ABSTRACT
Bone tumors may present as incidental findings, with pain or loss of function, or as fractures. There is a broad range of indications for transarterial embolization (TAE) in primary or metastatic bone tumors: to reduce operative hemorrhagic risks, to simplify or allow more definitive surgery, or in the context of pain palliation, fever, bleeding, or hypercalcemic and other rheological factors. Embolization may also increase tumor sensitivity to chemotherapy or radiation therapy. The procedure itself is often complex with significant risk to adjacent structures and is usually part of a wider treatment strategy. There are many options of embolic agent, techniques, and end points but all aim to devascularize the tumor. Catheter angiography at the time of TAE is used to determine the correct embolic agent and technique with care taken to isolate at risk structures. Many factors determine the best choice of embolic material, probably the most important of which is operator experience. In life-threatening situations or in preoperative embolizations of metastatic tumors, many operators opt for a combination of particulate emboli and stainless steel or platinum coils. Agents discussed include polyvinyl alcohol particles, trisacryl microspheres, gelatin sponge, liquid embolic agents, and embolization coils. Tumor types treated include vascular metastatic lesions, commonly renal cell or thyroid, particularly in locations prone to fracture; giant cell tumors; aneurysmal bone cysts; vertebral hemangiomas, osteosarcomas; arteriovenous malformations; and osteoblastomas. TAE should be considered in the treatment algorithm of primary or secondary bone tumors. Specific benefit is present where there is a high risk of bleeding at surgery, where there is spinal involvement and neural encroachment, where active bleeding is present or in awkward surgical locations where prolonged surgery is anticipated.
Keywords: Embolization, bone tumors, metastases
Embolization of tumors has been widely practiced in medicine and particularly interventional radiology for many years. Initially, this involved the treatment of hemorrhage and trauma with rapid implementation in the treatment of organ-based tumors.1 Refinements in technique led to its use in preoperative embolization of metastatic tumors to bone using gelfoam.2 Since those early days, indications have widened to include both benign and malignant bone tumors in palliative and potentially curative situations. There are now many options of embolic agent, techniques, and end points, but all aim to devascularize the tumor either as a primary treatment or as an adjunct to surgery. The addition of chemotherapy and sclerosants has also gained favor in some areas. There is also a role for direct intralesional embolization in some types of tumor where the transarterial embolization (TAE) route is not feasible. For example, cementoplasty has produced some promising results used either alone or in conjunction with radiofrequency ablation in the treatment of symptomatic extraspinal metastatic lesions.3,4,5
Musculoskeletal tumors include primary and metastatic tumors of benign or malignant etiology occurring within the skeleton, joint structures, and muscles. For the purposes of this article, we will be confined to those occurring in the skeleton. A wide spectrum of tumors can be encountered and many are rare entities. As a consequence, the literature-based evidence is at best level II; the bulk of evidence is at level III, with many case reports and case series from individual centers. There are no randomized controlled trials.
PRESENTATION
The presence of tumor within bone presents in several ways; where destruction of normal adjacent bone, infiltration of the periosteum, and expansion occur, pain is a common presenting feature. When adjacent structures such as joints, neural structures, and soft tissues are infiltrated or compressed, loss of function and pain may occur, and finally, where the structural integrity of the bone is threatened, microtrabecular fractures or complete fractures may occur; both are commonly accompanied by pain and disability. The presence of a bone tumor may also be noted as an incidental finding or in the course of investigations to establish the extent of disease.
PROCEDURAL GOALS
The management of patients with bone tumors is often complex; multiple specialties including interventional and diagnostic radiology, orthopedic surgery, neurosurgery and general surgery, oncology, and radiotherapy may be involved. The embolization procedure itself is often complex with significant risk to adjacent structures and is usually part of a wider treatment strategy. It is therefore vital to clearly establish the goals of treatment, potential side effects, and complications, as well as the treatment calendar, prior to embarking on a treatment regimen.
TECHNIQUE
In the case of TAE, the primary aim is to devascularize the tumor with subsequent tissue necrosis and tumor lysis, avoiding nontarget embolization. The procedure may be used in the setting of a palliative procedure to alleviate symptoms, as a preoperative measure to reduce operative blood loss, to allow more definitive surgery, or in some cases as a sole or adjunctive curative therapy. Outcomes will vary from complete tumor necrosis to degrees of ischemia and hypovascularity. Preprocedural planning with magnetic resonance imaging, computed tomography (CT), and ultrasound is essential, particularly in complex lesions to identify arterial blood supply, venous drainage, extent into adjacent tissues, and proximity of vital structures potentially sharing arterial supply. CT angiography is particularly useful in large and complex lesions with multiple arterial feeders where multiple treatments are planned.
Prerequisite tests to safe angiography include prothrombin time (international normalized ratio) or partial thromboplastin time in patients on heparin, platelet count, and hemoglobin. Abnormal coagulation should be corrected where possible as many of the particulate embolic agents—embolization coils and injectable thrombogenic agents—require a functioning intrinsic clotting cascade. This is particularly relevant in a patient with active hemorrhage or after multiple transfusions where the coagulation profile should be corrected prior to treatment. In some cases, the tumor may be associated with derangement of coagulation as in the case of Kasabach-Merritt phenomenon.6
Preprocedural planning catheter angiography performed at the time of TAE will confirm imaging findings and ultimately determine the safety of embolization. For example, prior to embolization of spinal lesions, identification of arterial supply to the spinal cord will determine whether TAE is feasible with acceptable safety parameters. Once the arterial supply to the tumor is identified, the vessels can be cannulated and embolization performed. The choice of catheter will depend upon the situation and size of feeding vessel. Our practice is to use a 4- or 5-French diagnostic catheter for the initial angiogram and either continue to use this catheter for embolization where the feeding vessel is large or add a standard 2.3- to 2.5-French microcatheter as a coaxial technique. In some situations, a larger-caliber guide catheter has advantages, for example, where frequent road mapping is required, where high flow saline flush is desired in the parent vessel as may be the case in spinal embolizations, or where a larger-diameter high-flow microcatheter is required. Microcatheters are generally recommended and offer several advantages: the embolic agent can be delivered further from the parent vessel and thus potentially reduce the chance of nontarget embolization, the feeding vessels to these tumors are often hypertrophied unnamed vessels, and cannulation with the larger diagnostic catheters may be more difficult. Arterial spasm and parent vessel occlusion with larger-caliber catheters may also lead to vessel injury and to false end points for embolization and reduce efficacy of these procedures. A case of renal cell carcinoma metastases embolized prior to intramedullary nail placement is shown in Fig. 1.
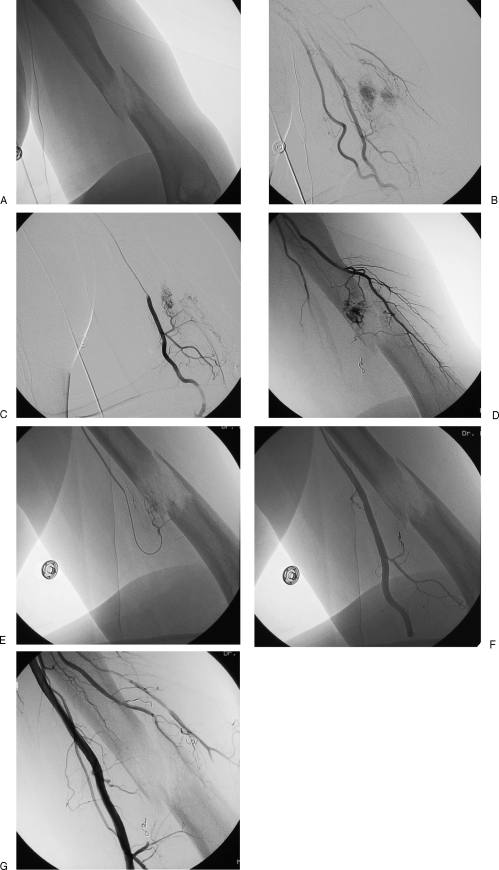
Figure 1.
(A) Pathological fracture through a renal cell metastases in the left humoral diaphysis. (B) Brachial angiogram demonstrating multiple arterial feeders from brachial and profunda brachii. (C,D) Selective catheterization and angiographic imaging of multiple arterial feeding vessels. (E) Distal catheterization of feeding vessel with particulate embolization. (F) Angiogram following embolization with single embolization coil in proximal feeding vessel. (G) Completion angiogram demonstrating effective devascularization; patient proceeded to intramedullary nail placement without incident and minimal blood loss.
CHOICE OF EMBOLIC MATERIAL
There are many factors that determine the best choice of embolic material, probably the most important of which is operator experience; liquid embolics are notoriously tricky and an experienced operator is a prerequisite. In life-threatening situations or in preoperative embolizations of metastatic tumors, many operators may opt for a combination of particulate emboli and stainless steel or platinum coils. There is very little published data on comparative results from embolic material; in one study looking at operative blood loss after embolization, no clinically significant difference was observed between trisacryl gelatin microspheres and polyvinyl alcohol (PVA) particles.7 It has been suggested that Contour SE® Microspheres (Boston Scientific, Natick, MA, USA) are less effective in the setting of uterine leiomyomata with consequential early recanalization,8 but in the acute setting, there is no evidence of decreased effectiveness and there are some procedural benefits (see following section on PVA). Liquid embolics; glue (N-butyl cyanoacrylate [NBCA]), absolute alcohol, Ethibloc® [(Ethicon, Norderstedt, Germany], sodium tetradecyl sulfate, Onyx® [(Microtherapeutics, Irvine, CA]) may achieve more tumor necrosis than particulates and be advantageous where definitive treatment is desired but carry an increased risk of nontarget embolization and nontarget necrosis compared with particulate emboli.9 Encouraging results, however, have been seen using NBCA in the treatment of aneurysmal bone cysts,10 and this technique may emerge as a viable alternative to surgery in this context.
PVA Particles
PVA is ground from blocks of foam and then separated into different size groupings. Available sizes range from 50 to 1000 μm; the commonest size used is in the 300- to 500-μm range. PVA has several desirable characteristics. It is a particulate material capable of penetrating the tumor blood supply and occluding it, and it is relatively inexpensive and easy to deliver. Most interventional radiologists have extensive experience in its use, and both animal studies and the published experience in patients suggest that it is safe without any known long-term side effects. The traditional preparation (Contour®) characteristically has a very irregular outline and as a result occludes vessels larger than particulate diameters due to particle aggregation.11 Some of the newer preparations (Cook Inc., Bloomington, IN) have a smoother outline and are less prone to catheter occlusion. Contour SE® Microspheres and Bead block® (Biocompatables, Surrey, UK) are engineered PVA particles with a more uniform particle size and due to their microporous nature are compressible with some delivery benefits through small catheters.
Embosphere® Microspheres
Embosphere® Microspheres (Biosphere Medical Inc., Rockland, MA, USA) are clear acrylic copolymer (trisacryl) microspheres that were previously used as a microcarrier for cell culture, which helped confirm their biocompatibility. Similar to PVA, they are available in sizes from 40 to 1200 μm. There are some attractive characteristics of this material, which is widely used in the treatment of uterine fibroids. The spheres are compressible and allow easy passage through a microcatheter with a luminal diameter smaller than that of the spheres; the spheres are more uniform in size than PVA, and particle sizes does not change in liquids. They have little tendency to clump after injection, and animal studies indicate that they have less tissue reaction than is typically seen with PVA.
Gelfoam (Gelatin Sponge)
Gelfoam or gelatin sponge is a dissolvable spongelike material that has been used for many years in surgery. It comes in small flat rectangular blocks that can be either cut with scissors into elongated rectangles or rolled into pledgets and injected via diagnostic catheters or microcatheters. Alternatively, the material can be cut into small cubes and mixed vigorously in a syringe (two syringes connected via a stopcock works well) to form a slurry. Gelfoam is considered a temporary occluding agent12 with the occluded vessel recannalizing in 2 to 4 weeks, although the evidence for this exact time frame is limited.
Once stasis or near stasis has been achieved with a particulate agent, many operators employ coil embolization for final and complete vessel occlusion. As many branches as can be safely embolized will be treated, avoiding occluding vessels supplying vital structures. Complications relating to the use of particulate emboli are generally secondary to nontarget embolization. Particular care should be taken to ensure correct suspension with the resultant suspension injected using arterial flow to carry the material to the target. Road map techniques allow identification of backflow, and in general embolization is continued until complete stasis is achieved. Particular attention should be taken in the vicinity of the spinal cord to avoid the spinal arteries, in the femoral region to avoid embolizing the vasa nervorum of the sciatic nerve and the lateral cutaneous nerve of the thigh, and in the humoral area to avoid the circumflex femoral nerve. In general, thought should be given to the location and vascular supply of at-risk vital structures.
Liquid Agents
Liquid embolic agents include NBCA, absolute alcohol, Ethibloc®, sodium tetradecyl sulfate, and Onyx®. Published results involving the use of these agents in embolization of bone tumors are limited; in a non–end organ such as bone with multiple arterial feeders, liquid agents may increase the potential for nontarget embolization. However, ethylic, cyanoacrylates, and Onyx® have all been used successfully for tumor embolization and in a potential cure situation may have a role to play. In general, where the goal is to devascularize the tumor as a palliative or presurgical procedure, there is little advantage over particulate emboli.
Embolization Coils
Stainless steel fibered coils and platinum coils have been used for many years for vascular occlusion. Coils can be delivered through standard catheters or microcatheters, and the adoption of neurointerventional techniques has led to the development of a variety of detachable coils specifically designed for use outside the central nervous system. Coils are usually reserved for larger-vessel occlusion but do have a role in tumor embolization after particulate emboli have been used and in the emergency situation where either the expertise or time is lacking for other forms of embolization.
CONTRAINDICATIONS
The usual contraindications to intravascular procedures apply with attention to the presence of coagulopathy, thrombocytopenia, or anemia.
PREOPERATIVE EMBOLIZATION
Tumor within bone may present as pain, an incidental finding, loss of function, or a pathological fracture. In many cases, surgery to debulk the tumor, completely resect the tumor, or augment the affected bone will be planned; in the case of pathological fracture, the fractures will not heal, and where tumor deposits are present, continued growth will often lead to fractures if left untreated. Embolization of a femoral metastatic lesion with prophylactic TAE is shown in Fig. 2; the lesion progressed to fracture.
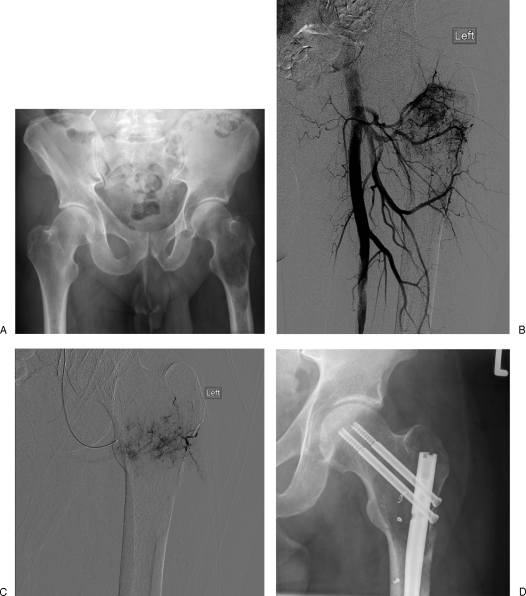
Figure 2.
(A) Renal cell metastases in left femoral head, preoperative appearances. (B) Appearances at angiography with a hypervascular lesion in the greater trochanter. (C) Embolization with 2.3-French microcatheter in a circumflex femoral feeding vessel; the lesion is partly embolized. (D) Post–gamma nail placement, multiple embolization coils can be seen; these were placed following particulate embolization with polyvinyl alcohol to completely occlude the vessels.
Many metastatic lesions, notably renal cell carcinoma and primary bone tumors, are highly vascular with excessive bleeding reported at surgery. One of the earliest reports of selective TAE was in 1975 when the technique was employed to reduce perioperative blood loss.13 There is now a broad range of indications for TAE of primary or metastatic bone tumors: reduction of operative hemorrhagic risks, simplification of surgery, pain palliation, fever, bleeding, and hypercalcemic and other rheological indications. Embolization may also increase tumor sensitivity to chemotherapy or radiation therapy.
Major blood loss can be avoided during surgery,14,15,16 and the resultant smaller tumor volume and reduced perioperative bleeding enable safer, easier, and more complete resection.16 In addition, preoperative TAE reduces the size of inoperable metastases prior to other therapies, such as radioiodine therapy, and is effective as a purely palliative measure for pain. A retrospective review published in 2007 of 21 patients who underwent embolization of painful renal cell carcinoma bone metastases demonstrated effective palliation in a group where therapeutic options are limited. Thirty procedures were performed to treat 39 lesions (18 pelvic, eight lower extremity, three upper extremity, five rib/chest wall and five vertebral). A clinical response was achieved at 36/39 sites with a mean duration of treatment response 5.5 months.17 When TAE is to be followed by surgery, it has been recommended that the surgery is performed within 3 days to avoid revascularization; in one study, blood loss was increased in patients operated on 3 or more days following the embolization procedure.18 In our own experience, tumors may rapidly revascularize following particulate embolization, recruiting hitherto unidentified vessels with rapid hypertrophy. Fig. 3 demonstrates a case where embolization to stasis was followed by rapid revascularization at 6 weeks.
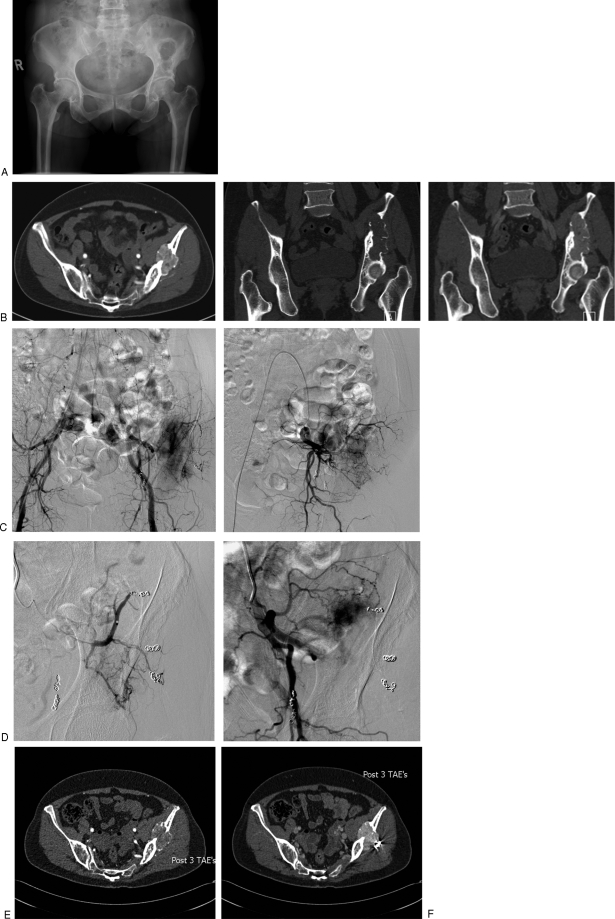
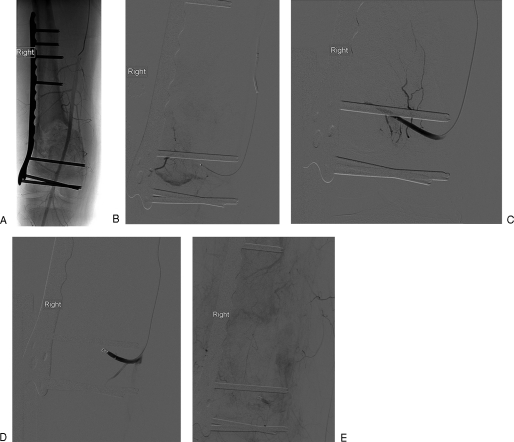
Figure 3.
Transarterial embolization (TAE) in a functional phosphaturic mesenchymal tumor causing oncogenic osteomalacia. The patient presented with pelvic pain with a 10-year history of a lytic lesion in the pelvis (A) thought originally to represent a brown tumor. Three separate embolization procedures were performed. Temporary improvement in fibroblast growth factor 23 occurred, but the tumor rapidly revascularized. (B) Coronal computed tomography (CT) pre- and postcontrast and axial contrast-enhanced CT demonstrating the hypervascular lesion in the left ileum extending to the acetabular surface. (C) Second embolization visit: flush aortogram demonstrates multiple coils in the superior gluteal artery with persistent arterial blush in the ileum; selective internal iliac angiogram demonstrates remaining arterial feeders. (D) Superior gluteal artery embolization: superior gluteal artery is occluded, with residual tumor blush from unnamed ileal artery. (E,F) CT 6 weeks after third TAE. Early arterial image demonstrated reduced vascularity but this fills in in late arterial phase (F).
METASTASES
Renal cell and thyroid carcinoma metastases are hypervascular, and TAE is frequently requested to alleviate symptoms. Treatment is aimed at improving quality of life, and there is evidence that patients experience a rapid reduction in pain levels, thought related to decompression of the periosteum, and a reduction in neurological symptoms following TAE.19 In renal cell carcinoma, spinal neurological symptoms have been treated in this manner.20,21,22 Bleeding at sites of pathological fractures or impending fractures is common and may be severe and may complicate surgery; in 16 cases of renal cell carcinoma, expected bleeding was significantly reduced when tumor enhancement was reduced by >70%.21 Embolization has also been utilized in the palliative treatment of metastatic lesions in patients with hepatocellular carcinoma. In one case, a biopsy precipitated massive hemorrhage, which was successfully treated with TAE.23 In one series of 33 patients with hepatocellular carcinoma, 39 metastatic bone lesions were treated.24 Each lesion had TAE alone (n = 11), TAE in conjunction with radiotherapy (n = 17), or radiotherapy alone (n = 11). Based on the results, TAE was found to be effective in relieving pain immediately and improving quality of life, but a combination of TAE and radiotherapy was recommended for permanent pain relief. Another series of seven patients with hepatocellular carcinoma underwent TAE for osseous metastases; of the five patients who had successful embolization, all became symptom free within 1 week.25
METASTATIC SPINAL TUMORS
Hypervascular spinal and pelvic metastases represent a difficult surgical group. In a series of 32 patients treated with TAE prior to anterior resection of spinal metastases (n = 21) or pelvic metastases (n = 11), there was a significant difference in blood loss and transfusion requirements in both groups compared with controls. Operating time was shorter, and there were no neurological deficits attributable to the embolization procedure.26 Another study of 24 patients undergoing preoperative embolization concluded that radical tumor resection was facilitated and that the procedure could be performed without permanent neurological, skin, or muscle deficit. PVA was the embolic material used, and in two cases partial embolization was performed due to the proximity of the artery of Adamkiewicz.27 Direct injection of these hypervascular spinal metastases preoperatively is an alternative route; in one series, direct intralesional injection of NBCA mixed with Lipiodol was used in conjunction with TAE, and there were no neurological deficits and subsequent surgery was again thought to have been facilitated.28 Vertebroplasty and kyphoplasty will be discussed elsewhere in this issue, but both have a role in the treatment of symptomatic spinal metastases. Fig. 4 demonstrates a case of preoperative spinal embolization.
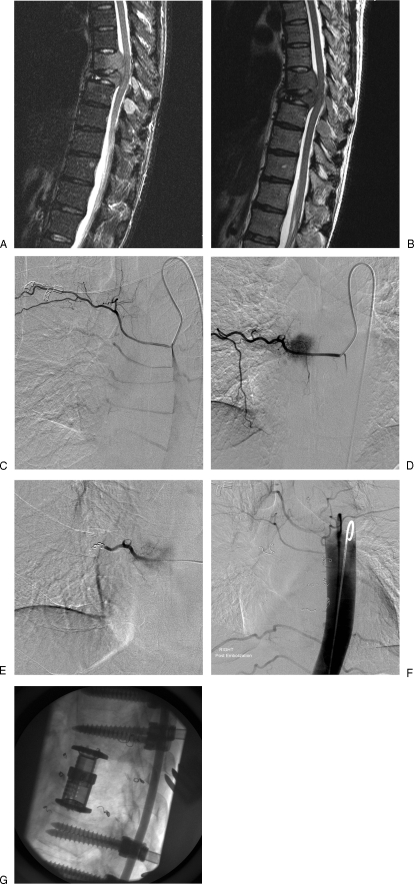
Figure 4.
(A, B) Sagittal T2-weighted magnetic resonance images pre- and postcontrast demonstrating tumor within two midthoracic vertebral bodies; impingement on the spinal cord is demonstrated with loss of integrity of the posterior wall of the two vertebrae. Postcontrast images demonstrate involvement of the posterior processes. (C) Simmonds two-catheter injection of right sixth intercostal artery demonstrates the intercostal vessels. Note crowding of T7 and T8 vessels in keeping with vertebral body collapse at these levels. (D) Right T7 intercostal angiogram; tumor blush seen in right side of the vertebral body. (E) Same level as Fig. 4D following coil deposition to protect the distal intercostal circulation. Particulate embolization followed with 150- to 250-μm particles; finally the vessel origin was coil embolized. (F) Flush aortogram following bilateral T7, 8, and 9 intercostal artery embolization with a combination of coils and polyvinyl alcohol particles. (G) Postoperative appearances with T7 and T8 corpectomies.
PRIMARY BONE TUMORS
Giant Cell Tumors
Giant cell tumors (GCTs) are benign but locally aggressive tumors that usually respond well to surgical resection, but when they occur in the spine and sacrum, resection is associated with excessive blood loss, significant complications of surgery and radiotherapy, as well as a local recurrence rates as high as 33%.29,30,31 Preoperative embolization has proven to be an effective therapy in these situations, reducing blood loss using either particulate embolization or NBCA.32 Several authors have gone further and suggested that serial arterial embolizations are a useful primary treatment modality for sacral GCTs. One series of nine cases treated with repeated TAE (mean 4.8 treatments per patient) demonstrated a substantial improvement in pain scores and no tumor progression in seven of nine cases, with mean follow-up of 8.96 years; minimal progression was seen in two cases over the study period. Various embolic techniques were used over the study period (1984 to 2006) but with a similar aim, to reduce tumor vascularity; embolization was continued until no further tumor supply could be seen.33
A case series published in 2002 described the results in 18 patients aged 17 to 59 years with sacral GCT managed with selective intra-arterial embolization over a 26-year period. Results indicated a durable response in 50% of patients with local recurrence rates of 31% at 10 years and 43% at 15 and 20 years.34 Fifty percent of the patients receive adjunctive cisplatin therapy. Typically, embolizations were performed at 2- to 4-month intervals until there was no longer a hypervascular mass or the patient's condition was improved; the mean number of treatments was 4.4. In this study, embolization involved the middle sacral, superior and inferior lateral sacral, lumbar segmental, internal iliac, iliolumbar, and superior and inferior rectal arteries. Embolization was continued to stasis using Gelfoam or PVA. One patient died the day after embolization (cause of death was not specified but was considered treatment related), three patients experienced neurological complications, and there were two cases of unilateral weakness of ankle dorsiflexion and one case of transient numbness involving the great toe, all associated with embolization of the lateral sacral artery. Transient pain and fever occurred in nearly all patients. No patient had compromised bowel or bladder function as a result of embolization. Of 18 patients, 14 (78%) demonstrated an objective, favorable radiographic response characterized by a decrease in vascularity and increase in ossification on CT and plain films. Other studies support this favorable response to embolization.35,36,37
GCT may also occur in the remaining areas of the spine, and again TAE has been shown to be useful in these circumstances, particularly in newly diagnosed cases.38 In another case, a GCT of the atlas was treated with combined surgical and preoperative embolization of the right vertebral artery.39 Where vertebral artery sacrifice is to be considered a test, balloon occlusion may be required. Spinal embolization, however, has significant risks; in one case, although the patient was disease free following TAE, he had permanent lower-limb paralysis.40
These results are encouraging, and embolization should be considered in the treatment algorithm of patient with sacral or spinal GCT where surgical treatment and radiation are associated with high morbidity and high recurrence rates. TAE may be used alone or in conjunction with other therapies.
Aneurysmal Bone Cysts
Curettage and resection have been considered the treatments of choice for aneurysmal bone cysts (ABCs); however, there is a high risk of local recurrence, and other treatments such as radiation, cryotherapy, and embolization have been used for inaccessible or recurrent lesions.41 TAE has also been used in combination with surgery to reduce operative blood loss,42,43 and excessive blood loss during surgery without prior embolization has been reported.44 Fig. 5 demonstrates a case where continued bleeding post–surgical curettage was successfully treated with TAE.
Figure 5.
(A) Angiogram in a case of giant cell tumor, with curettage and packing of the resultant cavity with postoperative bleeding. The buttress plate had been placed previously to avoid fracture. (B) Selective catheterization of a superior geniculate arterial feeder to the aneurysmal bone cyst demonstrates hemorrhage into the cavity. (C) Following polyvinyl alcohol embolization, the distal vessel is occluded. (D) The vessel is finally occluded with a coil. (E) Femoral arteriogram demonstrates cessation of hemorrhage.
In one case,45 a sacral ABC confirmed with cytology was embolized with PVA 500 to 700 μm in diameter following catheterization of the main feeding artery arising from the left inferior epigastric. The embolization procedure was followed by curettage and bone grafting with no evidence of recurrence at 6-month follow-up. Another article describes embolization of ABCs in the thoracic spine and long bones.42 Peripheral ABCs may also be treated with embolization; in three cases, embolization was followed by surgery, and in one case of a distal femur lesion, this was considered cured by embolization at 2 years.46
The most encouraging results have involved the use of NBCA: in one case report, the treated thoracic ABC was stable at 3 years following transcatheter embolization.47 A recent publication of ABCs treated with transcatheter embolization using NBCA reported on 55 embolizations in 36 patients. A single treatment was utilized in 61% and two treatments in 25%; the treatment was considered effective in 94% of cases with a minimum follow-up of 2 years. Reported complications were rare: 6% of cases had two episodes of skin necrosis and one case of transient paresis. The authors suggest that TAE with NBCA may be the treatment of choice in ABCs, particularly where the surgical risks are high.10
Vertebral Hemangioma
Vertebroplasty for vertebral hemangiomas will be discussed elsewhere in this issue; however, this remains one option for treating these highly vascular lesions and is generally reserved for lesions without neurological deficit. Traditionally, where presentation has involved spinal pain or cord compression with neurological deficit, radiation or decompression surgery has been the treatment of choice. Surgery, however, is often associated with massive hemorrhage from these highly vascular lesions,48,49 and preoperative TAE has been found to be a useful adjunctive step to reduce perioperative blood loss.50 Results support the role of TAE in this context as an adjunctive to surgery.51 Although TAE has been advocated by some as a stand-alone treatment,52 long-term data are lacking.
In one case, the patient with multicentric intraosseous hemangiomas presented with intractable retromolar hemorrhage. The bleeding was effectively controlled with transoral direct lesion puncture and injection of NBCA.53 Direct lesional injection with ethanol has also been described.54
Osteosarcoma
Surgery remains the standard treatment for osteosarcomas with adjunctive systemic chemotherapy, but transarterial chemoembolization in combination with limb salvage surgery yielded encouraging results when used to treat 32 patients with osteosarcoma prior to limb salvage surgery.55 An intra-arterial triple-drug regimen was given: methotrexate (1 to 2 g), pharmorubicin (30 to 50 mg), and cisplatin (60 to 100 mg). Vascular isolation of the treated limb was performed using a tourniquet for a period of 15 minutes in each case. Following the infusion of the chemotherapeutic agent, the vessels were embolized with a variety of embolic agents: adriblastina gelatin microspheres, anhydrous alcohol, gelatin sponge particles, and common bletilla tuber. These results confirm that tumor necrosis occurs, and the addition of a chemotherapeutic agent appears to increase tumor necrosis and result in a reduced incidence of local recurrence. However, convincing survival benefits have yet to be demonstrated. TAE without chemotherapy has also been shown to be effective as an adjunct to surgery56,57,58 and may have a place in awkward surgical cases or where excessive hemorrhage is predicted.
ARTERIOVENOUS MALFORMATION OF BONE
There are several references to TAE or transvenous embolization of arteriovenous malformations involving the bone59,60 and in one case by direct lesional puncture following failure of TAE to control hemorrhage.61 TAE appears to be a valuable primary or adjunctive treatment in these rare cases.
Cervical Spine Osteoblastomas
Three cases of TAE for cervical spine osteoblastoma are reported in the literature with favorable adjunctive results with surgery, where the procedure was thought to reduce intraoperative bleeding, increase the chance of complete resection, and reduce postoperative complications with the potential to improve patient outcomes62; these results were supported by another small study.63 PVA was used as the embolic agent (150 to 250 μm) in these cases, there were no reported complications.
Other Bone Tumors
Isolated reports involving other pathologies have been reported. TAE in hemangiopericytoma has been described,64,65 as has postoperative embolization in a case of angiosarcoma.66 Fig. 5 demonstrates TAE in a rare case of a functional phosphaturic mesenchymal tumor causing oncogenic osteomalacia. The patient presented with pelvic pain with a 10-year history of a lytic lesion in the pelvis thought originally to represent a brown tumor. Three separate embolization procedures were performed. Temporary improvement in fibroblast growth factor 23 occurred, but the tumor rapidly revascularized.
CONCLUSION
TAE should be considered in the treatment algorithm of primary or secondary bone tumors. Specific benefit is present where there is a high risk of bleeding at surgery, where there is spinal involvement and neural encroachment, where active bleeding is present, or in awkward surgical locations where prolonged surgery is anticipated.
REFERENCES
- Bücheler E, Hupe W, Hertel E U, Klosterhalfen H. [Catheter embolisation of renal tumours (author's transl)] Rofo. 1976;124:134–138. doi: 10.1055/s-0029-1230298. [DOI] [PubMed] [Google Scholar]
- Carpenter P R, Ewing J W, Cook A J, Kuster A H. Angiographic assessment and control of potential operative hemorrhage with pathologic fractures secondary to metastasis. Clin Orthop Relat Res. 1977;123:6–8. [PubMed] [Google Scholar]
- Basile A, Giuliano G, Scuderi V, et al. Cementoplasty in the management of painful extraspinal bone metastases: our experience. Radiol Med (Torino) 2008;113:1018–1028. doi: 10.1007/s11547-008-0314-1. [DOI] [PubMed] [Google Scholar]
- Anselmetti G C, Manca A, Ortega C, Grignani G, Debernardi F, Regge D. Treatment of extraspinal painful bone metastases with percutaneous cementoplasty: a prospective study of 50 patients. Cardiovasc Intervent Radiol. 2008;31:1165–1173. doi: 10.1007/s00270-008-9396-3. [DOI] [PubMed] [Google Scholar]
- Kodama H, Aikata H, Uka K, et al. Efficacy of percutaneous cementoplasty for bone metastasis from hepatocellular carcinoma. Oncology. 2007;72:285–292. doi: 10.1159/000113040. [DOI] [PubMed] [Google Scholar]
- Rodriguez V, Lee A, Witman P M, Anderson P A. Kasabach-Merritt phenomenon: case series and retrospective review of the mayo clinic experience. J Pediatr Hematol Oncol. 2009;31:522–526. doi: 10.1097/MPH.0b013e3181a71830. [DOI] [PubMed] [Google Scholar]
- Basile A, Rand T, Lomoschitz F, et al. Trisacryl gelatin microspheres versus polyvinyl alcohol particles in the preoperative embolization of bone neoplasms. Cardiovasc Intervent Radiol. 2004;27:495–502. doi: 10.1007/s00270-003-0147-1. [DOI] [PubMed] [Google Scholar]
- Siskin G P, Beck A, Schuster M, Mandato K, Englander M, Herr A. Leiomyoma infarction after uterine artery embolization: a prospective randomized study comparing tris-acryl gelatin microspheres versus polyvinyl alcohol microspheres. J Vasc Interv Radiol. 2008;19:58–65. doi: 10.1016/j.jvir.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Munk P L, Legiehn G M. Musculoskeletal interventional radiology: applications to oncology. Semin Roentgenol. 2007;42:164–174. doi: 10.1053/j.ro.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rossi G, Rimondi E, Bartalena T, et al. Selective arterial embolization of 36 aneurysmal bone cysts of the skeleton with N-2-butyl cyanoacrylate. Skeletal Radiol. 2010;39:161–167. doi: 10.1007/s00256-009-0757-z. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Imai S, Kobatake M, Yamashita T, Tamada T, Umetani K. Evaluation of tris-acryl gelatin microsphere embolization with monochromatic X Rays: comparison with polyvinyl alcohol particles. J Vasc Interv Radiol. 2006;17(11 Pt 1):1797–1802. doi: 10.1097/01.RVI.0000243614.87529.b0. [DOI] [PubMed] [Google Scholar]
- Vlahos L, Benakis V, Dimakakos P, Dimopoulos C, Pontifex G. A comparative study of the degree of arterial recanalization in kidneys of dogs following transcatheter embolization with eight different materials. Eur Urol. 1980;6:180–185. doi: 10.1159/000473322. [DOI] [PubMed] [Google Scholar]
- Feldman F, Casarella W J, Dick H M, Hollander B A. Selective intra-arterial embolization of bone tumors. A useful adjunct in the management of selected lesions. Am J Roentgenol Radium Ther Nucl Med. 1975;123:130–139. doi: 10.2214/ajr.123.1.130. [DOI] [PubMed] [Google Scholar]
- Fenoy A J, Greenlee J D, Menezes A H, et al. Primary bone tumors of the spine in children. J Neurosurg. 2006;105(4 Suppl):252–260. doi: 10.3171/ped.2006.105.4.252. [DOI] [PubMed] [Google Scholar]
- Reuter M, Heller M, Heise U, Beese M. [Transcatheter embolization of tumors of the muscular and skeletal systems] Rofo. 1992;156:182–188. doi: 10.1055/s-2008-1032861. [DOI] [PubMed] [Google Scholar]
- Gellad F E, Sadato N, Numaguchi Y, Levine A M. Vascular metastatic lesions of the spine: preoperative embolization. Radiology. 1990;176:683–686. doi: 10.1148/radiology.176.3.2389026. [DOI] [PubMed] [Google Scholar]
- Forauer A R, Kent E, Cwikiel W, Esper P, Redman B. Selective palliative transcatheter embolization of bony metastases from renal cell carcinoma. Acta Oncol. 2007;46:1012–1018. doi: 10.1080/02841860701280725. [DOI] [PubMed] [Google Scholar]
- Barton P P, Waneck R E, Karnel F J, Ritschl P, Kramer J, Lechner G L. Embolization of bone metastases. J Vasc Interv Radiol. 1996;7:81–88. doi: 10.1016/s1051-0443(96)70738-8. [DOI] [PubMed] [Google Scholar]
- Tol K M Van, Hew J M, Jager P L, Vermey A, Dullaart R P, Links T P. Embolization in combination with radioiodine therapy for bone metastases from differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2000;52:653–659. doi: 10.1046/j.1365-2265.2000.00998.x. [DOI] [PubMed] [Google Scholar]
- Guzman R, Dubach-Schwizer S, Heini P, et al. Preoperative transarterial embolization of vertebral metastases. Eur Spine J. 2005;14:263–268. doi: 10.1007/s00586-004-0757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Lang E V. Bone metastases from renal cell carcinoma: preoperative embolization. J Vasc Interv Radiol. 1998;9:263–269. doi: 10.1016/s1051-0443(98)70267-2. [DOI] [PubMed] [Google Scholar]
- Chatziioannou A N, Johnson M E, Pneumaticos S G, Lawrence D D, Carrasco C H. Preoperative embolization of bone metastases from renal cell carcinoma. Eur Radiol. 2000;10:593–596. doi: 10.1007/s003300050969. [DOI] [PubMed] [Google Scholar]
- Hansch A, Neumann R, Pfeil A, et al. Embolization of an unusual metastatic site of hepatocellular carcinoma in the humerus. World J Gastroenterol. 2009;15:2280–2282. doi: 10.3748/wjg.15.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura A, Fujimoto H, Yasuda S, et al. Transcatheter arterial embolization for bone metastases from hepatocellular carcinoma. Eur Radiol. 2001;11:1457–1462. doi: 10.1007/s003300000792. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Nakano Y, Abe M, Takahashi M, Kohno S. Osseous metastases from hepatocellular carcinoma: embolization for pain control. Cardiovasc Intervent Radiol. 1989;12:149–153. doi: 10.1007/BF02577380. [DOI] [PubMed] [Google Scholar]
- Wirbel R J, Roth R, Schulte M, Kramann B, Mutschler W. Preoperative embolization in spinal and pelvic metastases. J Orthop Sci. 2005;10:253–257. doi: 10.1007/s00776-005-0900-1. [DOI] [PubMed] [Google Scholar]
- Guzman R, Dubach-Schwizer S, Heini P, et al. Preoperative transarterial embolization of vertebral metastases. Eur Spine J. 2005;14:263–268. doi: 10.1007/s00586-004-0757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer C M, Malek A M, Kwan E S, et al. Preoperative embolization of hypervascular spinal metastases using percutaneous direct injection with n-butyl cyanoacrylate: technical case report. Neurosurgery. 2006;59:E431–E432. doi: 10.1227/01.NEU.0000223503.92392.CE. [DOI] [PubMed] [Google Scholar]
- Turcotte R E, Sim F H, Unni K K. Giant cell tumor of the sacrum. Clin Orthop Relat Res. 1993;291:215–221. [PubMed] [Google Scholar]
- Simpson A H, Porter A, Davis A, Griffin A, McLeod R S, Bell R S. Cephalad sacral resection with a combined extended ilioinguinal and posterior approach. J Bone Joint Surg Am. 1995;77:405–411. doi: 10.2106/00004623-199503000-00010. [DOI] [PubMed] [Google Scholar]
- Gokaslan Z L, Romsdahl M M, Kroll S S, et al. Total sacrectomy and Galveston L-rod reconstruction for malignant neoplasms. Technical note [technical note] J Neurosurg. 1997;87:781–787. doi: 10.3171/jns.1997.87.5.0781. [DOI] [PubMed] [Google Scholar]
- Mindea S A, Eddleman C S, Hage Z A, Batjer H H, Ondra S L, Bendok B R. Endovascular embolization of a recurrent cervical giant cell neoplasm using N-butyl 2-cyanoacrylate. J Clin Neurosci. 2009;16:452–454. doi: 10.1016/j.jocn.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Hosalkar H S, Jones K J, King J J, Lackman R D. Serial arterial embolization for large sacral giant-cell tumors: mid- to long-term results. Spine (Phila Pa 1976) 2007;32:1107–1115. doi: 10.1097/01.brs.0000261558.94247.8d. [DOI] [PubMed] [Google Scholar]
- Lin P P, Guzel V B, Moura M F, et al. Long-term follow-up of patients with giant cell tumor of the sacrum treated with selective arterial embolization. Cancer. 2002;95:1317–1325. doi: 10.1002/cncr.10803. [DOI] [PubMed] [Google Scholar]
- Wallace S, Granmayah M, deSantos L A, et al. Arterial occlusion of pelvic bone tumours. Cancer. 1979;43:322–328. doi: 10.1002/1097-0142(197901)43:1<322::aid-cncr2820430147>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Chuang V P, Soo C S, Wallace S, Benjamin R S. Arterial occlusion: management of giant cell tumor and aneurysmal bone cyst. AJR Am J Roentgenol. 1981;136:1127–1130. doi: 10.2214/ajr.136.6.1127. [DOI] [PubMed] [Google Scholar]
- Eftekhari F, Wallace S, Chuang V P, et al. Intraarterial management of giant-cell tumors of the spine in children. Pediatr Radiol. 1982;12:289–293. doi: 10.1007/BF00973194. [DOI] [PubMed] [Google Scholar]
- Luther N, Bilsky M H, Härtl R. Giant cell tumor of the spine. Neurosurg Clin N Am. 2008;19:49–55. doi: 10.1016/j.nec.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Tsuchiya H, Kokubo Y, Sakurada K, Sonoda Y, Saito S, Kayama T. [A case of giant cell tumor in atlas] No Shinkei Geka. 2005;33:817–823. [PubMed] [Google Scholar]
- Finstein J L, Chin K R, Alvandi F, Lackman R D. Postembolization paralysis in a man with a thoracolumbar giant cell tumor. Clin Orthop Relat Res. 2006;453:335–340. doi: 10.1097/01.blo.0000229304.59771.a3. [DOI] [PubMed] [Google Scholar]
- Guzey F K, Emel E, Aycan A, et al. Pediatric vertebral and spinal epidural tumors: a retrospective review of twelve cases. Pediatr Neurosurg. 2008;44:14–21. doi: 10.1159/000110657. [DOI] [PubMed] [Google Scholar]
- Fraser R K, Coates C J, Cole W G. An angiostatic agent in treatment of a recurrent aneurysmal bone cyst. J Pediatr Orthop. 1993;13:668–671. [PubMed] [Google Scholar]
- Kónya A, Szendröi M. Aneurysmal bone cysts treated by superselective embolization. Skeletal Radiol. 1992;21:167–172. doi: 10.1007/BF00242130. [DOI] [PubMed] [Google Scholar]
- Han X, Dong Y, Sun K, Lu Y. A huge occipital osteoblastoma accompanied with aneurysmal bone cyst in the posterior cranial fossa. Clin Neurol Neurosurg. 2008;110:282–285. doi: 10.1016/j.clineuro.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Yildirim E, Ersözlü S, Kirbaş I, Ozgür A F, Akkaya T, Karadeli E. Treatment of pelvic aneurysmal bone cysts in two children: selective arterial embolization as an adjunct to curettage and bone grafting. Diagn Interv Radiol. 2007;13:49–52. [PubMed] [Google Scholar]
- Börüban S, Sancak T, Yildiz Y, Sağlik Y. Embolization of benign and malignant bone and soft tissue tumors of the extremities. Diagn Interv Radiol. 2007;13:164–171. [PubMed] [Google Scholar]
- Marushima A, Matsumaru Y, Suzuki K, et al. Selective arterial embolization with n-butyl cyanoacrylate in the treatment of aneursymal bone cyst of the thoracic vertebra: a case report. Spine (Phila Pa 1976) 2009;34:E230–E234. doi: 10.1097/BRS.0b013e31818f8f7c. [DOI] [PubMed] [Google Scholar]
- Pastushyn A I, Slin'ko E I, Mirzoyeva G M. Vertebral hemangiomas: diagnosis, management, natural history and clinicopathological correlates in 86 patients. Surg Neurol. 1998;50:535–547. doi: 10.1016/s0090-3019(98)00007-x. [DOI] [PubMed] [Google Scholar]
- Fox M W, Onofrio B M. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg. 1993;78:36–45. doi: 10.3171/jns.1993.78.1.0036. [DOI] [PubMed] [Google Scholar]
- Bandiera S, Gasbarrini A, De Iure F, Cappuccio M, Picci P, Boriani S. Symptomatic vertebral hemangioma: the treatment of 23 cases and a review of the literature. Chir Organi Mov. 2002;87:1–15. [PubMed] [Google Scholar]
- Acosta F L, Jr, Dowd C F, Chin C, Tihan T, Ames C P, Weinstein P R. Current treatment strategies and outcomes in the management of symptomatic vertebral hemangiomas. Neurosurgery. 2006;58:287–295. discussion 287–295. doi: 10.1227/01.NEU.0000194846.55984.C8. [DOI] [PubMed] [Google Scholar]
- Jayakumar P N, Vasudev M K, Srikanth S G. Symptomatic vertebral haemangioma: endovascular treatment of 12 patients. Spinal Cord. 1997;35:624–628. doi: 10.1038/sj.sc.3100438. [DOI] [PubMed] [Google Scholar]
- Syal R, Tyagi I, Goyal A, Barai S, Parihar A. Multiple intraosseous hemangiomas-investigation and role of N-butylcyanoacrylate in management. Head Neck. 2007;29:512–517. doi: 10.1002/hed.20539. [DOI] [PubMed] [Google Scholar]
- Doppman J L, Oldfield E H, Heiss J D. Symptomatic vertebral hemangiomas: treatment by means of direct intralesional injection of ethanol. Radiology. 2000;214:341–348. doi: 10.1148/radiology.214.2.r00fe46341. [DOI] [PubMed] [Google Scholar]
- Chu J P, Chen W, Li J P, et al. Clinicopathologic features and results of transcatheter arterial chemoembolization for osteosarcoma. Cardiovasc Intervent Radiol. 2007;30:201–206. doi: 10.1007/s00270-005-0302-y. [DOI] [PubMed] [Google Scholar]
- Crews K R, Liu T, Rodriguez-Galindo C, et al. High-dose methotrexate pharmacokinetics and outcome of children and young adults with osteosarcoma. Cancer. 2004;100:1724–1733. doi: 10.1002/cncr.20152. [DOI] [PubMed] [Google Scholar]
- Philip T, Iliescu C, Demaille M C, et al. High-dose methotrexate and HELP [holoxan (ifosfamide), eldesine (vindesine), platinum]—doxorubicin in non-metastatic osteosarcoma of the extremity: a French multicentre pilot study. Fédération Nationale des Centres de Lutte contre le Cancer and Société Française d'Oncologie Pédiatrique. Ann Oncol. 1999;10:1065–1071. doi: 10.1023/a:1008395126800. [DOI] [PubMed] [Google Scholar]
- Wang M Q, Dake M D, Wang Z P, Cui Z P, Gao Y A. Isolated lower extremity chemotherapeutic infusion for treatment of osteosarcoma: experimental study and preliminary clinical report. J Vasc Interv Radiol. 2001;12:731–737. doi: 10.1016/s1051-0443(07)61445-6. [DOI] [PubMed] [Google Scholar]
- Katzen B T, Said S. Arteriovenous malformation of bone: an experience with therapeutic embolization. AJR Am J Roentgenol. 1981;136:427–429. doi: 10.2214/ajr.136.2.427. [DOI] [PubMed] [Google Scholar]
- Beek F J, ten Broek F W, Schaik J P van, Mali W P. Transvenous embolisation of an arteriovenous malformation of the mandible via a femoral approach. Pediatr Radiol. 1997;27:855–857. doi: 10.1007/s002470050254. [DOI] [PubMed] [Google Scholar]
- Resnick S A, Russell E J, Hanson D H, Pecaro B C. Embolization of a life-threatening mandibular vascular malformation by direct percutaneous transmandibular puncture. Head Neck. 1992;14:372–379. doi: 10.1002/hed.2880140506. [DOI] [PubMed] [Google Scholar]
- Trübenbach J, Nägele T, Bauer T, Ernemann U. Preoperative embolization of cervical spine osteoblastomas: report of three cases. AJNR Am J Neuroradiol. 2006;27:1910–1912. [PMC free article] [PubMed] [Google Scholar]
- Denaro V, Denaro L, Papalia R, Marinozzi A, Di Martino A. Surgical management of cervical spine osteoblastomas. Clin Orthop Relat Res. 2007;455:190–195. doi: 10.1097/01.blo.0000238846.34047.d9. [DOI] [PubMed] [Google Scholar]
- Findik S, Akan H, Baris S, Atici A G, Uzun O, Erkan L. Preoperative embolization in surgical treatment of a primary hemangiopericytoma of the rib: a case report. J Korean Med Sci. 2005;20:316–318. doi: 10.3346/jkms.2005.20.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneuve S, Lézy J P, Cyna-Gorse F, Vacher C. [Mandibular hemangiopericytoma, a malignant vascular tumor] Rev Stomatol Chir Maxillofac. 2007;108:146–149. doi: 10.1016/j.stomax.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mejia R O, Ojemann S G, Simko J, Chaudhary U B, Levy J, Lawton M T. Sacral epithelioid angiosarcoma associated with a bleeding diathesis and spinal epidural hematoma: case report. J Neurosurg Spine. 2006;4:246–250. doi: 10.3171/spi.2006.4.3.246. [DOI] [PubMed] [Google Scholar]