ABSTRACT
Image-guided musculoskeletal (MSK) biopsies are safe and effective procedures that yield diagnostic accuracies up to 97%. When performed in conjunction with a multidisciplinary team, they provide crucial information that will affect patient care and outcome. Computed tomography and ultrasound are the main modalities used to carry out MSK biopsies, and various needles and techniques are available to help the radiologist perform these procedures safely.
Keywords: Bone, CT, biopsy, ultrasound
Soft tissue masses and to a lesser degree bone lesions are not uncommon and at times require biopsy. The approach to performing these biopsies is in some ways no different from nonmusculoskeletal biopsies and requires a careful review of all relevant clinical and radiological information. Close collaboration with a multidisciplinary team that consists of a surgical oncologist, oncologist, and musculoskeletal (MSK) pathologist is vital. The purpose of this article is to illustrate the methodology and technique of MSK biopsies that will help guide the radiologist in performing these procedures.
INDICATIONS AND RATIONALE
The majority of biopsies are performed to determine whether there is an underlying primary or metastatic neoplasm. In some cases where patients are unresponsive to conventional antibiotic therapy (such as spondylodiscitis) for treatment of bone or soft tissue infections, a biopsy may be performed to isolate the organisms targeted for antimicrobial therapy. Also, biopsies are obtained in these patients prior to administration of antibiotics. The indications and contraindications for performing an osseous or soft tissue biopsy are similar and are outlined in Table 1.
Table 1.
Indications and Contraindications for MSK Biopsies
| Indications |
| Definitive diagnosis of a bone or soft tissue lesion with aggressive imaging features |
| Determination of a bone or soft tissue lesion with indeterminate imaging features |
| Confirm or exclude a metastasis in a patient with known primary malignancy |
| Isolation of microorganisms in a musculoskeletal infection |
| Exclude or confirm an underlying lesion causing a pathological fracture |
| Contraindications |
| Acute or ongoing non-MSK infection |
| Bleeding diathesis |
| Inaccessible site or uncooperative patient |
MSK, musculoskeletal.
As a general rule, obtaining tissue is indicated when the histopathology will alter patient management. Any MSK lesion that can be confidently diagnosed as “benign” based on radiological and clinical information (e.g., “Do not touch lesions”),1 should not be biopsied, and follow-up could be performed for cases where the lesion does not satisfy all the classical imaging criteria but lacks aggressive features. At our institution, most patients have already been evaluated by the surgical oncologist in an outpatient clinic or discussed at weekly sarcoma conferences attended by MSK pathologists, surgical oncologists, radiation oncologists, and oncologists. As a general rule, unless proven otherwise, all lesions should be treated as if they were sarcomas with the intent to perform curative resection.
The patient's clinical data are first reviewed, which includes any laboratory information such as coagulation profile and platelet count. Existing imaging is gathered and carefully reviewed. If the available imaging is suboptimal or another modality (computed tomography [CT], magnetic resonance imaging [MRI], or radiograph) is deemed necessary, then the biopsy is delayed until the imaging is complete and optimal. The role of imaging is not limited to diagnosing MSK lesions, but more importantly, includes staging of bone or soft tissue lesions that will help clinicians in determining the optimal therapy. The presence of distant metastases will often rule out surgical treatment, but a biopsy would still be indicated as the histology might decide which chemoradiation regimens would be suitable. Local invasion of deep fascial planes and/or encasement of a major neurovascular bundle must be assessed and conveyed to the surgeon to help determine if a limb-salvage procedure would be feasible.
TECHNIQUE FOR BONE BIOPSIES
After review of all imaging and clinical information, the next step is deciding the route and modality utilized for image-guided biopsy. Almost all bone biopsies are performed under CT guidance at our institution. CT provides excellent spatial localization of the lesion (superficial, deep, invasive, compartments involves, etc.), which helps determine the route that safely avoids vital neurovascular structures that are not involved by the neoplasm.2 The shortest distance to the lesion is not necessarily the optimal route. In general, avoiding crossing of more than one anatomic compartment is important in preserving a limb-salvage surgical plan, as the needle track is at risk of seeding tumor cells.3,4 Because the needle track is usually resected, the tissues that lie along the track will be removed.5 Minimizing compartmental tumoral contamination is therefore important, to provide some level of function for the patient postoperatively. For example, if a bone lesion has an exophytic component that involves the vastus lateralis muscle, then the biopsy route should never traverse the rectus femoris or patellar tendon (even though doing so would compromise only one anatomic compartment) as these would have to be resected during surgery, leaving the patient without any extensor function of the knee.
In general, all patients referred for bone biopsy at our institution have already been examined and had their skin marked by the surgical oncologist indicating the route desired for biopsy. If the marked area is such that biopsy would be technically difficult, then it is possible to biopsy slightly cranial or cephalad to area marked. Biopsy routes should always be reviewed with the surgical oncologist, and the radiologist should never hesitate to postpone the biopsy until all concerns are clarified.
Percutaneous biopsies have a lower risk of complications than do open biopsies. Reported percutaneous risk rates are 0 to 10% compared with up to 16% for open biopsies.6,7 Hemorrhage, nerve praxia (secondary to local anesthetic or direct trauma when the tumor is immediately adjacent to a nerve), and infection are the main complications. In addition to being minimally invasive, a percutaneous technique allows selective sampling of areas within the tumor that will provide the highest diagnostic yield (e.g., avoidance of cystic or necrotic areas). When placing the patient on the CT table, correct positioning is crucial as it will allow good access to the lesion while optimizing patient and operator comfort. Proper positioning will also enable the needles to fit through the gantry when confirming the needle tip's position. A strict sterile technique is adhered to at all times and includes sterile gown and surgical mask and cap.
Most bone biopsies are performed under conscious sedation with good local anesthetic infiltration to provide comfort during and after the procedure. General anesthesia is usually reserved for patients who cannot remain still or have exceedingly high narcotic tolerance levels. Patients are usually kept for 1 hour postprocedure to monitor for any immediate complications as well as provide additional pain control as needed.
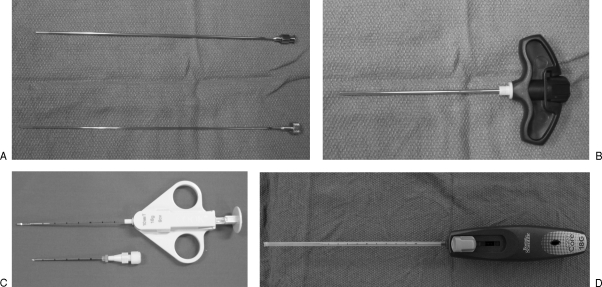
Depending on the location and nature of the osseous lesion, this will determine which needles are to be used. CT allows determination of the density of the osseous lesion as well as the amount and degree of dense osseous or chondroid matrix that will be targeted for biopsy. In general, when accessing an intraosseous lesion, a coaxial technique is preferred as multiple cores can be obtained through a single bone window with reduced radiation to the patient. A large variety of needles exist on the market, and selection will depend on personal preference and experience. In our institution, there are two biopsy needles that are mainly used. Trephine bone biopsy needles are used to sample dense osseous/chondroid lesions, and cutting needles are used to biopsy any soft tissue component of the osseous lesion (Fig. 1).
Figure 1.
Trephine bone biopsy needles. (A) A 14-gauge bone biopsy needle with inner stylet (Cook, Bloomington, IN). (B) An 11-gauge vertebroplasty needle (Cook) and cutting needles. (C) An 18-gauge QuickCore needle (Cook). (D) An 18-gauge Easycore needle (Boston Scientific, Natick, MA).
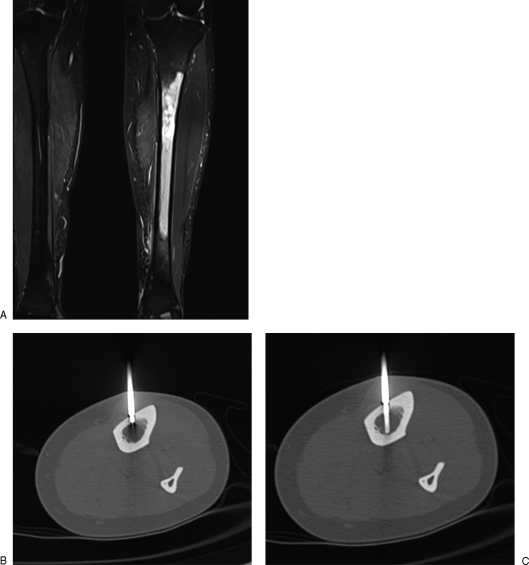
For dense osseous lesions, we use an 11-gauge vertebroplasty needle (Cook, Bloomington, IN) with a diamond tip to first cross the overlying cortex (this can be done manually or if the cortex is densely sclerotic, the needle can be attached to a pneumatic drill). Care is taken to keep the needle orthogonal to the plane of the cortex to prevent any slipping when drilling. Once the needle tip is adjacent to the lesion, the stylet is removed and a 14-gauge bone biopsy (Cook) needle is inserted coaxially to obtain a core of the lesion (Fig. 2). Soft tissue components are biopsied with spring-loaded cutting needles (Boston Scientific, Natick, MA) discussed later.
Figure 2.
(A) Coronal Short Tau Inversion Recovery (STIR) image shows abnormal left tibial diaphyseal marrow signal targeted for biopsy. (B) Axial computed tomography through tibial diaphysis shows tip of 11-gauge vertebroplasty needle (Cook, Bloomington, IN) used to advance through the cortex and placed adjacent to marrow space, after which the stylet is removed. (C) A 14-gauge bone biopsy needle inserted coaxially into marrow.
In the case of an osteolytic lesion that lacks matrix and the overlying cortex has been destroyed or is deficient, the lesion can be biopsied directly using a bone biopsy needle or a cutting needle.
Ideally, at least three cores are obtained at our institution, and samples are submitted in 10% formalin. It is important to discuss with the pathology department how the samples are to be properly submitted as this varies from one institution to another.
ULTRASOUND-GUIDED BIOPSIES
Ultrasound is a commonly used modality for performing musculoskeletal soft tissue biopsies.8,9,10 It is also used for lesions near a bone surface or bone lesion with an extraosseous component.11,12,13,14,15 Ultrasound is widely available, minimally invasive, and offers real-time and multiplanar imaging. The high spatial and contrast resolutions also allow easier visualization of lesions, particularly small lesions that are difficult to visualize on noncontrast CT. Although CT or MRI can be used, the dynamic nature of the imaging allows easier and more comfortable patient and operator positioning, and the real-time visualization of the needle provides reassurance that a safe and satisfactory biopsy has been performed and vital structures are avoided.16 Furthermore, ultrasound-guided biopsies are free from ionizing radiation. MRI-guided biopsy is an attractive method as it is outstanding in demonstrating soft tissue masses, but it is limited by issues of equipment compatibility, availability, cost, and longer procedure time. Contraindications to ultrasound-guided biopsies are similar to that of any other biopsies, such as bleeding diathesis, active infection in the region of biopsy, and nonvisualization of the lesion on ultrasound. Potential complications are the same as for any other musculoskeletal biopsies, such as bleeding, infection, and neuropraxia.
Ultrasound-guided musculoskeletal soft tissue biopsies have been reported to have high rates of accuracy. Recent studies demonstrate sensitivity of 95 to 96% and specificity of 100% in distinguishing benign and malignant lesions. Accurate identification of the specific cell type of malignancy has been reported in 83 to 98% of cases.14,17,18
Setup and Preparation
Most musculoskeletal soft tissue biopsies are best performed using a high-frequency linear array probe (7 to 12 MHz). In exceptional cases, such as a deep lesion in an obese patient, a lower-frequency probe may be used but at the expense of spatial resolution.
Cross-sectional imaging (whether CT or MRI) must be reviewed prior to the biopsy. The lesion and its relation to vital structures, such as the neurovascular bundle, underlying bone, and other muscular compartments, must be analyzed. This anatomic relationship should then be correlated with ultrasound prior to the biopsy. Color Doppler is routinely used to identify any neighboring vascular structures and any vascularity within the lesion. Solid and vascular regions of the lesion should be targeted in the biopsy. The preferred biopsy approach is then based upon all this information and the surgical implications of the approach.19,20,21,22 In our institution, almost all patients for soft tissue biopsies are seen by the oncological surgeon prior to the biopsy, and the surgeon marks on the patient where the surgical incision will be at the time of definitive surgery. Biopsy is then performed in this region. This method allows the biopsy route to be excised at the time of definitive surgery to remove any tumor that has seeded along the biopsy track.3,4,5
The patient should be in a comfortable position, preferably lying down, with the operator directly in front of the patient and the ultrasound monitor. Pillows are used to help position the patient and to relieve pressure areas. The procedure is done under local anesthetic, and sedation is rarely necessary. The patient's biopsy site is then prepared in a sterile manner. The ultrasound probe is covered with a sterile bag containing small amount of sterile gel as a coupling agent. Sterile gel is then placed on the patient's site of biopsy.
The type of needle used for the biopsy depends on the type of lesion and preference of the operator.23,24,25 Core biopsies are recommended, as mesenchymal lesions are often difficult for pathologists to diagnose, and core-needle biopsies are consistently associated with good results and allow assessment of histological pattern and histochemical analysis.5,26,27 In our institution, an 18-guage needle core biopsy cutting needle is used. The length of the needle and the length of the throw depend on the lesion and its location. Typically, a 20-mm throw is sufficient.
Technique
Once the approach has been decided, the biopsy path from the skin to the lesion should be as short as possible. Local anesthetic is infiltrated to the skin and along the path under ultrasound guidance. The biopsy needle is inserted along the same longitudinal plane as the ultrasound transducer to aid visualization of the needle. A 5-mm longitudinal skin incision in the craniocaudal direction is made, which facilitates the entry of the core biopsy needle as well as acts a marker for the surgeon of the location of the biopsy.
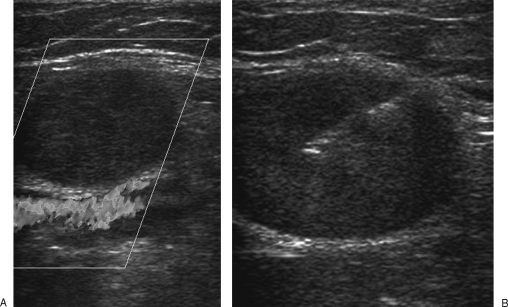
The biopsy needle is then inserted along the same path as the previously inserted local anesthetic needle. A minimum of three core samples is required, which are usually sent to pathology in 10% formalin. With small lesions, a spring-loaded needle with a 10-mm throw is preferred; the inner stylet is advanced first before the outer spring-loaded cutting sheath is deployed (such as the Cook Quick-core). This allows the operator more control over the biopsy. This type of needle is also useful in lesions where vital adjacent structures must not be traversed (Fig. 3).
Figure 3.
(A) Ultrasound demonstrates a small solid mass abutting the popliteal vessels. (B) A cutting core biopsy needle (the inner stylet is advanced first before the outer cutting sheath is deployed) is used. This reduces the risk of the needle traversing the popliteal vessels and the potential risk of tumor seeding.
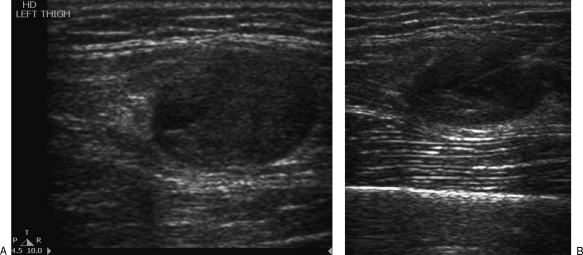
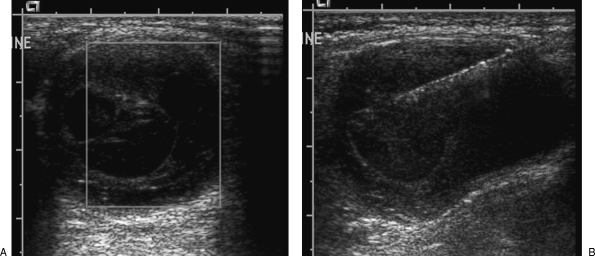
With cystic lesions, any focal nodular component or parts that show vascularity on Doppler should be targeted (Fig. 4 and Fig. 5). These regions will provide the highest yield in providing the histological diagnosis. Otherwise, the wall should be sampled. The cystic area should be avoided.11 The cystic nature of the lesion should also be documented as it is a valuable finding that may aid in the diagnosis.
Figure 4.
(A) Ultrasound demonstrates a partially cystic soft tissue tumor in the thigh. (B) The core biopsy needle is placed within the solid component of the lesion.
Figure 5.
(A) Ultrasound demonstrates a cystic mass in the paraspinal region with several thin internal septations, which demonstrate vascularity on color Doppler. (B) Core biopsy needle targets these septations.
Small mobile lesions are easier to biopsy on ultrasound than on CT. The tip of the biopsy needle is embedded slightly into the lesion to stabilize it before the needle is deployed.
After the biopsy has been performed, ultrasound of the area should be performed to detect any complications such as a hematoma. The skin incision is closed with surgical tape such as Steri-strips (Steri-Strip, 3M Health Care, St. Paul, MN). The uncomplicated patient is observed for 15 minutes before discharge. The patient is advised to be aware of potential complications such as bleeding, infection, and neuropraxia.
Limitations of Ultrasound
Ultrasound-guided biopsies are often operator-dependent, and one of the challenges is the visualization of the needle tip. Having the needle in the same longitudinal direction as the transducer is very helpful. The needle is best seen when it is perpendicular to the ultrasound beam. Rocking movement (or heel-toe angling) of the transducer will help in bringing the beam more perpendicular to the needle. Having a small amount of air in the needle makes a small needle more echogenic. The needle tip position can also be confirmed by injecting a small amount of local anesthetic or normal saline. Some manufacturers offer core biopsy needle with echogenic tips. Under no circumstances should the biopsy needle be deployed without confirmation of the needle tip.
Dense calcifications may obscure the lesion. If a satisfactory biopsy window cannot be found, then CT-guided biopsy would be more appropriate.
Some lesions are difficult to visualize on ultrasound as they have similar echogenicity to the neighboring structures such as muscle. These lesions are often much better depicted on MRI, and CT-guided biopsy would be more appropriate as correlation of lesion's location is much easier.
OUTCOMES AND COST
We routinely perform core biopsies of solid tumors, as many authors have shown a high diagnostic accuracy of core samples compared with fine-needle aspirates.28,29,30,31,32 However, in lesions that are purely cystic and/or in suspected infection, aspiration of contents is additionally performed and sent for cytological analysis to increase diagnostic yield. Percutaneous biopsies have a high diagnostic accuracy, ranging from 68 to 97%,29,33,34,35,36,37 and because the minimally invasive technique allows better recovery times and lower patient morbidity, this lowers cost compared with open biopsies.38,39,40,41 The underlying nature of the lesion also affects the diagnostic accuracy. Altuntas et al have shown that the biopsy accuracy was 81.4% for histologically proven malignant tumors and 78% for benign lesions.30
CONCLUSION
Image-guided MSK biopsies are safe, minimally invasive, and effective procedures with lower risks compared with open biopsies and offer high diagnostic results. They play a pivotal role in patient workup and management and should always be approached by a multidisciplinary team.
REFERENCES
- Helms C A. “Don't touch” lesions. Philadelphia: WB Saunders; 1995. In: Fundamentals of Skeletal Radiology. 2nd ed. pp. 56–77.
- Peh W CG. CT-guided percutaneous biopsy of spinal lesions. Biomed Imaging Interv J. 2006;2:e25. doi: 10.2349/biij.2.3.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N M, Livesley P J, Cannon S R. Recurrence of an osteosarcoma in a needle biopsy track. J Bone Joint Surg Br. 1993;75:977–978. doi: 10.1302/0301-620X.75B6.8245097. [DOI] [PubMed] [Google Scholar]
- Schwartz H S, Spengler D M. Needle tract recurrences after closed biopsy for sarcoma: three cases and review of the literature. Ann Surg Oncol. 1997;4:228–236. doi: 10.1007/BF02306615. [DOI] [PubMed] [Google Scholar]
- Wafa H, Grimer R J. Surgical options and outcomes in bone sarcoma. Expert Rev Anticancer Ther. 2006;6:239–248. doi: 10.1586/14737140.6.2.239. [DOI] [PubMed] [Google Scholar]
- Welker J A, Henshaw R M, Jelinek J, Shmookler B M, Malawer M M. The percutaneous needle biopsy is safe and recommended in the diagnosis of musculoskeletal masses. Cancer. 2000;89:2677–2686. doi: 10.1002/1097-0142(20001215)89:12<2677::aid-cncr22>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Mankin H J, Mankin C J, Simon M A, Members of the Musculoskeletal Tumor Society The hazards of the biopsy, revisited. J Bone Joint Surg Am. 1996;78:656–663. doi: 10.2106/00004623-199605000-00004. [DOI] [PubMed] [Google Scholar]
- Cardinal E, Beauregard C G, Chhem R K. Interventional musculoskeletal ultrasound. Semin Musculoskelet Radiol. 1997;1:311–318. doi: 10.1055/s-2008-1080154. [DOI] [PubMed] [Google Scholar]
- Dodd G D, III, Esola C C, Memel D S, et al. Sonography: the undiscovered jewel of interventional radiology. Radiographics. 1996;16:1271–1288. doi: 10.1148/radiographics.16.6.8946535. [DOI] [PubMed] [Google Scholar]
- Rubens D J, Fultz P J, Gottlieb R H, Rubin S J. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16:831–842. doi: 10.7863/jum.1997.16.12.831. [DOI] [PubMed] [Google Scholar]
- Ahrar K, Himmerich J U, Herzog C E, et al. Percutaneous ultrasound-guided biopsy in the definitive diagnosis of osteosarcoma. J Vasc Interv Radiol. 2004;15:1329–1333. doi: 10.1097/01.RVI.0000141347.75125.D1. [DOI] [PubMed] [Google Scholar]
- Gupta S, Takhtani D, Gulati M, et al. Sonographically guided fine-needle aspiration biopsy of lytic lesions of the spine: technique and indications. J Clin Ultrasound. 1999;27:123–129. doi: 10.1002/(sici)1097-0096(199903/04)27:3<123::aid-jcu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Civardi G, Livraghi T, Colombo P, Fornari F, Cavanna L, Buscarini L. Lytic bone lesions suspected for metastasis: ultrasonically guided fine-needle aspiration biopsy. J Clin Ultrasound. 1994;22:307–311. doi: 10.1002/jcu.1870220504. [DOI] [PubMed] [Google Scholar]
- Konermann W, Wuisman P, Ellermann A, Gruber G. Ultrasonographically guided needle biopsy of benign and malignant soft tissue and bone tumors. J Ultrasound Med. 2000;19:465–471. doi: 10.7863/jum.2000.19.7.465. [DOI] [PubMed] [Google Scholar]
- Saifuddin A, Mitchell R, Burnett S J, Sandison A, Pringle J A. Ultrasound-guided needle biopsy of primary bone tumours. J Bone Joint Surg Br. 2000;82:50–54. doi: 10.1302/0301-620x.82b1.10141. [DOI] [PubMed] [Google Scholar]
- Kandarpa K, Aruny J E. Handbook of Interventional Radiologic Procedures. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 765.
- Torriani M, Etchebehere M, Amstalden E M. Sonographically guided core needle biopsy of bone and soft tissue tumors. J Ultrasound Med. 2002;21:275–281. doi: 10.7863/jum.2002.21.3.275. [DOI] [PubMed] [Google Scholar]
- Yeow K M, Tan C F, Chen J S, Hsueh C. Diagnostic sensitivity of ultrasound-guided needle biopsy in soft tissue masses about superficial bone lesions. J Ultrasound Med. 2000;19:849–855. doi: 10.7863/jum.2000.19.12.849. [DOI] [PubMed] [Google Scholar]
- Liu P T, Valadez S D, Chivers F S, Roberts C C, Beauchamp C P. Anatomically based guidelines for core needle biopsy of bone tumors: implications for limb-sparing surgery. Radiographics. 2007;27:189–205. discussion 206. doi: 10.1148/rg.271065092. [DOI] [PubMed] [Google Scholar]
- Anderson M W, Temple H T, Dussault R G, Kaplan P A. Compartmental anatomy: relevance to staging and biopsy of musculoskeletal tumors. AJR Am J Roentgenol. 1999;173:1663–1671. doi: 10.2214/ajr.173.6.10584817. [DOI] [PubMed] [Google Scholar]
- Toomayan G A, Robertson F, Major N M. Lower extremity compartmental anatomy: clinical relevance to radiologists. Skeletal Radiol. 2005;34:307–313. doi: 10.1007/s00256-005-0910-2. [DOI] [PubMed] [Google Scholar]
- Toomayan G A, Robertson F, Major N M, Brigman B E. Upper extremity compartmental anatomy: clinical relevance to radiologists. Skeletal Radiol. 2006;35:195–201. doi: 10.1007/s00256-005-0063-3. [DOI] [PubMed] [Google Scholar]
- Haaga J R, LiPuma J P, Bryan P J, Balsara V J, Cohen A M. Clinical comparison of small- and large-caliber cutting needles for biopsy. Radiology. 1983;146:665–667. doi: 10.1148/radiology.146.3.6828680. [DOI] [PubMed] [Google Scholar]
- Coucher J R, Dimmick S J. Coaxial cutting needle biopsies: how important is it to rotate the cutting needle between passes? Clin Radiol. 2007;62:808–811. doi: 10.1016/j.crad.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Bickels J, Jelinek J S, Shmookler B M, Neff R S, Malawer M M. Biopsy of musculoskeletal tumors. Current concepts. Clin Orthop Relat Res. 1999;368:212–219. [PubMed] [Google Scholar]
- Domanski H A, Akerman M, Carlén B, et al. Core-needle biopsy performed by the cytopathologist: a technique to complement fine-needle aspiration of soft tissue and bone lesions. Cancer. 2005;105:229–239. doi: 10.1002/cncr.21154. [DOI] [PubMed] [Google Scholar]
- Powers C N, Berardo M D, Frable W J. Fine-needle aspiration biopsy: pitfalls in the diagnosis of spindle-cell lesions. Diagn Cytopathol. 1994;10:232–240. discussion 241. doi: 10.1002/dc.2840100309. [DOI] [PubMed] [Google Scholar]
- Dupuy D E, Rosenberg A E, Punyaratabandhu T, Tan M H, Mankin H J. Accuracy of CT-guided needle biopsy of musculoskeletal neoplasms. AJR Am J Roentgenol. 1998;171:759–762. doi: 10.2214/ajr.171.3.ajronline_171_3_001. [DOI] [PubMed] [Google Scholar]
- Hau A, Kim I, Kattapuram S, et al. Accuracy of CT-guided biopsies in 359 patients with musculoskeletal lesions. Skeletal Radiol. 2002;31:349–353. doi: 10.1007/s00256-002-0474-3. [DOI] [PubMed] [Google Scholar]
- Altuntas A O, Slavin J, Smith P J, et al. Accuracy of computed tomography guided core needle biopsy of musculoskeletal tumours. ANZ J Surg. 2005;75:187–191. doi: 10.1111/j.1445-2197.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- Ayala A G, Ro J Y, Fanning C V, Flores J P, Yasko A W. Core needle biopsy and fine-needle aspiration in the diagnosis of bone and soft-tissue lesions. Hematol Oncol Clin North Am. 1995;9:633–651. [PubMed] [Google Scholar]
- Bennert K W, Abdul-Karim F W. Fine needle aspiration cytology vs. needle core biopsy of soft tissue tumors. A comparison. Acta Cytol. 1994;38:381–384. [PubMed] [Google Scholar]
- Bommer K K, Ramzy I, Mody D. Fine-needle aspiration biopsy in the diagnosis and management of bone lesions: a study of 450 cases. Cancer. 1997;81:148–156. doi: 10.1002/(sici)1097-0142(19970625)81:3<148::aid-cncr4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Kattapuram S V, Rosenthal D I. Percutaneous biopsy of skeletal lesions. AJR Am J Roentgenol. 1991;157:935–942. doi: 10.2214/ajr.157.5.1927811. [DOI] [PubMed] [Google Scholar]
- Khuu H, Moore D, Young S, Jaffe K A, Siegal G P. Examination of tumor and tumor-like conditions of bone. Ann Diagn Pathol. 1999;3:364–369. doi: 10.1016/s1092-9134(99)80015-x. [DOI] [PubMed] [Google Scholar]
- Leffler S G, Chew F S. CT-guided percutaneous biopsy of sclerotic bone lesions: diagnostic yield and accuracy. AJR Am J Roentgenol. 1999;172:1389–1392. doi: 10.2214/ajr.172.5.10227522. [DOI] [PubMed] [Google Scholar]
- Logan P M, Connell D G, O'Connell J X, Munk P L, Janzen D L. Image-guided percutaneous biopsy of musculoskeletal tumors: an algorithm for selection of specific biopsy techniques. AJR Am J Roentgenol. 1996;166:137–141. doi: 10.2214/ajr.166.1.8571862. [DOI] [PubMed] [Google Scholar]
- Ward W G, Sr, Kilpatrick S. Fine-needle aspiration biopsy of primary bone tumors. Clin Orthop Relat Res. 2000;(373):80–87. doi: 10.1097/00003086-200004000-00011. [DOI] [PubMed] [Google Scholar]
- Ruhs S A, el-Khoury G Y, Chrischilles E A. A cost minimization approach to the diagnosis of skeletal neoplasms. Skeletal Radiol. 1996;25:449–454. doi: 10.1007/s002560050113. [DOI] [PubMed] [Google Scholar]
- Ashford R U, McCarthy S W, Scolyer R A, Bonar S F, Karim R Z, Stalley P D. Surgical biopsy with intra-operative frozen section. An accurate and cost-effective method for diagnosis of musculoskeletal sarcomas. J Bone Joint Surg Br. 2006;88:1207–1211. doi: 10.1302/0301-620X.88B9.17680. [DOI] [PubMed] [Google Scholar]
- Fraser-Hill M A, Renfrew D L, Hilsenrath P E. Percutaneous needle biopsy of musculoskeletal lesions. 2. Cost-effectiveness. AJR Am J Roentgenol. 1992;158:813–818. doi: 10.2214/ajr.158.4.1546598. [DOI] [PubMed] [Google Scholar]