ABSTRACT
Interventional radiology plays a major role in the management of symptomatic intervertebral disc herniations. In the absence of significant pain relief with conservative treatment including oral pain killers and anti-inflammatory drugs, selective image-guided periradicular infiltrations are generally indicated. The precise control of needle positioning allows optimal distribution of steroids along the painful nerve root. After 6 weeks of failure of conservative treatment including periradicular infiltration, treatment aiming to decompress or remove the herniation is considered. Conventional open surgery offers suboptimal results and is associated with significant morbidity. To achieve minimally invasive discal decompression, different percutaneous techniques have been developed. Their principle is to remove a small volume of nucleus, which results in an important reduction of intradiscal pressure and subsequently reduction of pressure inside the disc herniation. However, only contained disc herniations determined by computed tomography or magnetic resonance are indicated for these techniques. Thermal techniques such as radiofrequency or laser nucleotomy seem to be more effective than purely mechanical nucleotomy; indeed, they achieve discal decompression but also thermal destruction of intradiscal nociceptors, which may play a major role in the physiopathology of discal pain. The techniques of image-guided spinal periradicular infiltration and percutaneous nucleotomy with laser and radiofrequency are presented with emphasis on their best indications.
Keywords: Disc herniation, periradicular infiltration, percutaneous nucleotomy, laser, radiofrequency
Disc herniation is defined as rupture of the fibrocartilaginous annulus fibrosus that surrounds the intervertebral disc, associated with the release of the central gelatinous nucleus pulposus. Although first described by Wirshow in 1857, the physiopathology and the mechanisms of pain due to disc herniation remain controversial.1 There is no clear single explanation as to why disc rupture causes back and/or radicular pain. Indeed, physical pressure on a peripheral nerve root produces paresthesia but no pain. Biochemical factors may play a major role in the genesis of pain due to disc herniation. High levels of proinflammatory mediators such as interleukins, prostaglandins, and tumor necrosis factor are found inside degenerative discs.2,3 Histological studies have shown extensive innervation in the severely degenerated human lumbar disc compared with normal discs, with an increase of substance P immunoreactive nerve fibers known for their nociceptive properties.4
Pain management in symptomatic disc herniation relies mainly on conservative care combining rest, physiotherapy, analgesics, and anti-inflammatory drugs. Periradicular steroid injection can provide efficient pain relief on radicular pain caused by disc herniation. For spinal injection, image-guided techniques allow a precise control of the procedure to achieve optimal distribution of the steroids. After 6 weeks of failure of conservative therapies, including selective image-guided periradicular infiltration, treatment turns to disc decompression. The suboptimal results of conventional open surgery with significant morbidity have led to the development of minimally invasive techniques that avoid opening the spinal canal and extensive disc ablation.
The minimally invasive percutaneous techniques in use today aim at removing a small amount of central nucleus pulposus, so as to reduce intradiscal pressure and thus obviate discoradicular compression. Chemonucleolysis with papain was introduced in 1963 by Smith and colleagues.5 It was used for treating sciatica due to disc herniation and proved the concept of disc decompression for treating disc herniation. Although the technique showed good results with a 70 to 80% success rate, the product had unacceptable adverse reactions and is no longer in use in United States and Europe. Since then, several alternative techniques of percutaneous nucleotomy have been developed, relying either on pure mechanical (automated percutaneous lumbar discectomy), chemical (alcohol, oxygen-ozone), or thermal (laser, radiofrequency) decompression.6,7
PERIRADICULAR INFILTRATIONS
General Principles
Radicular pain due to disc herniation cannot be explained by a purely mechanical approach. The physiopathology of pain has a close relationship with the release of mediators from macrophages. Inflammatory cytokines, prostaglandins, nitrous oxide, phospholipase A2, and cyclooxygenase-2 may be involved in radiculitis caused by mechanical compression.8 Injection of steroids is probably effective because it decreases inflammation of the periradicular space.
Indications
The major indication for periradicular steroid injection is radicular symptoms due to discal compression that is resistant to conventional medical treatment. Precise clinical examination is mandatory and must be supported by discal abnormalities on computed tomography (CT) or magnetic resonance (MR) imaging at the corresponding level. Indeed, extradiscal causes of neuralgia should not be misdiagnosed.
Contraindications of steroid injection include patients with diabetes, gastric ulcers, and pregnancy. In patients with coagulation disorders, epidural puncture is contraindicated, and if needed, foraminal approach should be preformed carefully.
Technique
The procedure is performed in an outpatient basis. Image guidance is provided by CT, MR, or fluoroscopy. CT guidance is often preferred as it allows exact planning and positioning of the needle extradurally, preventing intradural or intravascular injection. The needle tip should be positioned as close as possible to the discoradicular interface. Periradicular steroid injection requires strict asepsis. Restricted sodium intake is recommended for the following week.
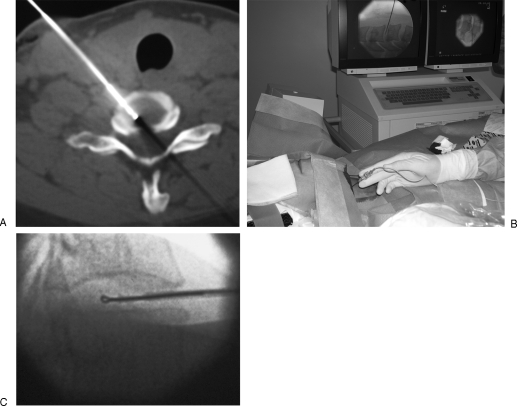
For lumbar level, injections are performed in the epidural lateral space for posterolateral disc herniations (Fig. 1) or in the foramen for foraminal and extraforaminal disc herniations (Fig. 2). For epidural lateral infiltrations, an interlaminar puncture is performed with a 22-gauge needle via a straight posterior approach. After passing the ligamentum flavum, 1 mL of iodine contrast is injected to confirm the epidural position of the needle tip. Indeed, using only flash or positive blood aspirate to predict intravascular injection is poorly sensitive.9 Then, 1.5 mL of long-acting steroid (cortivazol 3.75 mg, Aventis Pharma, Strasbourg, France) solution mixed with 1 mL of lidocaine 1% is injected. Long-acting synthetic steroids should not be injected intrathecally as they may precipitate in the cerebrospinal fluid and induce chemical arachnoiditis. In case of severe spinal canal stenosis, hydrocortisone is preferred as long-acting synthetic steroids may transiently worsen the symptoms due to their hyperosmotic effect.
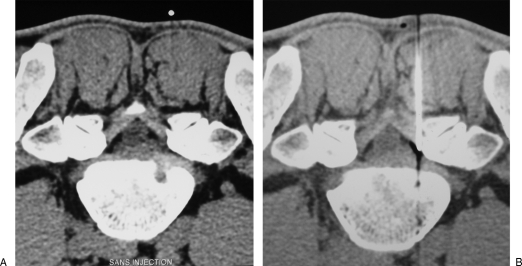
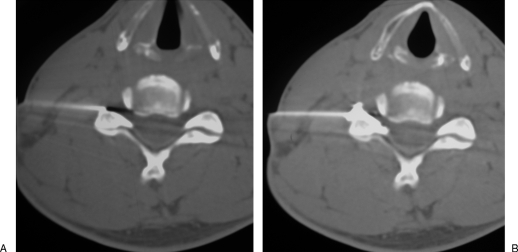
Figure 1.
Lumbar epidural infiltration. (A) Axial computed tomography scan shows a right posterolateral disc herniation at L5–S1 level, responsible for sciatic pain. (B) A 22-gauge needle is inserted into the right lateral epidural space; before steroid injection, proper position of the needle tip is confirmed by diffusion of a small air bubble into the epidural space.
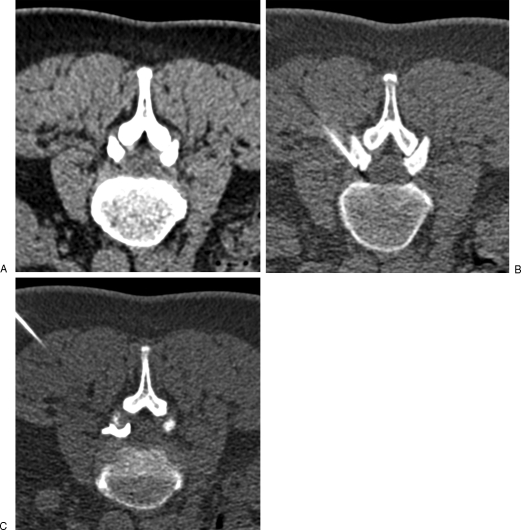
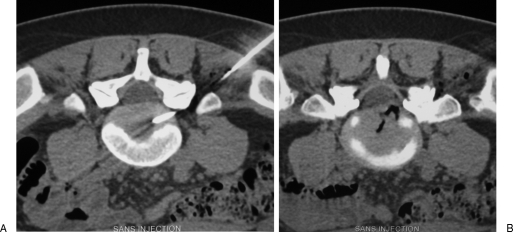
Figure 2.
Lumbar foraminal infiltration. (A) Axial computed tomography scan shows a left foraminal disc herniation at L2–L3 level, responsible for crural pain. (B) A 22-gauge needle is inserted into the low part of the foramen, behind the nerve root. (C) Contrast injection is performed to confirm appropriate needle position before steroid injection.
For foraminal infiltration, a 22-gauge needle is advanced toward the foramen via an oblique posterolateral approach. The needle must slip along the lateral border of the facet joint. The puncture has to be performed in the low part of the foramen, and the needle should not be pushed further than the anterior margin of the facet, so that its tip remains posterior and below the nerve root. Indeed, exceptional paraplegias have been reported with lumbar steroid injections performed under fluoroscopic guidance; they may be related to a low emerging radiculomedullary artery embolized with steroid crystals, and prior surgery at the same level seems to be a significant cofactor.10,11 However, this artery is positioned in the anterosuperior part of the foramen and should be avoided by proper positioning of the needle.
Thoracic periradicular steroid injections have few indications as intercostal radicular pain due to disc herniation is rare. However, a foraminal injection of long-acting steroids mixed with 1 mL of lidocaine can be safely done by CT-guided posterolateral approach (Fig. 3). As for lumbar foraminal infiltrations, the needle tip should remain behind and inferior to the nerve root. Its proper position is systematically checked by injection of 1 mL of iodine contrast before steroid injection. For high thoracic infiltrations, foraminal access may be blocked by large transverse process; in such cases, posterior interlaminar approach is possible, similar to lumbar epidural infiltration (Fig. 4).
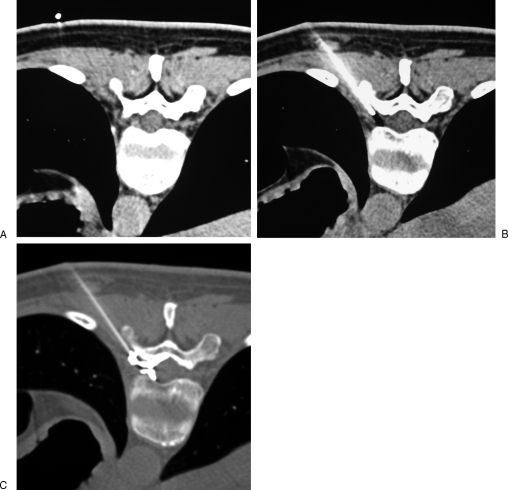
Figure 3.
Thoracic foraminal infiltration. (A) Axial computed tomography scan shows a left posterolateral disc herniation responsible for intercostal radicular pain. (B) A 22-gauge needle is inserted into the low part of the foramen, keeping behind the foraminal nerve root. (C) Contrast injection confirms the proper diffusion around the nerve root toward the spinal canal, before steroid injection.
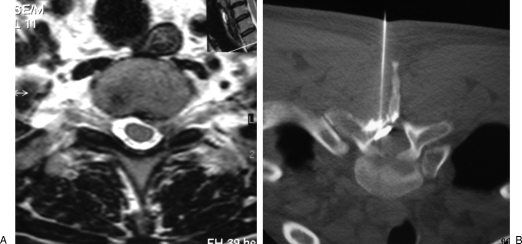
Figure 4.
Thoracic epidural infiltration. (A) Axial T2-weighted magnetic resonance image shows a left posterolateral disc herniation at T2–T3 level, responsible for axillary radicular pain. (B) Periradicular steroid injection is performed in the lateral epidural space via a posterior approach.
For cervical periradicular steroid injection, the patient is positioned in supine position with the head slightly turned to the opposite side. Hyperextension of the head with cushions under the shoulders can be useful for low cervical infiltrations or short necks. A 22-gauge needle in inserted toward the foramen via a lateral approach, near the painful nerve, under CT guidance. The tip of the needle is positioned in contact with the anterior side of the facet joint, behind the nerve root, avoiding the anteriorly situated vertebral artery (Fig. 5). A flushed connecting tube is fixed to the needle to avoid unintentional displacement of its tip during injection. After aspiration to check for blood, 1 to 2 mL of iodine contrast is injected to confirm the position of the needle and exclude intravascular injection. Then 1.5 mL of long-acting steroid (cortivazol 3.75 mg) solution is injected. Lidocaine should not be injected in the foramen as it may diffuse around the spinal cord and induce transient phrenic nerve palsy.
Figure 5.
Cervical infiltration. (A) A 22-gauge needle is inserted into the foramen, behind the nerve root, with its tip in close contact with the anterior border of the facet joint, away from the vertebral artery. (B) Before steroid injection, contrast injection is performed to confirm proper distribution around the nerve root, toward the foramen.
Results
Although periradicular steroid injections have been used for decades, the results are still controversial. However, the technique is not standardized as various puncture techniques are used. Moreover, most of the injections are performed without image guidance, which leads to high rate of inaccurate needle placement. For epidural infiltration, epidurography should also systematically performed to verify the injection site, as simple loss of air pressure resistance leads to 25 to 30% of inaccurate needle tip placement.12,13 Noncontrolled studies report success rate of 33 to 72% of patients. Short-term benefit of percutaneous nerve root block is quite high with good pain relief especially in irritative radiculopathy. Irritative radiculopathy is described as sciatica alone, and compressive radiculopathy is described as sciatica with sensory, motor, or reflex disturbances. There is a significantly better response in the irritative group than in the compressive group. Periradicular steroid infiltrations seem to be less beneficial for more chronic radicular pain.14 Epidural steroid injection provides mild to moderate improvement in leg pain and sensory deficits and reduces the need for analgesics for up to 6 weeks. It also prevents surgery for contained disc herniations.15,16 Steroids speed the rate of recovery and return to function, allowing a reduction of medication, while awaiting the natural improvement expected in most spinal disorders.17 Combining the results of several controlled studies, it is suggested that at long-term follow-up, no difference may be noted between the steroid-treated groups and the control groups. This can be explained by the favorable long-term prognosis in these nonsurgically treated patients. However, a beneficial effect is seen in patients with radicular pain syndromes at intermediate-term follow-up.
Severe adverse events following periradicular steroid injection are rare, occurring in less than 0.05%.18 The use of image guidance for periradicular infiltrations significantly increases accuracy and decreases complication rates.19 Major infectious complications such as epidural abscesses, meningitis, and spondylodiscitis have been reported. Vascular complications are mainly due to vertebral artery injury or intra-arterial injection at cervical level. Paraplegia following lumbar foraminal infiltrations has exceptionally been reported and is probably related to a low emerging radiculomedullary artery.10,11 Hematomas at the puncture site remain exceptional and generally minor (Fig. 6). Mild reactions to steroids like transient flushing or increase of pain at the injection site are reported in less than 5% of cases. Symptomatic epidural lipomatosis following local steroid infiltration has been reported but remains exceptional.20 However, no more than four infiltrations in a year should be performed at the same site to avoid secondary lipodystrophy.
Figure 6.
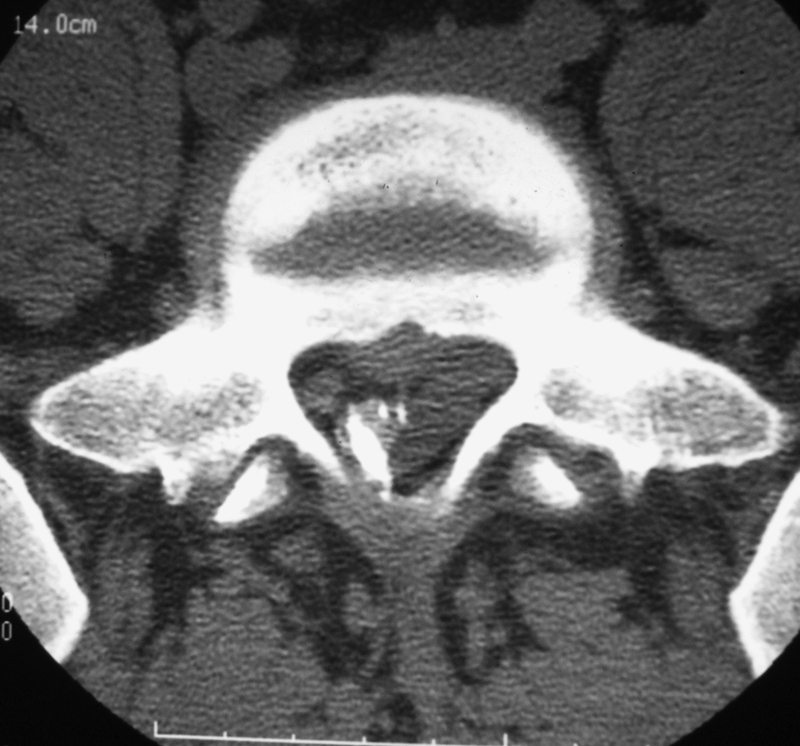
Epidural hematoma following epidural periradicular infiltration. Myelo-computed tomography shows a small left posterolateral collection displacing the dural sac (opacified with contrast). The patient remained asymptomatic.
PERCUTANEOUS NUCLEOTOMY
General Principles
When conservative therapies—including selective image-guided steroid injection—fail to control radicular pain due to disc herniation, treatment is directed to the disc with the aim of decompressing or removing the herniation. If conventional surgery is considered as the gold standard, its results are suboptimal and the morbidity is high, particularly with the risk of epidural fibrosis. Several minimally invasive percutaneous techniques have been developed to achieve intradiscal decompression. Removing a small amount of central nucleus pulposus results in a dramatic drop of pressure in the contained disc herniation, based on the principle that the intervertebral disc is a closed hydraulic space. However, this is not the case for extruded disc herniations or free discal fragments, which are contraindicated for percutaneous techniques.
Nucleolysis with chemopapain was the first percutaneous disc decompression technique, described in 1963.5 The results were good, but severe and definitive complications could occur in case of leakage toward the epidural space. Chemopapain is no longer manufactured. Since then, several mechanical and thermal nucleotomy techniques have emerged. Percutaneous techniques offer many advantages compared with conventional surgery: surrounding tissues are protected, there is no direct contact with the spinal canal, there is no scar, and the procedure is performed under local anesthesia in an outpatient basis. The cost is much reduced (25 to 30% of conventional surgical cost).
Thermal techniques (laser and radiofrequency nucleotomy) combine purely mechanical decompression with thermally induced modifications of intradiscal cytokines involved in disc degeneration. They also destroy nociceptors in the periphery of the annulus. Moreover, fusion of collagen fibers in the annulus occurs with temperatures over 70°C, with visible shrinkage at the periphery of the disc.21 Combined therapeutic effects with more promising results may explain why thermal techniques have overcome percutaneous mechanical discectomy.
Indications of percutaneous disc decompression are radicular pain due to contained disc herniation determined by CT or MR imaging and failure of 6 weeks of conservative treatment including selective steroid injection. Contraindications include nerve paralysis, hemorrhagic diathesis, spinal stenosis or instability, severe disc collapse >50%, and infection. Previous surgery at the same level is considered as a relative contraindication. Extruded disc herniations and free discal fragments are not indicated for percutaneous treatment.
Technique of Disc Puncture
For lumbar levels, the disc puncture is performed with a posterolateral approach, under fluoroscopic guidance. To open up the posterior aspect of the disc space, pillows are positioned under the abdomen to place the lumbar spine in a semiflexed position. The C-arm fluoroscope first is rotated craniocaudally in the plane of the disc and then obliquely, so that the articular process projection is centered midway between the anterior and posterior aspects of the vertebral body (“Scotty dog view”). Disc puncture is then performed in the axis of the X-ray beam, just lateral to the articular process. The needle must systematically slip along the articular process to avoid the nerve root in its extraforaminal course. For L5–S1, prominent iliac wings may block direct puncture and bended needle may be required. After puncturing the disc, both anteroposterior and lateral fluoroscopic projections are needed to confirm the proper positioning of the needle.
For thoracic levels, the disc puncture is performed with a posterolateral approach, under fluoroscopic guidance. The C-arm is rotated craniocaudally in the plane of the disc; then it is rotated 35 degrees laterally from the anteroposterior view. With this projection, the head of the rib and the pedicle project as two rings; the puncture is performed in the axis of the X-ray beam, between the two rings, at the level of the disc.
For cervical levels, the disc puncture is performed with an anterolateral approach, under fluoroscopic guidance. Cushions are positioned under the upper thoracic spine to hyperextend the neck and open up the anterior aspect of the cervical discs. With two fingers, the operator presses against the spine, moving the carotid artery and the jugular vein laterally. Then, the disc is punctured directly between the two fingers. The needle passes between the esophagus medially and the major cervical vessels laterally.
Percutaneous Thermal Disc Decompression
LASER DISC DECOMPRESSION
This technique was first described by Choy et al.22 The principle of laser disc decompression is to vaporize a small amount of nucleus with laser energy and achieve decompression. Transient increase of temperature also spoils chemical factors and intradiscal nociceptors responsible for pain. Laser energy is transmitted into the disc via a 400-μm diameter optical fiber inserted coaxially through an 18-gauge spinal needle. Laser disc decompression produces high temperatures, and the risk of thermal damage to the adjacent vertebral end plates increases when the discal height is reduced. Moreover, laser energy penetrates 5 to 8 mm from the tip of the optical fiber, which can be dangerous for the spinal cord and foraminal nerve roots at the cervical level with an anterior approach.23 For these reasons, low energy (300 J) and particular caution are required to treat thoracic and cervical disc herniations.
The laser energy used to treat disc is provided by lasers near the infrared region or with visible green radiation, with wavelength varying from 514 to 2150 nm.24,25,26 After puncturing the disc with an 18-gauge needle, the tip of the laser fiber is positioned at the posterior annulus/nucleus junction (Fig. 7). Then, short pulses of 1 second with a power of 15 W are applied, separated by free intervals of 5 to 10 seconds to achieve vaporization. If back pain occurs during the intervention, it may be due to the heating of the adjacent vertebral end plates or hyperpressure within the disc from trapped gas; in such cases, the position of the fiber should be checked and the interval between the pulses increased. Moreover, aspiration is applied via the side arm fitting to avoid excessive intradiscal gas trapping (Fig. 8).
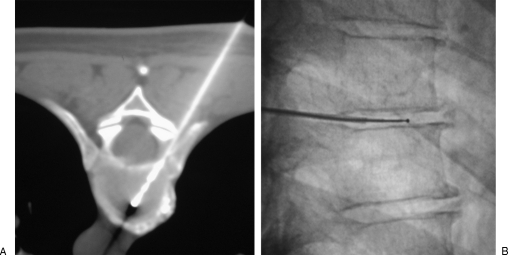
Figure 7.
Percutaneous laser lumbar nucleotomy at L5–S1 level. (A) Laser fiber is inserted into the disc coaxially through a bended 18-gauge needle. Close contact with the facet joint is mandatory to avoid the foraminal nerve root. (B) Computed tomography scan shows the vaporization with gas diffusion into the herniation.
Figure 8.
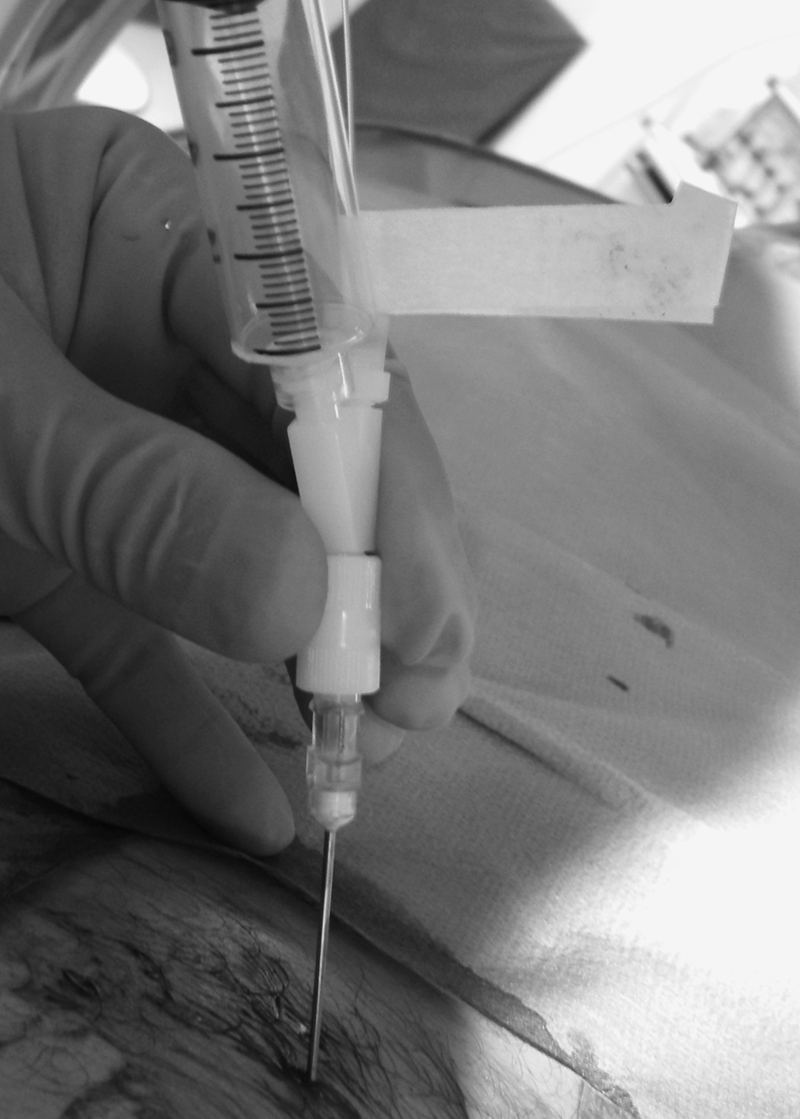
Percutaneous laser nucleotomy. Aspiration is applied via the side arm fitting to avoid excessive intradiscal gas trapping and lumbar pain during vaporization.
The recommended energy for laser disc decompression ranges from 1200 to 1500 J for L2–L3, L3–L4, and L5–S1 and 1500 to 2000 J for L4–L5.
For 2 weeks after intervention, sitting, bending, and stooping are restricted. Athletic activities should be halted for a minimum of 6 weeks. Follow-up relies mainly on clinical symptoms. The proper disc decompression is confirmed by the relief of radicular pain. CT or MR control is only indicated if a complication is suspected. The shrinkage if the herniation is generally moderate and only visible after several months.
Many studies report a success rate of 70 to 89% cases for radicular pain.27,28,29 The decrease of pain is fast and stabilizes after 6 weeks. The success rate is similar for lumbar and cervical levels if precise indications are respected. The overall rate of complication is low: 0.4 to 0.5% for lumbar levels and 0.6 to 1% for cervical levels.30 The major complication is infectious spondylodiscitis, but this should be avoided with strict sterility. Thermal injury to the adjacent vertebral end plates leading to aseptic spondylitis with severe back pain has been reported; if any back pain occurs during vaporization, optical fiber position should be checked and/or laser energy delivered should be slowed.
RADIOFREQUENCY NUCLEOTOMY (NUCLEOPLASTY)
Radiofrequency nucleotomy relies on Coblation® (ArthroCare, Austin, TX) technology to ablate soft tissue. A bipolar radiofrequency electrode is inserted within the disc via a conventional percutaneous approach. The electrode ionizes the sodium atoms in the nucleus, leading to creation of a high-energy ionic plasma field. This plasma disintegrates the intramolecular bonds in the nucleus. Unlike other radiofrequency devices that are temperature-driven, this system does not rely on heat energy to ablate tissue, so thermal damage and tissue carbonization is avoided.31 Radiofrequency nucleoplasty works in a much lower range of temperature compared with laser disc decompression. However, as with laser energy, the ionized plasma field may also induce intradiscal biochemical modifications, which may play an important additional therapeutic role.32,33 Coblation® technology requires sodium to transmit energy. This process cannot work if the disc is dehydrated. Thus, pressure reduction is highly dependent on the degree of spine degeneration.34
The indications and contraindications are the same as for laser disc decompression.
For lumbar levels, a dedicated 17-gauge introducer needle is inserted into the disc via a conventional posterolateral approach and placed at the posterior annulus/junction. The radiofrequency electrode is inserted coaxially (Fig. 9). Its curved tip allows creation of different channels within the nucleus when the axis of the electrode is rotated. Six to 10 channels are created in total, depending on the desired amount of tissue reduction. The gas produced by nucleus disintegration escapes from the disc via the introducer needle. Particular caution should be taken to keep the electrode parallel to the adjacent vertebral end plates to avoid touching them during the procedure. The ablation procedure is very fast, less than 2 minutes once the electrode is in position.
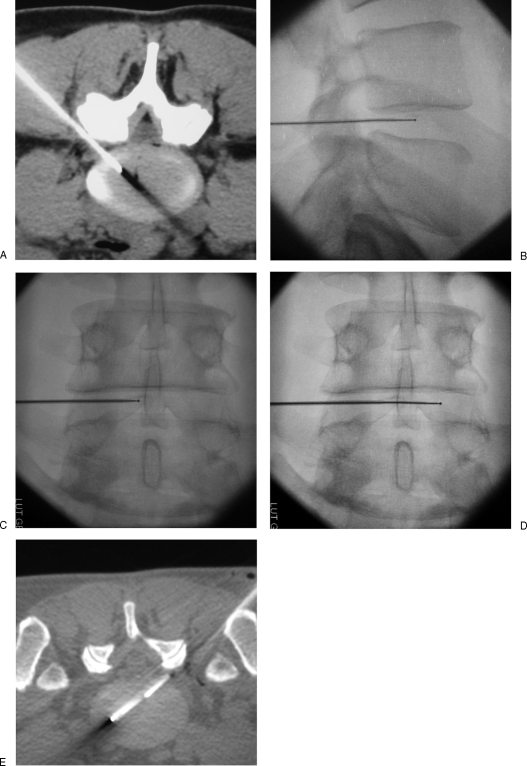
Figure 9.
Percutaneous lumbar radiofrequency nucleoplasty. (A) A 17-gauge introducer needle is positioned via a posterolateral approach at the posterior annulus/nucleus junction. (B, C) Fluoroscopic views show the radiofrequency electrode inserted coaxially into the nucleus, in proximal position. (D, E) Fluoroscopy and computed tomography show the electrode advanced in distal position, in contact with the anterior annulus. Six to 10 Coblation® channels are dug to achieve discal decompression. The tip of the electrode should not touch the vertebral end plates to avoid thermal damage to their cartilage.
For thoracic levels, the technique is similar, using a conventional fluoroscopy-guided posterolateral approach. Due to the small height of thoracic discs, a maximum of six channels are created: three on the left side, three on the right side. Indeed, pointing the tip of the electrode cranially or caudally may harm the adjacent vertebral end plates (Fig. 10).
Figure 10.
Percutaneous thoracic radiofrequency nucleotomy. (A) The electrode in inserted into the disc, parallel to the adjacent vertebral end plates, between the head of the rib and the pedicle. (B) Lateral fluoroscopic view shows the tip of the electrode, away from the vertebral end plates. Due to reduced height of thoracic discs, only lateral Coblation® channels are dug to avoid damage to the vertebral end plates.
For cervical levels, a dedicated 19-gauge electrode with a looped tip is used. This electrode creates small spherical voids in the annulus when the electrode is rotated on its axis. The electrode is advanced to the mid part of the disc, but never beyond the midthird/posterior-third junction of the disc to avoid damage to the spine cord or the nerve roots (Fig. 11). Sedation is prohibited to allow complete neurological monitoring of the patient during the whole procedure. A series of three spherical voids is generally sufficient to achieve disc decompression.
Figure 11.
Percutaneous cervical radiofrequency nucleotomy. (A, B) A dedicated 19-gauge radiofrequency electrode is inserted into the cervical disc via an anterolateral approach, just medial to the carotid artery. (C) Three spherical voids are created in the disc by rotation of the looped-tip radiofrequency electrode in the nucleus. The electrode should not be advanced beyond the midthird/posterior-third junction of the disc to avoid damage to the neurological structures.
Postoperative instructions following radiofrequency nucleoplasty are very similar to laser nucleotomy. For cervical decompression, a surgical collar is prescribed for the first week, and neck rotation or bending is limited. Follow-up relies mainly on clinical symptoms, and imaging is generally performed only if any complication is suspected.
Few studies of nucleoplasty are available. The selection criteria of those studies vary widely, with patients suffering from pure back pain and/or radicular pain included. Many authors report a success rate ranging from 75 to 80% with significant improvement in quality of life.32,35,36 At the cervical level, the promising results are similar, with faster return to work compared with conservative care.37,38 Nucleoplasty allows fast, effective, and safe disc decompression of contained disc herniations when conservative therapies fail. The specially designed cervical electrode allows for a safer treatment of cervical disc herniations compared with mechanical or laser decompression systems, as the device avoids neural structures and no heat is transmitted toward the spinal canal.
Compared with laser disc decompression, the overall results seem very similar. However, the disc decompression is much faster (less than 2 minutes once the electrode is in the disc), and the risk of thermal damage to the adjacent vertebral end plates is reduced as the radiofrequency plasma field works in a low range of temperature. One drawback of radiofrequency nucleoplasty is the cost of the electrode, which is significantly higher than the cost of the laser fiber. Finally, the dedicated 17-gauge introducer needle is very stiff and difficult to bend for complex L5–S1 approach; in this situation, the flexible laser fiber introduced coaxially through a bended 18-gauge spinal needle is more easily positioned into the disc.
CONCLUSION
Disc herniation causing axial and radicular pain is a very common pathology with huge social and economic consequences. Initial conservative treatment relies on rest and oral medication with analgesics and nonsteroidal anti-inflammatory drugs. When conservative treatment fails to rapidly control pain, selective periradicular steroid injection should be considered first. Image-guided infiltrations are precise and safe, particularly for cervical level. They allow contrast opacification to control the perfect positioning of the needle before steroid deposition. Short-term results are satisfying, but the benefit often decreases after several weeks.
After failure of 6 weeks of conservative therapy including steroid infiltrations, percutaneous disc decompression therapies may be considered. Several techniques are available, all based on removing part of the nucleus pulposus to achieve decompression of the herniation. Among them, percutaneous laser disc decompression and radiofrequency nucleoplasty may be more efficient as they not only act by mechanical effect but also induce modifications in the disc biochemistry and innervations. These decompression techniques are much less invasive than conventional open surgery but can only be performed in cases of contained disc herniation. Excellent pain relief is achieved in 75 to 80% of patients with long-term stable effects.
Interventional radiology plays an important role in pain management due to disc herniation. However, selection of patients after precise clinical examination remains essential to achieve successful treatment.
REFERENCES
- Castro I, Santos D P, Christoph DdeH, Landeiro J A. The history of spinal surgery for disc disease: an illustrated timeline. Arq Neuropsiquiatr. 2005;63(3A):701–706. doi: 10.1590/s0004-282x2005000400030. [DOI] [PubMed] [Google Scholar]
- Burke J G, Watson R W, McCormack D, Dowling F E, Walsh M G, Fitzpatrick J M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- Peng B, Hou S, Wu W, Zhang C, Yang Y. The pathogenesis and clinical significance of a high-intensity zone (HIZ) of lumbar intervertebral disc on MR imaging in the patient with discogenic low back pain. Eur Spine J. 2006;15:583–587. doi: 10.1007/s00586-005-0892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppes M H, Marani E, Thomeer R T, Groen G J. Innervation of “painful” lumbar discs. Spine (Phila Pa 1976) 1997;22:2342–2349. discussion 2349–2350. doi: 10.1097/00007632-199710150-00005. [DOI] [PubMed] [Google Scholar]
- Smith L, Garvin P J, Gesler R M, Jennings R B. Enzyme dissolution of the nucleus pulposus. Nature. 1963;198:1311–1312. doi: 10.1038/1981311a0. [DOI] [PubMed] [Google Scholar]
- Andreula C, Muto M, Leonardi M. Interventional spinal procedures. Eur J Radiol. 2004;50:112–119. doi: 10.1016/j.ejrad.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Gangi A, Dietemann J L, Gasser B, et al. Interventional radiology with laser in bone and joint. Radiol Clin North Am. 1998;36:547–557. doi: 10.1016/s0033-8389(05)70043-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Baba H, Uchida K, et al. Effect of mechanical compression on the lumbar nerve root: localization and changes of intraradicular inflammatory cytokines, nitric oxide, and cyclooxygenase. Spine (Phila Pa 1976) 2005;30:1699–1705. doi: 10.1097/01.brs.0000171910.97937.0e. [DOI] [PubMed] [Google Scholar]
- Furman M B, O'Brien E M, Zgleszewski T M. Incidence of intravascular penetration in transforaminal lumbosacral epidural steroid injections. Spine (Phila Pa 1976) 2000;25:2628–2632. doi: 10.1097/00007632-200010150-00014. [DOI] [PubMed] [Google Scholar]
- Houten J K, Errico T J. Paraplegia after lumbosacral nerve root block: report of three cases. Spine J. 2002;2:70–75. doi: 10.1016/s1529-9430(01)00159-0. [DOI] [PubMed] [Google Scholar]
- Huntoon M A, Martin D P. Paralysis after transforaminal epidural injection and previous spinal surgery. Reg Anesth Pain Med. 2004;29:494–495. doi: 10.1016/j.rapm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Bartynski W S, Grahovac S Z, Rothfus W E. Incorrect needle position during lumbar epidural steroid administration: inaccuracy of loss of air pressure resistance and requirement of fluoroscopy and epidurography during needle insertion. AJNR Am J Neuroradiol. 2005;26:502–505. [PMC free article] [PubMed] [Google Scholar]
- Johnson B A, Schellhas K P, Pollei S R. Epidurography and therapeutic epidural injections: technical considerations and experience with 5334 cases. AJNR Am J Neuroradiol. 1999;20:697–705. [PMC free article] [PubMed] [Google Scholar]
- Cyteval C, Fescquet N, Thomas E, Decoux E, Blotman F, Taourel P. Predictive factors of efficacy of periradicular corticosteroid injections for lumbar radiculopathy. AJNR Am J Neuroradiol. 2006;27:978–982. [PMC free article] [PubMed] [Google Scholar]
- Karppinen J, Malmivaara A, Kurunlahti M, et al. Periradicular infiltration for sciatica: a randomized controlled trial. Spine (Phila Pa 1976) 2001;26:1059–1067. doi: 10.1097/00007632-200105010-00015. [DOI] [PubMed] [Google Scholar]
- Kolstad F, Leivseth G, Nygaard O P. Transforaminal steroid injections in the treatment of cervical radiculopathy. A prospective outcome study. Acta Neurochir (Wien) 2005;147:1065–1070. discussion 1070. doi: 10.1007/s00701-005-0542-2. [DOI] [PubMed] [Google Scholar]
- McLain R F, Kapural L, Mekhail N A. Epidural steroid therapy for back and leg pain: mechanisms of action and efficacy. Spine J. 2005;5:191–201. doi: 10.1016/j.spinee.2004.10.046. [DOI] [PubMed] [Google Scholar]
- Ma D J, Gilula L A, Riew K D. Complications of fluoroscopically guided extraforaminal cervical nerve blocks. An analysis of 1036 injections. J Bone Joint Surg Am. 2005;87:1025–1030. doi: 10.2106/JBJS.D.02139. [DOI] [PubMed] [Google Scholar]
- Watanabe A T, Nishimura E, Garris J. Image-guided epidural steroid injections. Tech Vasc Interv Radiol. 2002;5:186–193. doi: 10.1053/tvir.2002.36426. [DOI] [PubMed] [Google Scholar]
- Sandberg D I, Lavyne M H. Symptomatic spinal epidural lipomatosis after local epidural corticosteroid injections: case report. Neurosurgery. 1999;45:162–165. doi: 10.1097/00006123-199907000-00037. [DOI] [PubMed] [Google Scholar]
- Wang J C, Kabo J M, Tsou P M, Halevi L, Shamie A N. The effect of uniform heating on the biomechanical properties of the intervertebral disc in a porcine model. Spine J. 2005;5:64–70. doi: 10.1016/j.spinee.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Choy D S, Case R B, Fielding W, Hughes J, Liebler W, Ascher P. Percutaneous laser nucleolysis of lumbar disks. N Engl J Med. 1987;317:771–772. doi: 10.1056/NEJM198709173171217. [DOI] [PubMed] [Google Scholar]
- Schmolke S, Kirsch L, Gossé F, Flamme C, Bohnsack M, Rühmann O. Risk evaluation of thermal injury to the cervical spine during intradiscal laser application in vitro. Photomed Laser Surg. 2004;22:426–430. doi: 10.1089/pho.2004.22.426. [DOI] [PubMed] [Google Scholar]
- Knappe V, Frank F, Rohde E. Principles of lasers and biophotonic effects. Photomed Laser Surg. 2004;22:411–417. doi: 10.1089/pho.2004.22.411. [DOI] [PubMed] [Google Scholar]
- Choy D S. Percutaneous laser disc decompression (PLDD) update: focus on device and procedure advances. J Clin Laser Med Surg. 1993;11:181–183. doi: 10.1089/clm.1993.11.181. [DOI] [PubMed] [Google Scholar]
- Gangi A, Dietemann J L, Ide C, Brunner P, Klinkert A, Warter J M. Percutaneous laser disk decompression under CT and fluoroscopic guidance: indications, technique, and clinical experience. Radiographics. 1996;16:89–96. doi: 10.1148/radiographics.16.1.89. [DOI] [PubMed] [Google Scholar]
- Bosacco S J, Bosacco D N, Berman A T, Cordover A, Levenberg R J, Stellabotte J. Functional results of percutaneous laser discectomy. Am J Orthop (Belle Mead NJ) 1996;25:825–828. [PubMed] [Google Scholar]
- Choy D S. Percutaneous laser disc decompression: a 17-year experience. Photomed Laser Surg. 2004;22:407–410. doi: 10.1089/pho.2004.22.407. [DOI] [PubMed] [Google Scholar]
- Gangi A, Basile A, Basille A, et al. Radiofrequency and laser ablation of spinal lesions. Semin Ultrasound CT MR. 2005;26:89–97. doi: 10.1053/j.sult.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Hellinger J. Complications of non-endoscopic percutaneous laser disc decompression and nucleotomy with the neodymium: YAG laser 1064 nm. Photomed Laser Surg. 2004;22:418–422. doi: 10.1089/pho.2004.22.418. [DOI] [PubMed] [Google Scholar]
- Chen Y C, Lee S H, Saenz Y, Lehman N L. Histologic findings of disc, end plate and neural elements after coblation of nucleus pulposus: an experimental nucleoplasty study. Spine J. 2003;3:466–470. doi: 10.1016/s1529-9430(03)00143-8. [DOI] [PubMed] [Google Scholar]
- Alexandre A, Coro L, Azuelos A, Pellone M. Percutaneous nucleoplasty for discoradicular conflict. Acta Neurochir Suppl. 2005;92:83–86. doi: 10.1007/3-211-27458-8_18. [DOI] [PubMed] [Google Scholar]
- O'Neill C W, Liu J J, Leibenberg E, et al. Percutaneous plasma decompression alters cytokine expression in injured porcine intervertebral discs. Spine J. 2004;4:88–98. doi: 10.1016/s1529-9430(03)00423-6. [DOI] [PubMed] [Google Scholar]
- Chen Y C, Lee S H, Chen D. Intradiscal pressure study of percutaneous disc decompression with nucleoplasty in human cadavers. Spine (Phila Pa 1976) 2003;28:661–665. doi: 10.1097/01.BRS.0000051920.45671.88. [DOI] [PubMed] [Google Scholar]
- Gerszten P C, Welch W C, King J T., Jr Quality of life assessment in patients undergoing nucleoplasty-based percutaneous discectomy. J Neurosurg Spine. 2006;4:36–42. doi: 10.3171/spi.2006.4.1.36. [DOI] [PubMed] [Google Scholar]
- Singh V, Derby R. Percutaneous lumbar disc decompression. Pain Physician. 2006;9:139–146. [PubMed] [Google Scholar]
- Bonaldi G, Baruzzi F, Facchinetti A, Fachinetti P, Lunghi S. Plasma radio-frequency-based diskectomy for treatment of cervical herniated nucleus pulposus: feasibility, safety, and preliminary clinical results. AJNR Am J Neuroradiol. 2006;27:2104–2111. [PMC free article] [PubMed] [Google Scholar]
- Nardi P V, Cabezas D, Cesaroni A. Percutaneous cervical nucleoplasty using coblation technology. Clinical results in fifty consecutive cases. Acta Neurochir Suppl. 2005;92:73–78. doi: 10.1007/3-211-27458-8_16. [DOI] [PubMed] [Google Scholar]