Balloon dilation has become a common method for treating a variety of esophageal conditions. When properly performed, it is an effective procedure with a high rate of success. Fluoroscopically guided balloon dilation offers distinct advantages over endoscopically performed balloon dilation or blindly performed bougienage, as it allows for better visual control of the procedure and morbidity is low. In our experience, fluoroscopically guided esophageal balloon dilation is safe and easy to perform, provided that the operators are aware of the potential complications and the procedure is properly tailored to the patient's underlying condition.
At our institution, over the course of 25 years we have performed more than 2300 fluoroscopically guided esophageal balloon dilations to treat a multitude of disorders including strictures of benign and malignant etiology, anastomotic strictures, radiation-induced strictures, tight gastric fundoplication wraps, esophageal webs and rings, achalasia, and anastomotic leaks. Over the years that we have performed the procedure, we have adapted our technique to maximize postprocedural outcomes and utilize available technology. In this article, we describe our current technique, present the various conditions amenable to balloon dilation, and emphasize modifications for each condition. We also discuss potential problems and complications that we have experienced in our practice.
TECHNIQUE
In general, the technique follows five steps (Fig. 1A). The first is to determine the nature of the stricture with an esophagogram prior to the balloon procedure. We prefer to use a barium sulfate suspension as the contrast medium because it usually allows better delineation of the stricture than does a water-soluble contrast agent. Careful attention should be directed to the length of the stricture, the site of maximal narrowing, and the severity of the strictured area. Additionally, if the stricture is malignant in etiology, esophageal stent placement may be a more appropriate long-term solution; in such cases, balloon dilation is frequently performed before inserting the stent to facilitate its placement.
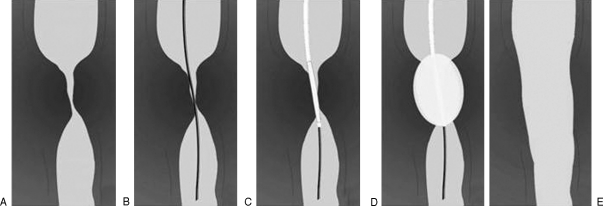
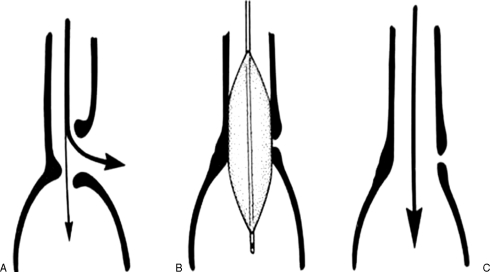
Figure 1.
Steps in fluoroscopically guided balloon dilation. (A) Stricture is identified on the preprocedural esophagogram. (B) Guide wire is advanced under fluoroscopic control across the lesion. (C) Balloon catheter is advanced over the wire and balloon is centered on the lesion. (D) Balloon is inflated, repeatedly if necessary. (E) Postprocedural esophagogram is performed to evaluate for esophageal perforation.
Before performing the procedure, it is important to properly position the patient and to provide appropriate sedation and analgesia. The patient should be placed in the supine or posterior oblique position (supported by a wedge cushion) on a fluoroscopy table. The head of the table is then raised ∼30 degrees from the horizontal position to make the patient more comfortable and reduce the risk of aspiration. Premedication with analgesic and sedative medicines makes the patient more comfortable. We prefer to give 1 to 4 mg of midazolam and 50 to 150 μg of fentanyl intravenously in divided doses during the procedure, with the amount titrated to achieve the desired sedation effect. A nurse assistant should closely monitor and record oximetry, electrocardiography, and blood pressure. To aid in the oral passage of catheters and to help inhibit the gag reflex, the patient's throat is anesthetized, either by spraying with a topical anesthetic or using viscous lidocaine that the patient gargles and then swallows. If necessary, salivary secretions can be reduced with atropine sulfate or removed by means of oral suctioning.
The second step is to pass a guide wire or feeding tube through the patient's mouth into the esophagus and advance it across the stricture under fluoroscopic control (Fig. 1B). If a feeding tube is used, a shortened 8- to 12-French feeding catheter (Kendall Argyle feeding tube, Tyco Healthcare, Mansfield, MA) is passed into the gut until it reaches the stricture. The angiographic guide wire is then advanced independently or through the tube to a point beyond the narrowed segment. During oral passage of the feeding tube or wire, it can be useful to have the patient in a chin-tucked position to help direct the tube or wire into the esophagus rather than the airway. Instructing the patient to swallow may also aid in passing it. We prefer to use a 0.035-inch exchange-length angiographic guide wire with a flexible, straight or “J-tipped” end (Amplatz stiff guide wire or Rosen curved guide wire, Cook Medical, Bloomington, IN). In general, the guide wire needs to be advanced well beyond the stricture to provide a stable purchase for catheter exchange. When the center of the stricture is at an angle relative to the longitudinal axis of the esophagus, or when there are multiple strictures, it may be necessary to use a relatively stiff guide wire or a curved “hockey stick” angiographic catheter (Soft-Vu Berenstein, Angiodynamics Inc., Queensbury, NY) to direct the tip of the wire into the orifice of the stricture. If a feeding tube was used to facilitate guide wire positioning, the tube should be removed once the guide wire is well beyond the stricture.
The third step is to advance the balloon catheter over the guide wire and center it on the lesion (Fig. 1C). The midportion of the balloon should straddle the area of maximum narrowing. The balloon diameter chosen for the procedure depends on the diameter of the stricture and that of the adjacent normal gut, as well as the esophageal condition being treated. For inflammatory strictures, esophageal webs, Nissen wraps, and anastomotic leaks, the choice of balloon size may vary in diameter from 6 to 20 mm and in length from 3 to 8 cm. A balloon diameter of 18 to 20 mm (C.R.E. Pulmonary Balloon or XXL Balloon Dilation Catheter, Boston Scientific, Natick, MA) is most commonly used for treatment of strictures in adults, and a large pneumatic balloon with a diameter of 30 to 40 mm and length of 15 cm (Rigiflex II, Boston Scientific) is used for treating patients with achalasia. In case of tight strictures, and depending on how the patient and stricture respond to the dilation, it may be necessary to use balloons of progressively larger size during the procedure.
The fourth step is to inflate the balloon under fluoroscopic control (Fig. 1D). During inflation, the balloon pressure is monitored and controlled with an in-line pressure gauge (Alliance II Integrated Inflation System, Boston Scientific, or Quantum Inflation Device, Cook Medical). As the balloon begins to inflate, a waistlike contour is usually noted (preferably at the balloon midpoint), corresponding to the site of the stricture. The balloon is slowly inflated until the “waist” disappears. In many cases, it is not possible or safe to stretch the stricture completely at the first inflation and, in these cases, the balloon is inflated only until resistance is felt. This inflation pressure is maintained for 30 to 180 seconds, after which the balloon is deflated for approximately 1 minute and subsequently reinflated for an additional 30- to 180-second period. Inflation is repeated as many times as necessary to obtain full distention of the stricture. The total number of inflations varies, but typically ranges from three to five. With each inflation, the pressure is increased by a small increment (usually one atmosphere) until either maximum balloon diameter or manufacturer-rated pressure tolerance (varying from 3 to 6 atm in the larger balloons) is achieved. In our experience, performing multiple balloon inflations with small incremental increases in pressure is preferable to dilating the stricture with a single inflation to maximum pressure as the latter method increases the risk of perforation. During each inflation, the balloon is watched fluoroscopically to ascertain that no complications have occurred. Additionally, the patient's level of pain is closely monitored, and further dilation is not performed until the patient's pain is adequately controlled.
The fifth step is to perform a postprocedural esophagogram to check for complications such as a deep esophageal laceration or perforation (Fig. 1). Immediately after the final balloon inflation, a limited esophagogram can be obtained by injecting water-soluble contrast material through the balloon catheter as it is withdrawn across the treated stricture. When no complications are identified, it is recommended to repeat the esophagogram with barium suspension after the patient is fully recovered and alert because small tears are better visualized with barium suspension than with water-soluble contrast agent. When there is suspicion of an esophageal perforation below the diaphragm, evaluation should be performed with water-soluble contrast medium only to avoid spillage of barium suspension into the abdominal cavity. On the postprocedural esophagogram, it is important to recognize that the stricture often appears unchanged in severity in comparison to the preprocedural radiographs. This lack of apparent change may be caused by edema and esophageal spasm induced by balloon dilation at the stricture site. The success of the procedure is therefore best determined by the patient's relief of symptoms rather than from the appearance of the stricture on the postprocedural esophagogram.
INDICATIONS
The conditions amenable to fluoroscopically guided balloon dilation are numerous. In the following section, we discuss the conditions that we have treated over the years and emphasize modifications of the technique for some of these cases.
Benign Inflammatory Strictures
Inflammatory strictures caused by gastroesophageal reflux are the most common benign strictures and present as areas of narrowing, most commonly in the distal one-third of the esophagus or at the gastroesophageal junction (i.e., Schatzki ring). Other benign causes of esophageal strictures include prior ingestion of caustic material (lye, certain medications, etc.), previous iatrogenic dissection or perforation with an instrument, prolonged presence of a nasogastric tube in the esophagus, and Crohn's disease. In mild strictures, a single, large-diameter balloon can be used without resorting to a sequence of progressively larger balloons (Fig. 2). The maximum balloon diameter that we use for these is typically 20 mm. In patients with tight strictures, the procedure is performed initially with small-diameter balloons followed by balloons of larger caliber, in a sequential fashion with rest periods of 1 minute between each inflation. When the stricture extends over several centimeters, we prefer to use a relatively long balloon as it is more likely to remain in position during inflation without sliding away. A long balloon is also useful in patients with sliding hiatal hernias and strictures near the gastroesophageal junction. This makes it easier to correctly position the balloon, as a stricture located above a hernia may slide back and forth across the diaphragm. A long balloon is also recommended when dilating multiple strictures that are close together because all of them can be treated simultaneously with the same inflation.
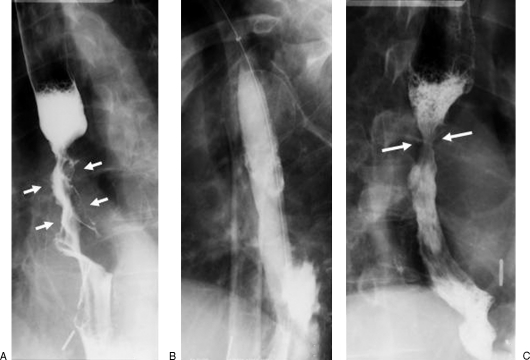
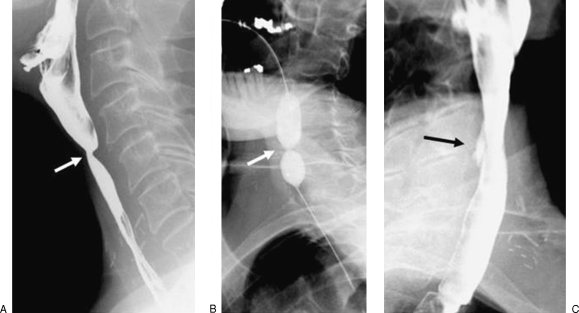
Figure 2.
A 62-year-old man with history of gastroesophageal reflux disease and progressive dysphagia. (A) Preprocedural esophagogram shows two areas of stricturing, including a distal esophageal stricture (between large arrows) and a tight Schatzki ring (small arrow). Note that there is a small sliding hiatal hernia. (B) Balloon dilation was performed with a 20-mm-diameter balloon across the two strictures. (C) Postprocedural esophagogram shows improvement of both strictures.
Malignant Strictures
Malignant esophageal strictures usually do not respond in the long term to balloon dilation, as most cancers continue to grow and cause recurrent narrowing of the esophagus. Balloon dilation plays an important adjunctive role, however, when placement of an esophageal stent is considered to improve the patient's ability to swallow or to cover an esophagotracheal fistula to protect the patient from aspiration pneumonia. In general, balloon dilation is performed before insertion of a stent to open the stricture and facilitate deployment of the stent. Care must be taken to choose a balloon of relatively small size, usually 12 to 15 mm in diameter, as a larger 20-mm balloon will increase the risk of perforating a necrotic tumor. Also, the balloon must be inflated slowly and with small increments in pressure to diminish the perforation risk (Fig. 3). Placement of stents in malignant strictures has been shown to provide relief of dysphagia in ∼90% of cases.1 Fluoroscopically guided balloon dilation is also useful to improve oral intake and nutrition in patients with malignant strictures who are scheduled for surgical resection. In our experience, dilation of malignant strictures is safe when carefully performed.
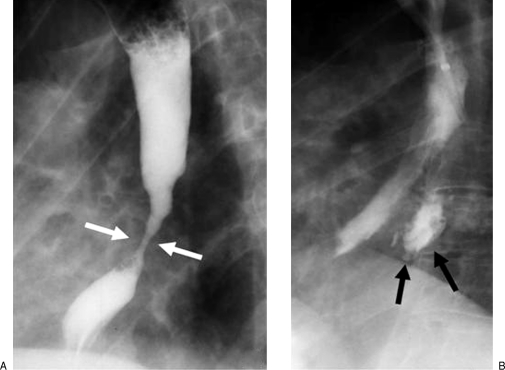
Figure 3.
A 68-year-old man with esophageal carcinoma. (A) Preprocedural esophagogram demonstrates a long irregular stricture in the midthoracic esophagus with shouldered margins caused by the tumor (arrows). (B) Under careful fluoroscopic control, the balloon is inflated to its maximum diameter of 20 mm. (C) Postprocedural esophagogram shows marked improvement of the stricture with an area of residual narrowing at the proximal end of the tumor (between arrows). There is no evidence of perforation.
Anastomotic Strictures
Stricture formation at an esophageal anastomosis is a common postoperative complication that requires treatment if it leads to obstruction or dysphagia. It may be difficult to pass a guide wire across a strictured anastomosis because the stricture is usually less tapered than a benign inflammatory stricture. Furthermore, when the esophageal wall is deformed at the anastomosis, the orientation of the stricture may be at an angle with respect to the longitudinal axis of the esophagus. This is especially the case when an end-to-side anastomosis was created. In such instances, the use of an angled-tip catheter may be helpful to direct the guide wire through the anastomosis. For dilating anastomotic strictures, the choice of balloon size is based on two factors: the diameter of the anastomosis originally created during surgery, if known, and the diameter of the normal lumen of the gut above and below the stricture as determined from a predilation esophagogram (Fig. 4). In practice, however, a tight stricture may prevent distention of the normal gut distal to the narrowing, and in these cases, it may be impossible to measure the true diameter of the distal esophagus. We usually base our choice of maximum balloon diameter on our estimation of the desirable anastomotic diameter to be restored. To minimize the risk of perforation, care must be taken not to dilate to a greater diameter than the original size of the surgically created anastomotic opening.
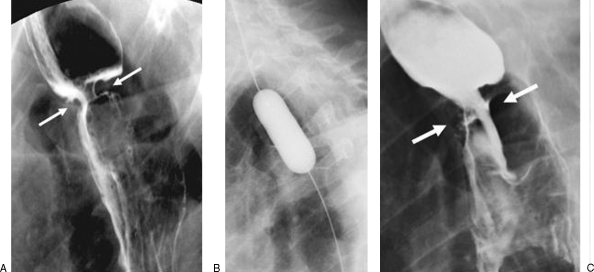
Figure 4.
A 74-year-old man with a stricture of a gastroesophageal anastomosis (following esophagectomy and gastric pull-up) causing dysphagia for solid food. (A) Preprocedural esophagogram showing a relatively tight stricture at the anastomotic site (between arrows). (B) Image showing a fully inflated 20-mm-diameter balloon crossing the anastomosis. (C) The postprocedural esophagogram shows no evidence of a perforation. Note that there appears to be relatively little improvement of the anastomotic diameter (between arrows) compared with the preprocedural esophagogram. However, clinically there was marked improvement of the patient's ability to swallow solid food.
Achalasia
Balloon dilation of the lower esophageal sphincter in patients with achalasia is performed with a large pneumatic balloon (30-mm diameter or greater). This large-diameter balloon is used to cause a controlled rupture of circular muscle fibers composing the sphincter. We typically start with a 30-mm-diameter balloon and, if necessary, may follow with a 35-mm-diameter balloon at a future date. The diameter of the balloon has not been proven to affect outcome based on symptom score or objective responses.2,3,4 However, it has been suggested that repeat dilation with progressively larger balloons increases the likelihood of long remissions.5
Esophageal Webs
Esophageal webs are folds of mucosa and submucosa that result in a fixed, shelflike structure on esophagography. They most commonly occur in the cervical esophagus and can cause difficulty in swallowing and the feeling of food getting stuck in the throat. To make the dilating procedure as comfortable as possible for the patient, a shorter balloon with a length of 3 to 5 cm and diameter of 18 to 20 mm is recommended. A longer balloon may press on the cricopharyngeus muscle during inflation, triggering the patient's urge to swallow and causing displacement of the balloon. After positioning the balloon over the web, it is inflated quickly to maximum diameter to disrupt the tissue, after which it is quickly deflated to relieve the pressure on the trachea (Fig. 5). Fluoroscopically, one can see an abrupt disappearance of the “waist” on the balloon when the web tears, and the patient may feel the sensation of a “snap.” It is important to have good sedation when dilating a web, as the patient may otherwise feel uncomfortable from having the expanded balloon deep in the throat pressing on the trachea. Many patients seem to tolerate the procedure better when asked to hold their breath for ∼15 seconds during the time of balloon inflation and deflation. In the majority of cases, only one dilation is necessary for tearing the web and typically, little or no residual stricture is visible on the postprocedural esophagogram. Patients usually experience prompt relief of the symptoms, and repeat dilations are rarely needed as the webs are unlikely to recur.6
Figure 5.
An 80-year-old woman with an esophageal web causing marked narrowing of the cervical esophagus. (A) Preprocedural esophagogram showing the web (arrow). (B) The web was dilated with a 20-mm-diameter, 5.5-cm-long balloon that was inflated three times to a maximum of 6 atm. (C) Postprocedural esophagogram shows marked improvement of the esophageal lumen at site of the web.
Nissen Fundoplication
A Nissen fundoplication is usually performed for treatment of severe gastroesophageal reflux. After surgery, some patients experience significant dysphagia7; this may be caused by a tight fundal wrap, the diaphragmatic hiatus may be too narrow, or there may be fibrosis from reflux esophagitis that was present preoperatively. The balloon dilation technique for Nissen fundoplication is identical to that used for other benign esophageal strictures. If the patient's dysphagia does not respond to dilation with a 20-mm-diameter balloon, we may perform a follow-up dilation with a 30-mm pneumatic balloon, which is used for the treatment of achalasia. Efficacy of a balloon dilation procedure in patients with Nissen fundoplication has not yet been fully documented in the literature. However, in our experience, many patients have marked improvement of dysphagia, and it seems to be a worthwhile therapy to consider prior to the alternative of surgical revision.
Anastomotic Leaks
In our practice, esophageal resection is most commonly associated with gastric pull-up and esophagogastrostomy or colonic interposition. Breakdown of the gastroesophageal anastomosis occurs in 6 to 33% of cases.8,9 If present, a disrupted suture or staple line becomes evident indirectly shortly after surgery from the appearance of esophageal secretions draining through the surgical wound in the patient's neck. Without treatment, such leak may persist for weeks or months. It is believed that narrowing of the anastomosis caused by postsurgical reactive tissue contributes to the maintenance of the leak, forcing the secretions to escape through the disrupted suture/staple line as the path of least resistance rather than through the anastomosis itself.10 In our experience, balloon dilation of a leaking anastomosis greatly accelerates healing, with the leak usually closing within a few days. This is because the procedure widens the anastomosis, thus facilitating the flow of secretions into the distal gut instead of through the disrupted suture/staple line (Fig. 6). The balloon dilation technique for a leaking anastomosis is similar to that described earlier for the general technique. The diameter of the balloon used for dilation should correspond to the diameter of the anastomosis created at surgery. In our experience, dilating a leaking anastomosis does not cause further disruption of the structures, provided that the appropriate balloon diameter is chosen.
Figure 6.
Schematic drawing of balloon dilation of anastomotic leak. (A) When there is a leak at the anastomotic suture or staple line, esophageal secretions are forced to escape chiefly through the leak (large curved arrow) as flow into the distal gut is hampered by the narrowing of the anastomosis. (B) Balloon dilation of the anastomosis increases its diameter, thus (C) facilitating the flow of secretions into the distal gut and promoting closure and healing of the anastomotic leak. (Reprinted with permission from de Lange EE, Shaffer HA Jr, Holt PD. Esophagoenteric anastomotic leaks: treatment with fluoroscopically guided balloon dilatation. AJR Am J Roentgenol 1994;162:51–54, figure 2.)
COMPLICATIONS
The risk of a perforation occurring during fluoroscopically controlled esophageal balloon dilation is very low. A review of our first 1157 consecutive procedures performed between 1983 and 1999 showed 17 complications that were directly related to the procedure itself, giving an overall complication rate of 1.4%. Low complication rates have also been reported by others performing fluoroscopically guided balloon dilation for esophageal strictures. Weintraub and Eubig reported a single perforation in 272 procedures, giving a complication rate of less than 1%.11 Said et al reported a 1.7% rupture rate in 115 procedures performed for anastomotic strictures after surgical repair of esophageal atresia.12 No complications were reported by Azizkhan et al in 92 procedures performed for strictures secondary to recessive dystrophic epidermolysis bullosa.13
Low rates of complications have also been reported for endoscopically performed balloon dilation. Piotet et al had a complication rate of less than 1% in over 1800 procedures for esophageal strictures14; Adamek et al had 1.7% in 101115; Fry et al had 2.2% in 36516; Drábek et al had 2.2% in 9017; and Chan et al had 4.5% in 66 patients.18 However, with endoscopically performed balloon dilations, the actual number of tears may be higher than reported because a postprocedural esophagogram is typically not routinely obtained after an endoscopic procedure; thus, asymptomatic intramural lacerations and contained perforations may have gone undetected.
An intramural laceration is seen on a postprocedural esophagogram as an extraluminal collection of contrast material confined within the esophageal wall (Fig. 7). Most intramural tears are managed conservatively with short-term dietary restriction to liquids and soft solids and, if needed, placement of a nasogastric tube. In these instances, the patient returns to a regular diet within a few days if no symptoms develop, and further follow-up is typically not needed.
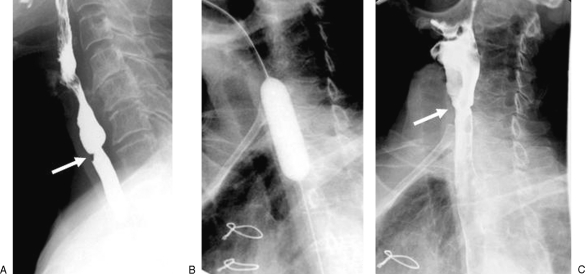
Figure 7.
A 59-year-old man with tight stricture of the cervical esophagus following laryngectomy. (A) Preprocedural esophagogram showing the stricture in the cervical esophagus. (B) The stricture was dilated with a 20-mm-diameter balloon. There is a waist corresponding to the stricture site (arrow). (C) An small intramural tear is demonstrated on the postprocedural esophagogram as a collection of extraluminal contrast material confined within the esophageal wall (arrow).
When a transmural perforation occurs during balloon inflation, there is an abrupt disappearance of the “waist” on the balloon and a rapid drop of the balloon pressure. In addition, the patient may experience a sudden, severe pain in the region of the stricture. When a transmural perforation is suspected, one should immediately evaluate for a leak by deflating the balloon, removing the guide wire, withdrawing the balloon catheter tip to a position just above the stricture, and injecting a water-soluble contrast agent through the guide wire lumen of the catheter (Fig. 8). If a perforation is detected, it is recommended to place the wire again through the main lumen of the balloon catheter and position it at a point in the gut beyond the stricture so as not to lose access and to facilitate the advancement of an orogastric catheter for decompression of the stomach. If no perforation is detected when injecting water-soluble contrast agent through the guide wire lumen of the balloon catheter, one should perform a more detailed esophagogram with a dilute barium suspension after the patient is fully recovered from sedation, because small tears may go undetected on the limited examination performed using the balloon catheter. The acute disappearance of the “waist” on the balloon does not always indicate a perforation; it can also occur with rapid tearing of the fibrous tissue that causes the stricture. In this circumstance, however, the wall of the esophagus remains intact and there is no transmural perforation.
Figure 8.
A 22-year-old woman with severe reflux stricture of the distal thoracic esophagus. (A) Preprocedural esophagogram showing marked luminal narrowing in the distal thoracic esophagus. (B) Following fluoroscopically guided balloon dilation, an extraluminal collection of contrast material in the mediastinum (arrows) was demonstrated on the postprocedural esophagogram, indicating the occurrence of a transmural perforation. The patient was managed nonsurgically. Esophagography performed 1 week later demonstrated complete healing of the leak.
A transmural perforation may need immediate surgical repair. However, in our experience, most transmural perforations can be treated medically, particularly if they are small. Other important considerations for medical treatment rather than surgery include that the patient is clinically stable during the balloon procedure and thereafter; the perforation is detected at the time of the occurrence and thus before the development of mediastinal contamination; and the disruption is contained within the mediastinum. In these instances, nonsurgical treatment consists of nothing by mouth, intravenous hydration, broad-spectrum antibiotics, and comfort measures.19
A potentially significant long-term consequence of dilating esophageal strictures, especially those in the region of the lower esophageal sphincter and gastroesophageal junction, is that the improved luminal diameter obtained by the procedure may actually increase the risk of gastroesophageal reflux. As a result, acute esophagitis may develop caused by the reflux, and the stricture may recur. It is therefore important after balloon dilation to consider providing prophylactic long-term acid-reducing medication, such as a proton pump inhibitor or H2 blocker, to reduce gastric acid production and esophageal reflux.
DISCUSSION
Fluoroscopically guided balloon dilation provides a safe and effective treatment for several esophageal conditions. The procedure should be modified to fit the pathology being treated to achieve optimal results. Complications rarely occur and are readily detected, and most can be treated nonsurgically.
Fluoroscopically guided balloon dilation offers several advantages over blindly performed bougienage and endoscopically guided procedures. The first and most notable advantage is that fluoroscopically guided balloon dilation allows for better visual control during placement of the guide wire and balloon catheter as both the stricture and the structures distal to it are visualized on the monitor of the fluoroscopic equipment. Fluoroscopy is not used during endoscopy, and as a result, the operator has no control over distal aspect of the balloon catheter and the esophagus beyond the stricture because these structures are obscured by the balloon after it is advanced into the stricture opening. Second, when a perforation occurs during fluoroscopically guided balloon dilation, it is detected promptly on the postprocedural esophagogram, a study that is usually not performed routinely after bougienage or endoscopically performed dilation. Early detection of a perforation is essential as it allows for immediate implementation of treatment before contamination and subsequent infection occurs.
The success rate of fluoroscopically guided balloon dilation reported in the literature ranges from 83 to 100%, although the techniques and patient populations vary.20,21,22,23,24 In our experience, the majority of patients experience clinical improvement with one procedure; however, in a substantial number of patients the symptoms may recur and repeat balloon dilation is needed. A few patients may require several repeat procedures for recurrent symptoms, and some may elect to have surgery. Nevertheless, fluoroscopically guided balloon dilation is a safe and effective procedure that can play an important role in the treatment of strictures and other conditions of the esophagus.
REFERENCES
- Baron T H. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med. 2001;344:1681–1687. doi: 10.1056/NEJM200105313442206. [DOI] [PubMed] [Google Scholar]
- Kadakia S C, Wong R K. Graded pneumatic dilation using Rigiflex achalasia dilators in patients with primary esophageal achalasia. Am J Gastroenterol. 1993;88:34–38. [PubMed] [Google Scholar]
- Wong R K. Pneumatic dilation for achalasia. Am J Gastroenterol. 2004;99:578–580. doi: 10.1111/j.1572-0241.2004.04117.x. [DOI] [PubMed] [Google Scholar]
- Zerbib F, Thétiot V, Richy F, Benajah D A, Message L, Lamouliatte H. Repeated pneumatic dilations as long-term maintenance therapy for esophageal achalasia. Am J Gastroenterol. 2006;101:692–697. doi: 10.1111/j.1572-0241.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- Mikaeli J, Bishehsari F, Montazeri G, Yaghoobi M, Malekzadeh R. Pneumatic balloon dilatation in achalasia: a prospective comparison of safety and efficacy with different balloon diameters. Aliment Pharmacol Ther. 2004;20:431–436. doi: 10.1111/j.1365-2036.2004.02080.x. [DOI] [PubMed] [Google Scholar]
- Huynh P T, de Lange E E, Shaffer H A., Jr Symptomatic webs of the upper esophagus: treatment with fluoroscopically guided balloon dilation. Radiology. 1995;196:789–792. doi: 10.1148/radiology.196.3.7644644. [DOI] [PubMed] [Google Scholar]
- Funch-Jensen P, Jacobsen B. Dysphagia after laparoscopic Nissen fundoplication. Scand J Gastroenterol. 2007;42:428–431. doi: 10.1080/00365520600955120. [DOI] [PubMed] [Google Scholar]
- Orringer M B, Orringer J S. Esophagectomy: definitive treatment for esophageal neuromotor dysfunction. Ann Thorac Surg. 1982;34:237–248. doi: 10.1016/s0003-4975(10)62492-7. [DOI] [PubMed] [Google Scholar]
- Orringer M B, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg. 1978;76:643–654. [PubMed] [Google Scholar]
- de Lange E E, Shaffer H A, Jr, Holt P D. Esophagoenteric anastomotic leaks: treatment with fluoroscopically guided balloon dilatation. AJR Am J Roentgenol. 1994;162:51–54. doi: 10.2214/ajr.162.1.8273689. [DOI] [PubMed] [Google Scholar]
- Weintraub J L, Eubig J. Balloon catheter dilatation of benign esophageal strictures in children. J Vasc Interv Radiol. 2006;17:831–835. doi: 10.1097/01.RVI.0000217964.55623.19. [DOI] [PubMed] [Google Scholar]
- Said M, Mekki M, Golli M, et al. Balloon dilatation of anastomotic strictures secondary to surgical repair of oesophageal atresia. Br J Radiol. 2003;76:26–31. doi: 10.1259/bjr/64412147. [DOI] [PubMed] [Google Scholar]
- Azizkhan R G, Stehr W, Cohen A P, et al. Esophageal strictures in children with recessive dystrophic epidermolysis bullosa: an 11-year experience with fluoroscopically guided balloon dilatation. J Pediatr Surg. 2006;41:55–60. discussion 55–60. doi: 10.1016/j.jpedsurg.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Piotet E, Escher A, Monnier P. Esophageal and pharyngeal strictures: report on 1,862 endoscopic dilatations using the Savary-Gilliard technique. Eur Arch Otorhinolaryngol. 2008;265:357–364. doi: 10.1007/s00405-007-0456-0. [DOI] [PubMed] [Google Scholar]
- Adamek H E, Jakobs R, Dorlars D, Martin W R, Krömer M U, Riemann J F. Management of esophageal perforations after therapeutic upper gastrointestinal endoscopy. Scand J Gastroenterol. 1997;32:411–414. doi: 10.3109/00365529709025073. [DOI] [PubMed] [Google Scholar]
- Fry L C, Mönkemüller K, Neumann H, Schulz H U, Malfertheiner P. Incidence, clinical management and outcomes of esophageal perforations after endoscopic dilatation. Z Gastroenterol. 2007;45:1180–1184. doi: 10.1055/s-2007-963558. [DOI] [PubMed] [Google Scholar]
- Drábek J, Keil R, Námesný I. The endoscopic treatment of benign esophageal strictures by balloon dilatation. Dis Esophagus. 1999;12:28–29. doi: 10.1046/j.1442-2050.1999.00018.x. [DOI] [PubMed] [Google Scholar]
- Chan K C, Wong S K, Lee D W, et al. Short-term and long-term results of endoscopic balloon dilation for achalasia: 12 years' experience. Endoscopy. 2004;36:690–694. doi: 10.1055/s-2004-825659. [DOI] [PubMed] [Google Scholar]
- Shaffer H A, Jr, Valenzuela G, Mittal R K. Esophageal perforation. A reassessment of the criteria for choosing medical or surgical therapy. Arch Intern Med. 1992;152:757–761. doi: 10.1001/archinte.152.4.757. [DOI] [PubMed] [Google Scholar]
- Inagake M, Yamane T, Kitao Y, et al. Balloon dilatation for anastomotic stricture after upper gastro-intestinal surgery. World J Surg. 1992;16:541–544. doi: 10.1007/BF02104467. [DOI] [PubMed] [Google Scholar]
- de Lange E E, Shaffer H A., Jr Anastomotic strictures of the upper gastrointestinal tract: results of balloon dilation. Radiology. 1988;167:45–50. doi: 10.1148/radiology.167.1.3347744. [DOI] [PubMed] [Google Scholar]
- Whitworth P W, Richardson R L, Larson G M. Balloon dilatation of anastomotic strictures. Arch Surg. 1988;123:759–762. doi: 10.1001/archsurg.1988.01400300105018. [DOI] [PubMed] [Google Scholar]
- Lindor K D, Ott B J, Hughes R W., Jr Balloon dilatation of upper digestive tract strictures. Gastroenterology. 1985;89:545–548. doi: 10.1016/0016-5085(85)90449-4. [DOI] [PubMed] [Google Scholar]
- Cho Y K, Shin J H, Kim B S, et al. Fluoroscopically guided balloon dilation of anastomotic strictures after total gastrectomy: long-term results. AJR Am J Roentgenol. 2007;188:647–651. doi: 10.2214/AJR.05.1291. [DOI] [PubMed] [Google Scholar]