Hyperkalemia is a common problem in patients with acute or chronic renal failure as the kidneys are typically responsible for 80 to 90% of daily potassium excretion.1 Those presenting for declotting procedures of dialysis access shunts are at elevated risk of hyperkalemia as they often have recently received incomplete dialysis.2 Noncardiac symptoms of hyperkalemia are usually subtle and may include fatigue, paresthesias, and skeletal muscle weakness.3 However, due to its effects on the cellular membrane resting potential, elevated serum potassium can lead to several cardiac arrhythmias, ranging from benign to ventricular fibrillation and asystole.4 Although not commonly associated with hyperkalemia, dialysis patients with elevated serum potassium may develop bradycardia,5 which as seen in this case can occur during a declotting procedure and is a potentially life-threatening sequela.
CASE REPORT
A 72-year-old woman with a past medical history significant for end-stage renal disease had been on hemodialysis for 8 years prior to presentation. She also had a history of rheumatic heart disease as well as significant coronary artery disease for which she underwent coronary artery bypass grafting of three vessels and replacement of the mitral and aortic valves with bioprosthetic valves 6 months prior to presentation. Two years prior to presentation, the patient had a left forearm loop arteriovenous (AV) graft placed after a previous brachiocephalic AV fistula thrombosed due to outflow stenosis.
On the day of admission, the patient had not been able to be dialyzed for 1 week due to AV graft thrombosis, and she presented for a percutaneous declotting procedure of her dialysis graft. She was anesthetized locally with 1% lidocaine and also received conscious sedation with midazolam and fentanyl. Venous access was obtained using a micropuncture set, and a 7-mm angioplasty balloon catheter was advanced over a J-wire to the axillary vein. A central venogram revealed no stenoses in the subclavian vein, brachiocephalic vein, or superior vena cava. The graft was then accessed toward the arterial side using a micropuncture set and a 6-mm angioplasty balloon catheter was inserted. Using the catheter directed toward the venous side, 5000 U of heparin was administered and the 7-mm angioplasty balloon catheter was withdrawn into the graft where 1.5 mg of tissue plasminogen activator was laced.
The patient immediately became bradycardic with a heart rate of 39 and hypotensive with a blood pressure of 92/52, which persisted after she received a total dose of 0.4 mg of naloxone and 0.5 mg of flumazenil. After the patient's heart rate declined to 27, 1 mg of atropine was given and the code team was called. At this point, all forearm catheters were removed and a left femoral vein vascular sheath was placed. A stat serum potassium at this time was found to be 6.5 mEq/L. The patient received 10 U of regular insulin as well as 1 ampoule of 50% dextrose and 2 ampoules of calcium chloride. The patient also received 1 mg of epinephrine and another 1-mg dose of atropine. The patient's rhythm changed to pulseless ventricular tachycardia, and defibrillation was attempted once at 50 J followed by two attempts at 150 J. The patient also received a 300-mg bolus of amiodarone and multiple rounds of cardiopulmonary resuscitation were performed. The patient's pulse returned, and she was transferred to the emergency department and subsequently to the cardiac catheterization laboratory. A temporary pacemaker was placed through the left subclavian vein, and the patient's vascular sheath in her left femoral vein was exchanged for a 13.5-French temporary dialysis catheter. The patient was then transferred to the cardiac intensive care unit, at which point her serum potassium had risen to 8.7 mEq/L for which she underwent emergent hemodialysis through a temporary catheter. Following dialysis, her serum potassium declined to 3.4 mEq/L, and the day following admission her temporary pacemaker was removed.
Eight days after admission, the patient was brought back to the interventional radiology suite where she was again anesthetized with 1% lidocaine locally as well as intravenous fentanyl and midazolam. She underwent a declotting procedure without complication. After the patient was dialyzed using the graft, she was discharged from the hospital.
Two months after discharge, a right elbow AV fistula was placed. Seven months after discharge, the patient presented to the interventional radiology suite for a fistulogram of the immature right AV fistula and another declotting procedure of the left AV graft. At the last dialysis session prior to her presentation, she had received only 30 minutes of hemodialysis before an electrical outage occurred; once power returned, her graft had clotted.
The patient was again anesthetized with 1% lidocaine as well as fentanyl and midazolam. The fistulogram was attempted first, but an ultrasound of the right arm revealed a very short and curved segment of the outflow vein that appeared to dive into the deep portion of the arm with a palpable thrill only detectable in a 2-cm segment. Neither this vein nor an even smaller and more superficial adjacent vein could be cannulated, making it impossible to perform the fistulogram. Prior to preparing the left arm for the declotting procedure, the patient was noted to be hypotensive and bradycardic with altered mental status. The code blue team was called, and the patient received 1 mg of atropine, 10 U of regular insulin, and one ampoule of 50% dextrose, calcium chloride, and sodium bicarbonate. A 13.5-French temporary dialysis access line was placed in the right femoral vein. The patient never lost consciousness, and she was immediately transferred to the emergency department. Her serum potassium was found to be 7.5 mEq/L and she received emergent dialysis. Her potassium declined to 4.1 mEq/L, and 3 days after admission the patient was able to undergo a successful repeat left AV graft declotting procedure, after which she was discharged from the hospital.
DISCUSSION
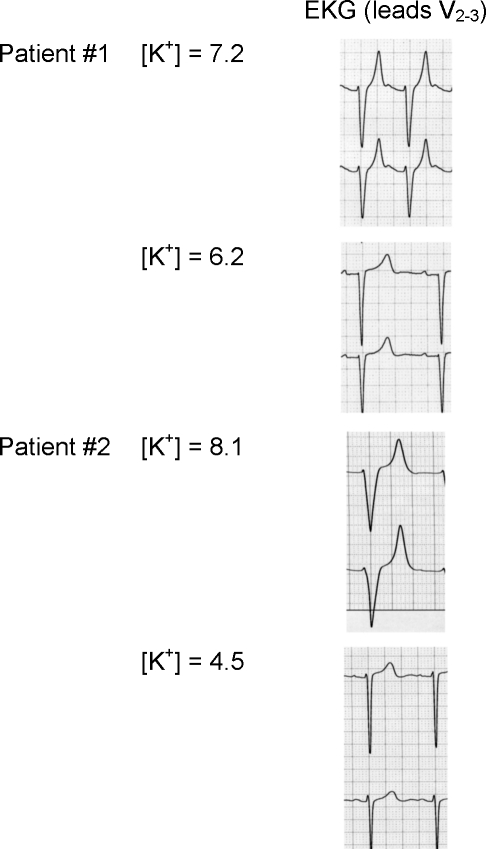
As illustrated, patients with elevated serum potassium may have few subjective complaints, but the cardiac manifestations of hyperkalemia may be life-threatening. At the cellular level, the membrane resting potential depends primarily on the ratio of intracellular to extracellular potassium, and therefore small disturbances in potassium homeostasis can result in large effects on electrically active cells.1 In the myocardium, hyperkalemia depresses electrical conduction velocity but increases the rate of repolarization, leading to peaking of the T waves, prolongation of the PR interval, flattening or absence of the P wave, widening of the QRS complex, and even merging of the QRS complex with the T wave causing a sine-wave pattern (Fig. 1).4
Figure 1.
Two representative patients with serum potassium levels (in mEq/L) and associated electrocardiograms (EKGs). In both patients, observed hyperkalemic EKG changes include peaking of the T waves, QRS widening, lengthening of the PR interval, and flattening of the P wave. Note in patient No. 1 that EKG changes resolve despite persistent elevated serum potassium.
Bradycardia is a less commonly recognized cardiac sequela of hyperkalemia. In one study of 12 patients with hyperkalemia and fascicular block, only one exhibited bradycardia.6 Abe et al described a series of seven patients with chronic or end-stage renal failure who presented with profound bradycardia.5 Interestingly, four of the seven patients had ischemic heart disease, as did our patient.
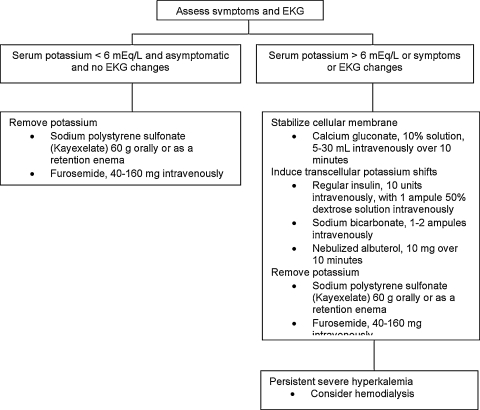
Aggressive treatment of hyperkalemia is indicated in those who are symptomatic or have electrocardiogram (EKG) changes consistent with hyperkalemia (Fig. 2). Furthermore, as there is an unpredictable correlation between serum potassium concentration and EKG changes, treatment is indicated in all patients with serum potassium concentrations above 6 mEq/L.3,7 There are three main aspects to the treatment of hyperkalemia: inducing the cellular uptake of potassium, counteracting the arrhythmogenic effects of hyperkalemia, and removing potassium from the body. Potassium may be driven into cells using insulin, bicarbonate, or nebulized albuterol, and its cardiac effects are blunted using intravenous calcium. The removal of potassium from the body may be achieved through the kidney using a loop diuretic, the gut using a cation exchange resin, or through hemodialysis.3
Figure 2.
Brief management algorithm for hyperkalemia. EKG, electrocardiogram.
Little evidence exists as to the benefit or cost-effectiveness of routinely preoperatively evaluating serum electrolytes in patients undergoing percutaneous declotting procedures. The American College of Radiology practice guideline for adult sedation/analgesia states that before moderate sedation, “relevant laboratory values, when appropriate, should be available for review,” and the practice guideline for diagnostic arteriography states, “[l]aboratory evaluation may be indicated, including . . . electrolytes.”8,9 Although the quality improvement guidelines on the percutaneous management of thrombosed or dysfunctional dialysis access lists severe hyperkalemia as a relative contraindication to intervention, the practice guideline regarding the same subject does not address the preoperative evaluation of serum electrolytes.10,11
However, patients presenting for declotting procedures have multiple risk factors for hyperkalemia and malignant arrhythmias. As seen in our patient, those in need of declotting procedures have often missed recent dialysis sessions, and even patients who have not missed recent sessions may have predialysis serum potassium levels of 5.0 mEq/L to 6.5 mEq/L.12 These patients are often sedated with fentanyl, which may cause bradycardia due to stimulation of the central vagal nucleus.13,14 These factors combined with the high prevalence of underlying cardiac pathology in the dialysis population15 increase these patients' risk of periprocedural arrhythmias. Some experts recommend that if the patient is hyperkalemic, the decision whether to perform a declotting procedure or acute dialysis through a temporary line should be made jointly between the patient's nephrologist and interventionalist,12,16 and acute dialysis should be strongly considered in cases of severe hyperkalemia.2 Even if acute dialysis is not performed, the above risks may indicate that in selected cases preprocedural evaluation of serum potassium and an EKG may be beneficial.
ACKNOWLEDGMENTS
Special thanks to Dr. Rory Childers for assistance with EKGs.
REFERENCES
- Weiner I D, Wingo C S. Hyperkalemia: a potential silent killer. J Am Soc Nephrol. 1998;9:1535–1543. doi: 10.1681/ASN.V981535. [DOI] [PubMed] [Google Scholar]
- Hammes M S. Medical complications in hemodialysis patients requiring vascular access radiology procedures. Semin Intervent Radiol. 2004;21:105–110. doi: 10.1055/s-2004-833683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H J, Han S W. Therapeutic approach to hyperkalemia. Nephron. 2002;92(Suppl 1):33–40. doi: 10.1159/000065375. [DOI] [PubMed] [Google Scholar]
- Mattu A, Brady W J, Robinson D A. Electrocardiographic manifestations of hyperkalemia. Am J Emerg Med. 2000;18:721–729. doi: 10.1053/ajem.2000.7344. [DOI] [PubMed] [Google Scholar]
- Abe R, Yonemura K, Takahashi T, Watanabe H, Sano K, Hishida A. Marked bradycardia associated with profound hyperkalemia in patients with end-stage renal disease. Nephron. 1998;80:355–356. doi: 10.1159/000045200. [DOI] [PubMed] [Google Scholar]
- Bashour T, Hsu I, Gorfinkel H J, Wickramesekaran R, Rios J C. Atrioventricular and intraventricular conduction in hyperkalemia. Am J Cardiol. 1975;35:199–203. doi: 10.1016/0002-9149(75)90001-6. [DOI] [PubMed] [Google Scholar]
- Martinez-Vea A, Bardají A, Garcia C, Oliver J A. Severe hyperkalemia with minimal electrocardiographic manifestations: a report of seven cases. J Electrocardiol. 1999;32:45–49. doi: 10.1016/s0022-0736(99)90020-1. [DOI] [PubMed] [Google Scholar]
- Towbin R B. American College of Radiology Practice Guideline for Adult Sedation/Analgesia. 2005. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/RadSafety/OtherSafetyTopics/adultsedation.aspx. Accessed April 13, 2010. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/RadSafety/OtherSafetyTopics/adultsedation.aspx
- Singh H. American College of Radiology Practice Guideline for Diagnostic Arteriography. 2007. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/iv/diagnostic_arteriography.aspx. Accessed April 13, 2010. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/iv/diagnostic_arteriography.aspx
- Gray R J. American College of Radiology Practice Guideline for Endovascular Management of the Thrombosed or Dysfunctional Dialysis Access. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/iv/mgmt_dialysisaccess.aspx. Accessed April 13, 2010. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/iv/mgmt_dialysisaccess.aspx
- Aruny J E, Lewis C A, Cardella J F, et al. Society of Interventional Radiology Standards of Practice Committee Quality improvement guidelines for percutaneous management of the thrombosed or dysfunctional dialysis access. J Vasc Interv Radiol. 2003;14(9 Pt 2):S247–S253. [PubMed] [Google Scholar]
- Scheel P J. In: Savader SJ, Trerotola SO, editor. Venous Interventional Radiology with Clinical Perspectives. 2nd ed. New York: Thieme; 2000. Vascular access for hemodialysis: a nephrologist's perspective. p. 321.
- Bowdle T A. Adverse effects of opioid agonists and agonist-antagonists in anaesthesia. Drug Saf. 1998;19:173–189. doi: 10.2165/00002018-199819030-00002. [DOI] [PubMed] [Google Scholar]
- Eaton M P, Bailey P L. In: Estafanous FG, Barash PG, Reves JG, editor. Cardiac Anesthesia: Principles and Practice. Philadelphia: Lippincott Williams & Wilkins; 2001. Cardiovascular pharmacology of anesthetics. p. 299.
- Stack A G, Bloembergen W E. Prevalence and clinical correlates of coronary artery disease among new dialysis patients in the United States: a cross-sectional study. J Am Soc Nephrol. 2001;12:1516–1523. doi: 10.1681/ASN.V1271516. [DOI] [PubMed] [Google Scholar]
- Turmel-Rodrigues L, Raynaud A, Louail B, Beyssen B, Sapoval M. Manual catheter-directed aspiration and other thrombectomy techniques for declotting native fistulas for hemodialysis. J Vasc Interv Radiol. 2001;12:1365–1371. doi: 10.1016/s1051-0443(07)61691-1. [DOI] [PubMed] [Google Scholar]