ABSTRACT
Vascular malformations (VM) are classified by flow characteristics and channel content. They can involve any organ and tissue plane, and occur in focal and diffuse forms. Slow-flow vascular malformations (venous and lymphatic malformations) are typically treated by sclerotherapy, whereas fast-flow lesions (arteriovenous malformations) are managed with embolizations. Some VMs, such as VMs of the rectum or uterus, are best dealt with surgically. This review will present a summary of the conditions, their imaging features, and some useful endovascular therapeutic techniques.
Keywords: Vascular malformation, venous malformation, arteriovenous malformation, sclerotherapy, therapeutic embolizations
Vascular malformations occur everywhere in the body, including the female pelvis. They are best categorized according to the biological classification proposed by Mulliken and Glowacki in 1982.1 This classification describes the entities according to their flow characteristics (fast flow or slow flow), and their vascular channel components (capillary, venous, lymphatic, arterial, and combined). Each type of malformation is distinguished by its own clinical and imaging features. In general, vascular malformations can be diagnosed by physical examination when the skin is involved, or when increased pulsatility, bruit, or thrill can be detected.2 Ultrasonography with color Doppler interrogation is useful to detect vascular malformations of the pelvis, but determination of the nature and extent of a deep vascular malformation is best made using magnetic resonance imaging (MRI).3,4,5,6,7,8 In centers specializing in the care of patients with vascular malformations, endovascular therapeutic techniques are often the primary treatment modality.
VENOUS MALFORMATIONS
Venous malformation (VM) is the most common symptomatic vascular malformation. Typically, these anomalies are caused by germline or somatic mutations in the TIE2 gene, which is involved in signaling between the endothelial and the mesenchymal cells during vasculogenesis and angiogenesis.9,10 These anomalous veins have endothelial cellular abnormalities and severe deficiencies of the smooth muscle layer, resulting in gradual stretching and expansion of the lumen over time. The malformed veins become distended with dependency or increased venous pressure. VMs can affect all tissue layers and can be focal, multifocal, or diffuse. The most common site involved in the female pelvis is the perineum, especially the labia majora.11 Symptoms include swelling and pain, especially during prolonged standing, walking, or exercising, and often worsen during menses or pregnancy. The skin, when involved, has a bluish-tinged color, and subcutaneous or cutaneous varices may also be evident. Although this anomaly may be limited to the labia majora, often it is part of a diffuse VM of the lower extremity, perineum, and buttock (Fig. 1).12,13
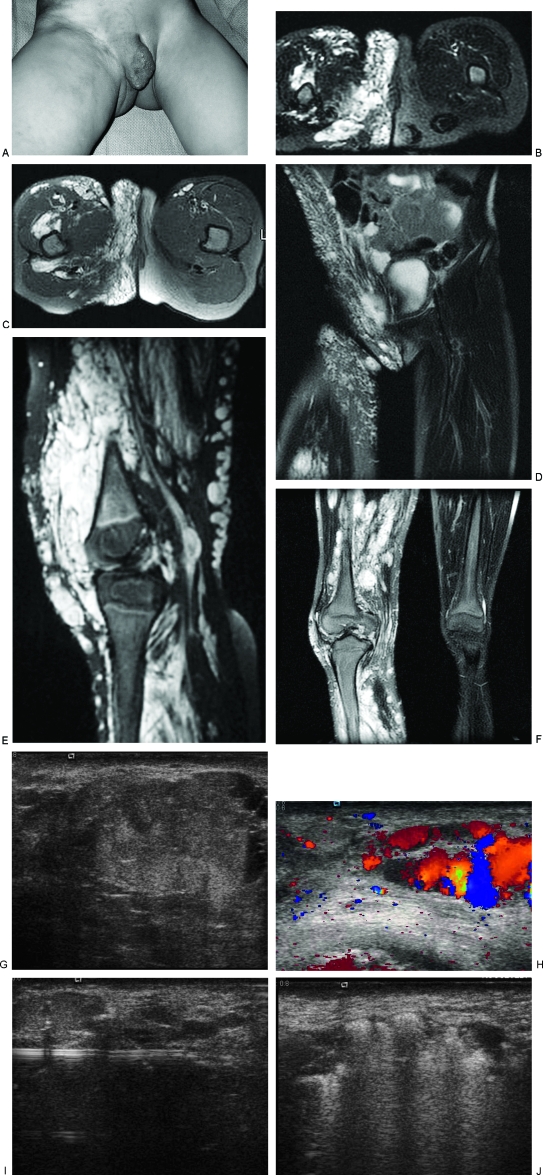
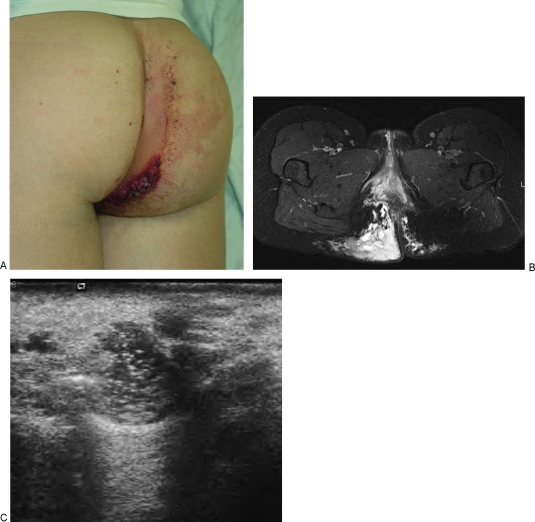
Figure 1.
Diffuse venous malformation (VM) of the right lower extremity and perineum. This young girl became symptomatic before one year of age and has undergone numerous therapeutic procedures including sclerotherapy, resection of intraarticular VM of the right knee, and contour resection of the right labia majora. (A) Clinical photograph demonstrates enlargement of the right labia majora and adjacent buttock with purplish discoloration. Note the prominent varicosities in the adjacent thigh and pelvic wall. (B) T2-weighted axial magnetic resonance image (MRI) of the perineum shows diffuse involvement of the perineum with serpiginous, T2-hyperintense venous channels. (C) T2-weighted axial MRI of the perineum one year later, after several sessions of sclerotherapy and contour resection. Although the contour is improved, there is still VM throughout the labium and buttock. (D) Coronal T2-weighted MRI, 5 years after (B), and following contour resection of the right labium majorum shows a more symmetrical perineum. Note the involvement of the wall of the right pelvis, which has also been treated. (E) Sagittal T2-weighted MRI of the right knee, prior to treatment, showing a massive VM filling the joint and surrounding tissues. (F) Coronal T2-weighted MRI of the lower extremities after sclerotherapy and excision of the intraarticular VM. Note the severe swelling of the right lower extremity compared with the left. (G) Ultrasonographic image of the labium majorum in another patient with a focal VM of the perineum. The lesion is composed of tubular spaces that are compressible. Note the echogenic contents of the abnormal venous spaces and the fluid levels. (H) Color Doppler ultrasonogram shows blood flow or movement with release of compression by the ultrasound probe. (I) Ultrasonographic image demonstrating placement of a 16-gauge angiocatheter into the abnormal venous spaces. The metal stylette has been removed and a bare laser fiber inserted through the cannula. (J) Sonographic image during endovenous laser treatment. The steam generated by the laser energy causes echogenicity within the VM.
Less commonly, the rectal wall, vagina, uterus, or bladder may be affected. Venous malformations of the rectum are frequently associated with varicosities and insufficiency of the hemorrhoidal, mesenteric, and portal veins (Fig. 2).14,15,16 In addition to the presence of rectal bleeding, which may be severe, affected patients may develop thrombosis of the hemorrhoidal veins with resultant portal vein thromboembolism and hepatic infarction. Venous malformations of the uterus and ovaries are typically associated with insufficiency of the ovarian vein and probably form a subtype of “pelvic congestion syndrome” (Fig. 3).17 Pure VM of the uterus is rare (Fig. 4). Venous malformations involving the urinary system can lead to severe problems with hematuria.15,16 Diffuse VMs of the lower extremities in conjunction with the perineum and buttock are typically associated with painful swelling, hemarthrosis, and occasionally pulmonary thromboembolism (Fig. 1). Patients with extensive VMs may develop localized intralesional coagulopathy resulting in a systemic disseminated intravascular coagulopathy, especially in association with surgery.13
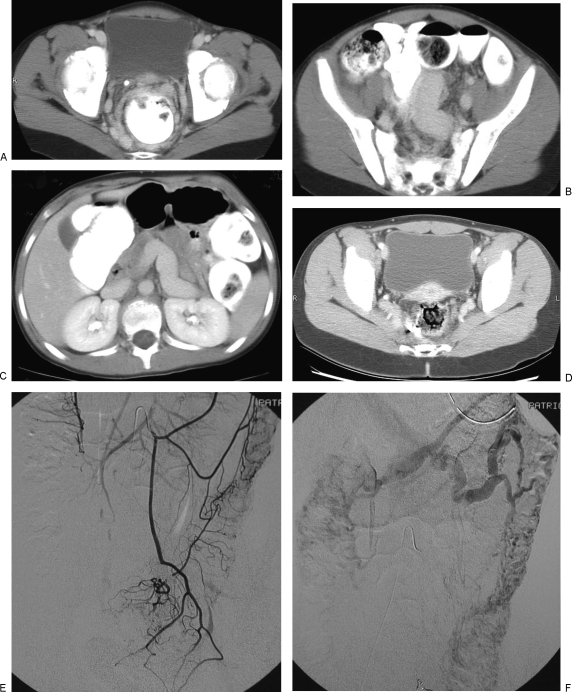
Figure 2.
Venous malformation (VM) involving the colon. This young girl presented with hematochezia. Imaging showed massive enlargement of the inferior mesenteric and hemorrhoidal veins. The inferior mesenteric vein was embolized through a transhepatic portal vein approach to decrease venous reflux into the malformation. Her bleeding diminished. However, 6 years later the patient developed acute portal vein thrombosis and required urgent endovascular recanalization. She remains anticoagulated. (A) Axial computed tomography (CT) image of the pelvis following intravenous and oral contrast administration demonstrates dilated venous channels arranged circumferentially around the rectum. Note, the phlebolith on the right anterior wall. (B,C) Axial CT images in the mid pelvis and abdomen show marked enlargement of the inferior mesenteric and portal veins. (D) Axial CT image of the pelvis 4 years after embolization shows some improvement in the venous engorgement around the rectum. (E,F) Inferior mesenteric arteriography 6 years after embolization shows a diffuse VM of the colon. Note the absence of filling of the inferior mesenteric vein.
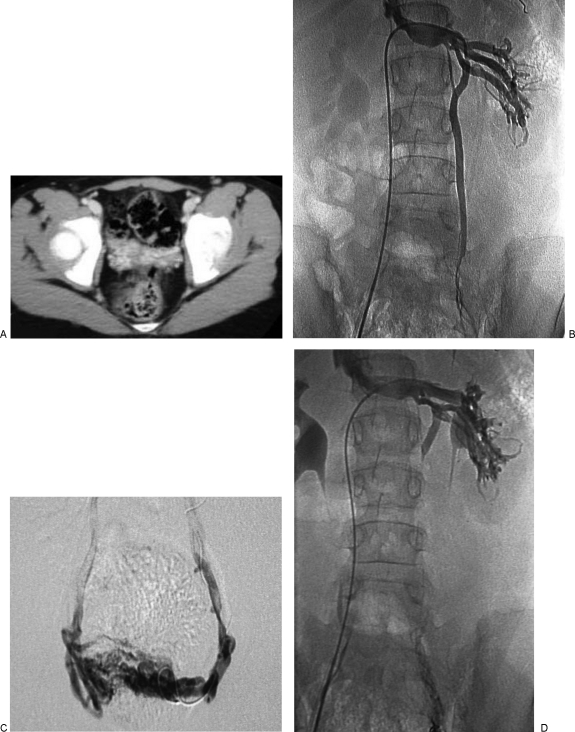
Figure 3.
Venous malformation (VM) of the uterus and ovaries in a 6-year-old girl, presenting with urinary frequency and pelvic discomfort with activities or prolonged walking. The patient's symptoms were improved after transvenous embolization of the left gonadal vein and sclerotherapy of the pelvic network. (A) Axial computed tomography (CT) image after intravenous contrast medium shows a network of venous channels in the adnexal region. (B) Left renal venogram shows insufficiency of the left gonadal vein. (C) Selective venography after placement of a microcatheter into the gonadal venous plexus. This was sclerosed with sodium tetradecyl sulfate; the gonadal vein was then embolized with coils and additional sclerosant. (D) Left renal venogram postembolization shows no further reflux.
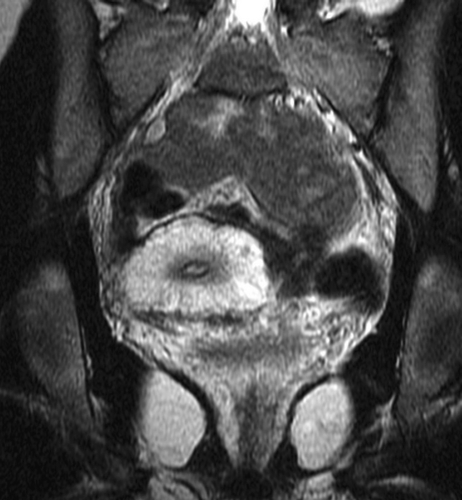
Figure 4.
Venous malformation (VM) of the uterus and vagina in a teenage girl with severe menorrhagia, partly controlled by taking Lupron (TAP Pharmaceutical Products, Inc. Lake Forest, IL). This coronal T2-weighted magnetic resonance image (MRI) of the pelvis shows diffuse enlargement of the uterus with abnormal T2 hyperintense thickening of the myometrium. Note, the continuation of the VM in the wall of the vagina and surrounding pelvic tissues. A hysterectomy was advised because it was felt that an adequate result could not be achieved by endovenous treatment.
Venous malformations are readily diagnosed by MRI. These lesions are highly hyperintense on T2-weighted images, often contain thrombi or phleboliths and enhance inhomogeneously.4 Therapeutic options include graded elastic compression garments (for the lower extremities), endovascular ablation, and resection. Patients prone to thromboembolism often require life-long anticoagulation. Inferior vena caval filters may also be necessary, although in some patients, the presence of collateral venous channels or severe dilatation of the inferior vena cava make this difficult.
ENDOVASCULAR TREATMENT OF VENOUS MALFORMATIONS OF THE PERINEUM
Direct cannulation and sclerosant injection, typically using either absolute ethanol or 3% sodium tetradecyl foam, constitutes the main endovascular approach to treatment of VMs.11,18 These sclerosing agents typically cause significant swelling; skin breakdown with subsequent infection is a risk. Endovenous laser therapy, performed by inserting a bare laser fiber directly through an intralesional cannula, can be combined with sclerosant injection. Because less sclerosant is needed, this technique results in less swelling than sclerotherapy alone. Contour resection of a grossly enlarged labium majorum is often an efficient way of reducing the bulk. All of these procedures result in good palliation, but recurrence is common (Fig. 1).
Some rectal VMs can be treated endoscopically or percutaneously by sclerotherapy (Fig. 2).19 Those that involve the rectum in a circumferential fashion are usually dealt with surgically. Resection or pull-through procedure may be needed to control bleeding.20,21
LYMPHATIC MALFORMATIONS
Lymphatic malformations (LMs) can also occur as a focal or diffuse anomaly. Pelvic LMs are composed of macrocysts (fluid collections > 2 cm diameter), microcysts (< 2 cm diameter) or a combination of both (Figs. 5 and 6). Some diffuse LMs actually consist of enlarged, incompetent lymphatic channels rather than cysts, the so-called lymphangiectatic pattern. Cutaneous involvement by these LMs consists of vesicles with or without a capillary stain. Whereas the cystic malformations cause pain and swelling, which can be intermittent in nature, the cutaneous vesicles are secondarily complicated by leakage of lymphatic fluid, blood, or chyle. Cystic lymphatic malformations are often associated with localized enlargement of the adjacent conducting veins or with persistent embryonic veins (lymphaticovenous [LVM]) (Fig. 6) or capillary-lymphaticovenous malformation (CLVM). The latter term, when it involves the adjacent limb with overgrowth, is often referred to as Klippel-Trenaunay syndrome.
Figure 5.
Predominantly microcystic lymphatic malformation (LM) of the right buttock in an 18-year-old woman with recent onset of severe pain and leakage of bloody fluid from cutaneous vesicles. Resection of intraperitoneal cysts had been performed in the neonatal period. (A) The photograph demonstrates a capillary stain on the surface of the skin and an enlarged cluster of friable vesicles responsible for the leakage of serosanguineous fluid. (B) T2-weighted axial magnetic resonance image (MRI) shows increased thickness of the subcutaneous fat of the right buttock, with diffuse increased signal representing microcystic lymphatic malformation. Note the focal fluid collections that represent lymphatic cysts or spaces. The LM extends through the ischiorectal space into the retroperitoneum. (C) Ultrasonographic image during sclerotherapy shows microbubbles in one of the focal lymphatic spaces. This patient experienced dramatic improvement in pain and drainage following sclerotherapy.
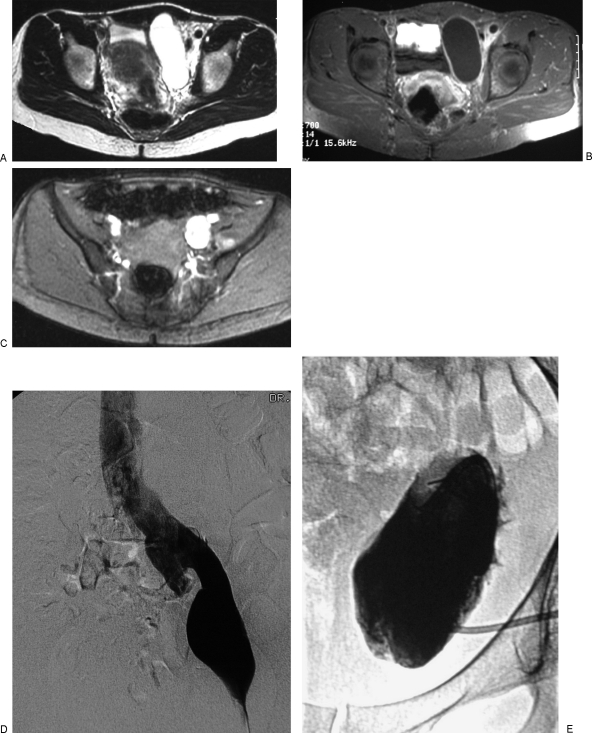
Figure 6.
Lymphaticovenous malformation (LVM) of the left pelvis in a 40-year-old woman with increasing pelvic fullness and discomfort. The symptoms improved after sclerotherapy of the lymphatic macrocysts. (A) T2-weighted axial magnetic resonance image (MRI) of the pelvis without fat suppression shows a large focal macrocyst to the left of the bladder and infiltration of the soft tissues around the iliac vessels with microcystic lymphatic malformation (LM). (B) Axial T1-weighted MRI after intravenous gadolinium and fat suppression shows enhancement of the rim of the macrocyst. (C) Gradient recalled echo image of the pelvis shows a large left iliac vein. (D) Left femoral venogram confirms the presence of dilatation of the left external iliac vein as well as the more proximal iliac veins and inferior vena cava. Dilatation of adjacent conducting veins is often seen in patients with macrocystic lymphatic malformations. (E) After placement of a pigtail catheter into the macrocyst, injection of contrast medium confirms the cystic nature of the malformation. Fluid was drained and the cyst was injected with sclerosant. Two procedures using ethanol as a sclerosant were ineffective. One treatment with OK-432 (picibanil) resulted in complete disappearance of the macrocyst.
MRI of an LM typically shows a focal or septated fluid collection in the case of macrocystic LM, or a diffuse T2 hyperintense area of tissue enlargement in a microcystic lesion.4 Unlike VM, the fluid collection does not enhance. Typically, there is rim or septal enhancement in cystic LM.
Macrocystic LMs can be treated by aspiration or drainage with injection of sclerosant, such as ethanol, doxycycline, OK-432 (picibanil), or bleomycin. Seventy-five to ninety percent of patients with a simple macrocystic LM have an excellent response to sclerotherapy, with a very low rate of recurrence, and a reduced morbidity compared with resection.18,22,23 However, microcystic or combined macrocystic–microcystic LMs demonstrate a less predictable response to treatment. Sclerotherapy or superficial laser treatment of the cutaneous vesicles can be helpful in decreasing the leakage of fluid and subsequent infection, but the results are not permanent and treatment often needs to be repeated frequently. Patients with diffuse tubular lymphatic anomalies, especially those with bone involvement (Gorham's disease) or chylous leakage fare least well in general, although some patients stop leaking, at least temporarily.24
FAST-FLOW VASCULAR MALFORMATIONS
Arteriovenous malformations (AVMs) and arteriovenous fistuli (AVF) can affect the pelvic wall and/or viscera (Fig. 7).25,26 Arteriovenous malformations typically evolve over time, changing from an asymptomatic lesion with minimal shunting to one with active shunting, venous engorgement, and venous hypertension. This evolution is often stimulated by trauma or hormonal change, such as that occurring in puberty or with pregnancy. It is fairly common for AVMs to become symptomatic during or after pregnancy. Symptoms range from mild discomfort and a feeling of pressure to significant vaginal or rectal bleeding to cardiac volume overload and congestive cardiac failure. Although most AVMs are sporadic, several mutations have been identified. These include ALK-1 and endoglin in hereditary hemorrhagic telangiectasia, RASA-1 in familial capillary malformation-AVM, and phosphatase and tensin (PTEN) in patients with Bannayan–Riley–Ruvalcaba syndrome or Cowden syndrome.27,28,29,30,31,32 Typically, an AVM consists of supplying arteries, a vascular network or “nidus” bypassing the capillary bed, and draining veins.
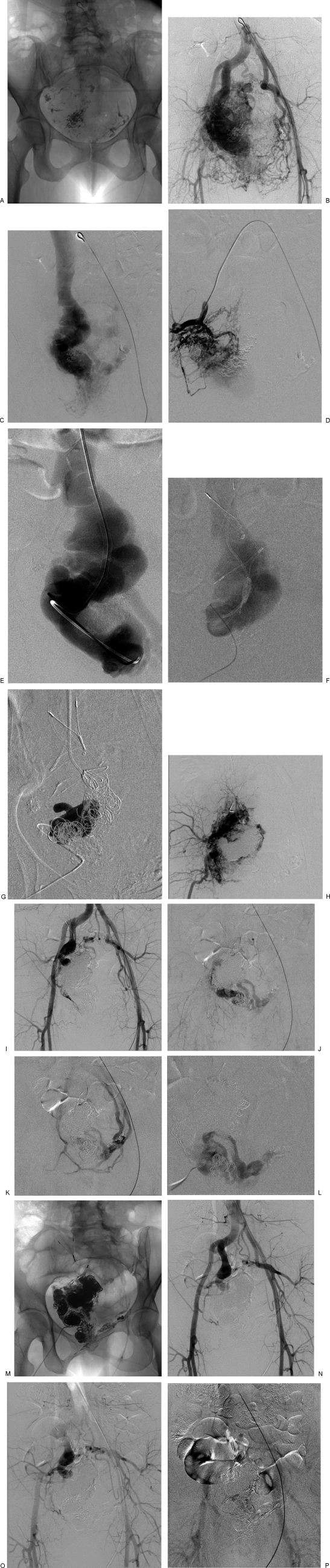
Figure 7.
Massive arteriovenous malformation (AVM) of the pelvis in a 46-year-old woman with vaginal bleeding following a cervical biopsy. The AVM was diagnosed after a pregnancy and had been embolized 6 times previously using NBCA through the arterial route. (A) Scout radiograph from an arteriogram shows radiopaque embolic material from previous arterial embolizations. (B) The abdominal aortogram shows massive flow through a right pelvic AVM. There is enlargement of all of the adjacent branches of the internal iliac arteries as well as the median sacral and superior hemorrhoidal arteries. (C) Venous drainage, however is solely into a large varix of the internal iliac vein, internal obturator vein. (D) Selective angiogram in the distal anterior division of the right internal iliac artery shows shunting through a myriad of tiny arterial branches in the wall of the varix. (E) Transvenous (via a right internal jugular vein) catheterization and venography of the draining varix shows marked dilatation and tortuosity of the right internal iliac vein. (F) Road map image of the varix obtained by injecting contrast medium through a catheter inserted percutaneously through the anterior pelvic wall into the varix. Note, the transvenous catheter is placed from above, as well as the radiopaque anchors of a bird's nest filter placed from the right internal jugular venous approach, to contain the embolic devices and prevent pulmonary embolism. (G) Road map image obtained during injection of n-butyl-2-cyanoacrylate (NBCA), after placement of numerous large Nester platinum fiber coils within the varix. (H) Right internal iliac arteriogram following transvenous embolizations. The shunt is dramatically reduced, but there is still opacification of small arteries and veins and part of the varix. (I,J,K) Aortography 2 months after transvenous embolization shows regression of most of the feeding arteries with a smaller residual shunt into the varix, now draining through the left obturator internal vein. (L) Percutaneous venogram after cannulation of the draining varix through the anterior pelvic wall confirms placement of the catheter within the residual draining varix. Hydrocoils (0.035 inch) were placed in the varix and after confirming slowing of the flow, 10 mL of absolute ethanol was injected. (M) Oblique radiograph of the pelvis at the end of the second transvenous embolization shows extensive packing of the varix with coils and NBCA. (N,O,P) Postembolization aortogram shows no further arteriovenous shunting. Late images demonstrated normal opacification of the iliofemoral veins and inferior vena cava.
Intrauterine AVMs typically arise after pregnancy or other uterine trauma.5,33,34,35,36 In many cases, hysterectomy or dilatation and curettage reveal retained products of conception, so these lesions are probably not true AVMs. Among asymptomatic women diagnosed by ultrasonography, the AVM resolves spontaneously in a high percentage, so conservative management is recommended.5 Women presenting with hemorrhage should undergo embolization. Embolization of uterine AVMs, usually through standard arterial microcatheter techniques, is usually effective in eliminating the lesion, with a very low recurrence rate.33,34,36,37,38,39 Successful pregnancy has been reported after embolization of uterine AVMs. Uterine AVM can also be seen in hereditary hemorrhagic telangiectasia.40
Many fast-flow vascular malformations are diagnosed by pelvic ultrasonography.5,33,35,36 Doppler interrogation of dilated vascular channels typically shows high velocity, low resistance flow in the feeding arteries and draining veins. MRI findings include presence of vascular flow voids within the effected tissues.6 MR angiography (MRA), best performed as a time-resolved contrast-enhanced three-dimensional acquisition can be very useful in demonstrating the extent and vascular anatomy of these anomalies.
AVMs of the pelvic wall and viscera can attain massive size and flow characteristics. Typically, they are supplied by all arteries in the pelvis including the anterior branches of the internal iliac arteries as well as the median sacral and inferior mesenteric arteries.25,26,41 This extensive arterial supply, and a tendency to recruit new supply through angiogenesis, results in a poor response to traditional arterial embolization as well as to surgical ligations. Fortunately, many of these AVMs represent arteriolovenous fistula in which many feeding arteries drain into a single draining vein, often a pudendal or obturator vein.42 Endovascular occlusion or ablation of the single draining vein can lead to complete obliteration of the arteriovenous shunts with long-term follow-up showing minimal, if any recurrence (Fig. 7).
COMBINED VASCULAR MALFORMATIONS
Klippel–Trenaunay syndrome (CLVM) and Parkes–Weber syndrome (CAVM or CLAVM) are, respectively, slow-flow and fast-flow combined vascular overgrowth malformations that typically affect a lower extremity and the adjacent pelvis.2
Individuals with Klippel–Trenaunay syndrome have a large capillary malformation or port wine stain as well as lymphatic and venous anomalies of variable severity.43,44,45,46,47,48,49 The venous component typically consists of persistent embryonic veins (marginal vein or vein of Servelle) that courses in the lateral subcutaneous tissue of the calf and thigh. Most of these represent a form of persistent sciatic vein.49 They communicate with the central circulation in a variety of ways, often coursing through the pelvis. The deep veins of the leg can be hypoplastic or interrupted, and even when they are intact, they demonstrate abnormal function on noninvasive imaging.50 Lymphatic involvement may be as minor as the presence of small vesicles in the capillary stain, or severe, with combined microcystic and macrocystic components in the limb and pelvis. Patients with predominately venous anomalies typically suffer from painful swelling and a risk of pulmonary thromboembolism, as well as bleeding from the vagina or rectum.51 These symptoms worsen during pregnancy, so many affected women are counseled against having children. Those who do become pregnant must be vigilant in wearing graded elastic stockings and may require anticoagulation. Patients with Klippel–Trenaunay syndrome who have severe lymphatic anomalies develop intermittent swelling and pain due to infection or intralesional bleeding. Many have lymphatic vesicles in the perianal region, which cause chronic leakage of serosanguinous fluid and can serve as a portal of infection.
Endovascular treatment of patients with Klippel–Trenaunay syndrome must be planned carefully.18,44 Ablation of anomalous veins in the lower extremities is feasible, using a combination of endovenous laser, microcatheter embolization, and sclerotherapy, but may not result in clinical improvement, particularly if the deep veins are inadequate. Macrocystic and some microcystic LMs in the limb or pelvis are amenable to sclerotherapy. Extremely bulky subcutaneous LMs can be managed by contour resection. However, partial resection of LMs may be associated with worsening of the residual lesion, leading to severe bleeding a few years later. Sclerotherapy is often helpful in managing such recurrences. In general, these complex patients should be managed in a center with an organized multidisciplinary vascular anomalies center.
Parkes–Weber syndrome typically consists of extensive cutaneous capillary malformation with diffuse small-vessel AVM and overgrowth of the limb and buttock.12,27,47 These patients develop leg length discrepancy and cardiac overload. Skin breakdown and bleeding can occur. A small percentage of these patients have a lymphatic component that can cause cellulitis or fluid leakage. Severe chylorrhea has been seen in a small group. These complications are difficult to manage. If the LM is large enough to cannulate, sclerotherapy may be helpful in decreasing the frequency of infection or fluid drainage.
SUMMARY
Pelvic vascular malformations can occur as focal, isolated lesions, but more commonly are part of a diffuse vascular malformation of the adjacent extremity and pelvic wall. Each type of vascular malformation has characteristic clinical and imaging findings. Endovascular treatment, combined with resection in some situations, can provide effective treatment or palliation in many cases.
REFERENCES
- Mulliken J B, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69(3):412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- Mulliken J B, Fishman S J, Burrows P E. Vascular anomalies. Curr Probl Surg. 2000;37(8):517–584. doi: 10.1016/s0011-3840(00)80013-1. [DOI] [PubMed] [Google Scholar]
- Burrows P E, Laor T, Paltiel H, Robertson R L. Diagnostic imaging in the evaluation of vascular birthmarks. Dermatol Clin. 1998;16(3):455–488. doi: 10.1016/s0733-8635(05)70246-1. [DOI] [PubMed] [Google Scholar]
- Laor T, Burrows P E. Congenital anomalies and vascular birthmarks of the lower extremities. Magn Reson Imaging Clin N Am. 1998;6(3):497–519. [PubMed] [Google Scholar]
- Timmerman D, den Bosch T Van, Peeraer K, et al. Vascular malformations in the uterus: ultrasonographic diagnosis and conservative management. Eur J Obstet Gynecol Reprod Biol. 2000;92(1):171–178. doi: 10.1016/s0301-2115(00)00443-7. [DOI] [PubMed] [Google Scholar]
- Diaz Candamio M J, Lee V S, Rofsky N M, Krinsky G A, Weinreb J C. Pelvic arteriovenous malformations: gadolinium-enhanced three-dimensional MR angiography findings. Eur Radiol. 2000;10(8):1257–1260. doi: 10.1007/s003300000329. [DOI] [PubMed] [Google Scholar]
- Wei C J, Tseng H S, Wu M H, et al. Congenital pelvic arteriovenous malformation: two cases and MR findings. Eur J Obstet Gynecol Reprod Biol. 2003;108(2):226–228. doi: 10.1016/s0301-2115(02)00427-x. [DOI] [PubMed] [Google Scholar]
- Jain K A, Jeffrey R B, Jr, Sommer F G. Gynecologic vascular abnormalities: diagnosis with Doppler US. Radiology. 1991;178(2):549–551. doi: 10.1148/radiology.178.2.1987622. [DOI] [PubMed] [Google Scholar]
- Vikkula M, Boon L M, Mulliken J B. Molecular genetics of vascular malformations. Matrix Biol. 2001;20(5–6):327–335. doi: 10.1016/s0945-053x(01)00150-0. [DOI] [PubMed] [Google Scholar]
- Boon L M, Mulliken J B, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971–976. doi: 10.1001/archderm.140.8.971. [DOI] [PubMed] [Google Scholar]
- Herman A R, Morello F, Strickland J L. Vulvar venous malformations in an 11-year-old girl: a case report. J Pediatr Adolesc Gynecol. 2004;17(3):179–181. doi: 10.1016/j.jpag.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Enjolras O, Chapot R, Merland J J. Vascular anomalies and the growth of limbs: a review. J Pediatr Orthop B. 2004;13(6):349–357. doi: 10.1097/01202412-200411000-00001. [DOI] [PubMed] [Google Scholar]
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219–225. doi: 10.1016/s0190-9622(97)70284-6. [DOI] [PubMed] [Google Scholar]
- Azizkhan R G. Life-threatening hematochezia from a rectosigmoid vascular malformation in Klippel-Trenaunay syndrome: long-term palliation using an argon laser. J Pediatr Surg. 1991;26(9):1125–1127. discussion 1128. doi: 10.1016/0022-3468(91)90687-o. [DOI] [PubMed] [Google Scholar]
- Azouz E M. Hematuria, rectal bleeding and pelvic phleboliths in children with the Klippel-Trenaunay syndrome. Pediatr Radiol. 1983;13(2):82–88. doi: 10.1007/BF02390107. [DOI] [PubMed] [Google Scholar]
- Servelle M, Bastin R, Loygue J, et al. Hematuria and rectal bleeding in the child with Klippel and Trenaunay syndrome. Ann Surg. 1976;183(4):418–428. doi: 10.1097/00000658-197604000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour M A, Soliman H E, Khougeer G A. Role of descending venography and endovenous embolization in treatment of females with lower extremity varicose veins, vulvar and posterior thigh varices. Saudi Med J. 2007;28(2):206–212. [PubMed] [Google Scholar]
- Burrows P E, Mason K P. Percutaneous treatment of low flow vascular malformations. J Vasc Interv Radiol. 2004;15(5):431–445. doi: 10.1097/01.rvi.0000124949.24134.cf. [DOI] [PubMed] [Google Scholar]
- Keljo D J, Yakes W F, Andersen J M, Timmons C F. Recognition and treatment of venous malformations of the rectum. J Pediatr Gastroenterol Nutr. 1996;23(4):442–446. doi: 10.1097/00005176-199611000-00015. [DOI] [PubMed] [Google Scholar]
- Fishman S J, Shamberger R C, Fox V L, Burrows P E. Endorectal pull-through abates gastrointestinal hemorrhage from colorectal venous malformations. J Pediatr Surg. 2000;35(6):982–984. doi: 10.1053/jpsu.2000.6947. [DOI] [PubMed] [Google Scholar]
- de la Torre L, Carrasco D, Mora M A, Ramirez J, Lopez S. Vascular malformations of the colon in children. J Pediatr Surg. 2002;37(12):1754–1757. doi: 10.1053/jpsu.2002.36714. [DOI] [PubMed] [Google Scholar]
- Alomari A I, Karian V E, Lord D J, Padua H M, Burrows P E. Percutaneous sclerotherapy for lymphatic malformations: a retrospective analysis of patient-evaluated improvement. J Vasc Interv Radiol. 2006;17(10):1639–1648. doi: 10.1097/01.RVI.0000239104.78390.E5. [DOI] [PubMed] [Google Scholar]
- Molitch H I, Unger E C, Witte C L, vanSonnenberg E. Percutaneous sclerotherapy of lymphangiomas. Radiology. 1995;194(2):343–347. doi: 10.1148/radiology.194.2.7529933. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Arima K, Matsuura H, et al. Percutaneous sclerotherapy for chyluria with leg edema: a case report. Hinyokika Kiyo. 1998;44(1):25–27. [PubMed] [Google Scholar]
- Mitty H A, Baron M G, Jacobson J H., II Pelvic arteriovenous malformations. Am J Roentgenol Radium Ther Nucl Med. 1968;102(2):427–430. [PubMed] [Google Scholar]
- Calligaro K D, Sedlacek T V, Savarese R P, Carneval P, DeLaurentis D A. Congenital pelvic arteriovenous malformations: long-term follow-up in two cases and a review of the literature. J Vasc Surg. 1992;16(1):100–108. [PubMed] [Google Scholar]
- Revencu N, Boon L M, Mulliken J B, et al. Parkes Weber syndrome, vein of Galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum Mutat. 2008;29(7):959–965. doi: 10.1002/humu.20746. [DOI] [PubMed] [Google Scholar]
- Zhou X P, Marsh D J, et al. Germline and germline mosaic PTEN mutations associated with a Proteus-like syndrome of hemihypertrophy, lower limb asymmetry, arteriovenous malformations and lipomatosis. Hum Mol Genet. 2000;9(5):765–768. doi: 10.1093/hmg/9.5.765. [DOI] [PubMed] [Google Scholar]
- Takaya N, Iwase T, Maehara A, et al. Transcatheter embolization of arteriovenous malformations in Cowden disease. Jpn Circ J. 1999;63(4):326–329. doi: 10.1253/jcj.63.326. [DOI] [PubMed] [Google Scholar]
- Guttmacher A E, Marchuk D A, White R I., Jr Hereditary hemorrhagic telangiectasia. N Engl J Med. 1995;333:918–924. doi: 10.1056/NEJM199510053331407. [DOI] [PubMed] [Google Scholar]
- Bourdeau A, Cymerman U, Paquet M E, et al. Endoglin expression is reduced in normal vessels but still detectable in arteriovenous malformations of patients with hereditary hemorrhagic telangiectasia type 1. Am J Pathol. 2000;156(3):911–923. doi: 10.1016/S0002-9440(10)64960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eerola I, Boon L M, Mulliken J B, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73(6):1240–1249. doi: 10.1086/379793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J J, Xiang Y, Wan X R, Yang X Y. Diagnosis and management of uterine arteriovenous fistulas with massive vaginal bleeding. Int J Gynaecol Obstet. 2005;89(2):114–119. doi: 10.1016/j.ijgo.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Maleux G, Timmerman D, Heye S, Wilms G. Acquired uterine vascular malformations: radiological and clinical outcome after transcatheter embolotherapy. Eur Radiol. 2005;16:299–306. doi: 10.1007/s00330-005-2799-5. [DOI] [PubMed] [Google Scholar]
- Jain K A, Gerscovich E O. Sonographic spectrum of pelvic vascular malformations in women. J Clin Ultrasound. 1999;27(9):523–530. doi: 10.1002/(sici)1097-0096(199911/12)27:9<523::aid-jcu6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kwon J H, Kim G S. Obstetric iatrogenic arterial injuries of the uterus: diagnosis with US and treatment with transcatheter arterial embolization. Radiographics. 2002;22(1):35–46. doi: 10.1148/radiographics.22.1.g02ja0735. [DOI] [PubMed] [Google Scholar]
- Kelly F W. Forceps delivery after molar malignancy in a woman with arteriovenous malformation. A case report. J Reprod Med. 2001;46(11):1013–1016. [PubMed] [Google Scholar]
- Ghai S, Rajan D K, Asch M R, et al. Efficacy of embolization in traumatic uterine vascular malformations. J Vasc Interv Radiol. 2003;14(11):1401–1408. doi: 10.1097/01.rvi.0000096761.74047.7d. [DOI] [PubMed] [Google Scholar]
- Lim A K, Agarwal R, et al. Embolization of bleeding residual uterine vascular malformations in patients with treated gestational trophoblastic tumors. Radiology. 2002;222(3):640–644. doi: 10.1148/radiol.2223010035. [DOI] [PubMed] [Google Scholar]
- Dahlgren L S, Effer S B, McGillivray B C, Pugash D J. Pregnancy with uterine vascular malformations associated with hemorrhagic hereditary telangiectasia: a case report. J Obstet Gynaecol Can. 2006;28(8):720–723. doi: 10.1016/S1701-2163(16)32224-1. [DOI] [PubMed] [Google Scholar]
- Jacobowitz G R, Rosen R J, Rockman C B, et al. Transcatheter embolization of complex pelvic vascular malformations: results and long-term follow-up. J Vasc Surg. 2001;33(1):51–55. doi: 10.1067/mva.2001.111738. [DOI] [PubMed] [Google Scholar]
- Cho S K, Do Y S, Shin S W, et al. Arteriovenous malformations of the body and extremities: analysis of therapeutic outcomes and approaches according to a modified angiographic classification. J Endovasc Ther. 2006;13(4):527–538. doi: 10.1583/05-1769.1. [DOI] [PubMed] [Google Scholar]
- Berry S A, Peterson C, Mize W, et al. Klippel-Trenaunay syndrome. Am J Med Genet. 1998;79(4):319–326. [PubMed] [Google Scholar]
- Kanterman R Y, Witt P D, Hsieh P S, Picus D. Klippel-Trenaunay syndrome: imaging findings and percutaneous intervention. AJR Am J Roentgenol. 1996;167(4):989–995. doi: 10.2214/ajr.167.4.8819399. [DOI] [PubMed] [Google Scholar]
- Baskerville P A, Ackroyd J S, Lea Thomas M, Browse N L. The Klippel-Trenaunay syndrome: clinical, radiological and haemodynamic features and management. Br J Surg. 1985;72(3):232–236. doi: 10.1002/bjs.1800720331. [DOI] [PubMed] [Google Scholar]
- Oduber C E, der Horst C M van, Hennekam R C. Klippel-Trenaunay syndrome: diagnostic criteria and hypothesis on etiology. Ann Plast Surg. 2008;60(2):217–223. doi: 10.1097/SAP.0b013e318062abc1. [DOI] [PubMed] [Google Scholar]
- Ziyeh S, Spreer J, Rössler J, et al. Parkes Weber or Klippel-Trenaunay syndrome? Non-invasive diagnosis with MR projection angiography. Eur Radiol. 2004;14(11):2025–2029. doi: 10.1007/s00330-004-2274-8. [DOI] [PubMed] [Google Scholar]
- Jacob A G, Driscoll D J, Shaughnessy W J, et al. Klippel-Trenaunay syndrome: spectrum and management. Mayo Clin Proc. 1998;73(1):28–36. doi: 10.1016/S0025-6196(11)63615-X. [DOI] [PubMed] [Google Scholar]
- Cherry K J, Gloviczki P, Stanson A W. Persistent sciatic vein: diagnosis and treatment of a rare condition. J Vasc Surg. 1996;23(3):490–497. doi: 10.1016/s0741-5214(96)80016-4. [DOI] [PubMed] [Google Scholar]
- Delis K T, Gloviczki P, Wennberg P W, Rooke T W, Driscoll D J. Hemodynamic impairment, venous segmental disease, and clinical severity scoring in limbs with Klippel-Trenaunay syndrome. J Vasc Surg. 2007;45(3):561–567. doi: 10.1016/j.jvs.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Walder B, Kapelanski D P, Auger W R, Fedullo P F. Successful pulmonary thromboendarterectomy in a patient with Klippel-Trenaunay syndrome. Chest. 2000;117(5):1520–1522. doi: 10.1378/chest.117.5.1520. [DOI] [PubMed] [Google Scholar]