebral lesions: study in swine. Radiology. 2008;247:374–380. doi: 10.1148/radiol.2472070808. [DOI] [PubMed] [Google Scholar]
- Callstrom M R, Charboneau J W, Goetz M P, et al. Image-guided ablation of painful metastatic bone tumors: a new and effective approach to a difficult problem. Skeletal Radiol. 2006;35:1–15. doi: 10.1007/s00256-005-0003-2. [DOI] [PubMed] [Google Scholar]
- Fan Q Y, Ma B A, Zhou Y, Zhang M H, Hao X B. Bone tumors of the extremities or pelvis treated by microwave-induced hyperthermia. Clin Orthop Relat Res. 2003;406:165–175. doi: 10.1097/01.blo.0000037439.23683.9c. [DOI] [PubMed] [Google Scholar]
- Gage A A, Baust J G. Cryosurgery for tumors. J Am Coll Surg. 2007;205:342–356. doi: 10.1016/j.jamcollsurg.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Theodorescu D. Cancer cryotherapy: evolution and biology. Rev Urol. 2004;6(Suppl 4):S9–S19. [PMC free article] [PubMed] [Google Scholar]
- Beland M D, Dupuy D E, Mayo-Smith W W. Percutaneous cryoablation of symptomatic extraabdominal metastatic disease: preliminary results. AJR Am J Roentgenol. 2005;184:926–930. doi: 10.2214/ajr.184.3.01840926. [DOI] [PubMed] [Google Scholar]
- Allaf M E, Varkarakis I M, Bhayani S B, Inagaki T, Kavoussi L R, Solomon S B. Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation—initial observations. Radiology. 2005;237:366–370. doi: 10.1148/radiol.2371040829. [DOI] [PubMed] [Google Scholar]
- Callstrom M R, Atwell T D, Charboneau J W, et al. Painful metastases involving bone: percutaneous image-guided cryoablation—prospective trial interim analysis. Radiology. 2006;241:572–580. doi: 10.1148/radiol.2412051247. [DOI] [PubMed] [Google Scholar]
- Ullrick S R, Hebert J J, Davis K W. Cryoablation in the musculoskeletal system. Curr Probl Diagn Radiol. 2008;37:39–48. doi: 10.1067/j.cpradiol.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Belov S V. [The technology of high-frequency cold-hot plasma ablation for small invasive electrosurgery] Med Tekh. 2004;2:25–30. [PubMed] [Google Scholar]
- Georgy B A, Wong W. Plasma-mediated radiofrequency ablation assisted percutaneous cement injection for treating advanced malignant vertebral compression fractures. AJNR Am J Neuroradiol. 2007;28:700–705. [PMC free article] [PubMed] [Google Scholar]