ABSTRACT
The diagnosis of pelvic congestion syndrome (PCS) continues to challenge all physicians involved especially those in such specialties as anesthesia, gastroenterology, general surgery, obstetrics and gynecology, and interventional radiology. When other pelvic pathology is ruled out, an interventional radiologist may be consulted for additional evaluation and treatment of PCS. A heightened awareness and clinical suspicion for the specific symptomatology and associated findings may bring about a more rapid progression toward treatment. For most interventional radiologists who treat PCS patients, magnetic resonance imaging/MR venography (MRI/MRV), diagnostic venogram, and embolotherapy are at the center of diagnosis and treatment of PCS.
Keywords: Chronic pelvic pain, embolization, ovarian vein embolotherapy, pelvic, congestion syndrome, pelvic varices
In the past, a diagnosis of chronic pelvic pain left many women frustrated with few treatment options and a lack of available resources. Their physicians were likewise perplexed, despite the endless acquisition of negative laboratory and imaging data as well as inconclusive consultations obtained. In the last 10 years, improved scientific understanding and increased physician awareness have lessened the confusion surrounding this condition and its distinct association with pelvic congestion syndrome (PCS). Furthermore, refinements of medical and minimally invasive surgical solutions give affected patients more therapeutic choices today.
As an unusual diagnosis of exclusion, the first step in the treatment of PCS related to chronic pelvic pain requires a multidisciplinary approach because the differential diagnosis is quite long and varied (Table 1). Clearly, evaluation by an obstetrics/gynecology (Ob/Gyn) specialist is a fundamental part of the patient's assessment, but input from other specialties including anesthesiology, gastroenterology, general surgery, neurology, hematology/oncology, psychiatry, and urology may also be necessary. The standard workup usually includes an abdominal and pelvic examination, Pap smear test, routine laboratory blood work, and some cross-sectional imaging.
Table 1.
Chronic Pelvic Pain Differential Diagnosis
| Bowel pathology |
| Cancer/metastases |
| Endometriosis |
| Fibroids |
| Fibromyalgia |
| Neurologic pathology |
| Orthopedic pathology |
| Ovarian cyst |
| Pelvic congestion syndrome |
| Pelvic inflammatory disorder |
| Porphyria |
| Urologic pathology |
| Uterine prolapse |
Millions of women worldwide may suffer with chronic pelvic pain at some time in their life, and the occurrence may be as high as 39.1%. Chronic pelvic pain may account for 10 to 15% of outpatient gynecologic visits in the United States.1 First described clinically by the French in 1857, the association with pelvic varices was initially documented in 1949.2 Now commonly referred to as PCS, the typical age of patients with this condition ranges from 20 to 45 years. It is unclear whether there is any genetic or ethnic predilection.
PATHOPHYSIOLOGY
Multiple factors contribute to the pathogenesis of PCS. The characteristic severe dull aching pain of PCS is thought to be a direct result of the presence of ovarian and pelvic varicosities, much like the leg pain resulting from lower extremity varicose veins. Multiparous women seem to be predisposed to develop PCS. In patients with multiple previous pregnancies, there may have been a significant increase in intravascular volume at each term of gestation. Vein capacity can increase by 60%. Over time, venous distension can render the valves incompetent. Additionally, the weight gain and anatomic changes in the pelvic structures during pregnancy may cause chronic intermittent venous obstruction. Blood pooling in the pelvic and ovarian veins may cause further engorgement, thrombosis, and mass effect on nearby nerves, collectively contributing to pelvic pain.3 The majority of women affected are premenopausal, and a relationship between PCS and endogenous estrogen levels is suggested, as estrogen is known to weaken the vein walls.
Obstructing anatomic anomalies may also lead to secondary PCS. In patients with a retroaortic left renal vein, there may be obstruction of the left ovarian vein leading to symptomatic pelvic varices. Additionally, the left ovarian vein and the left renal vein may by compressed by the superior mesenteric artery (Nutcracker phenomenon) as well. Finally, compression from the right common iliac artery on the left common iliac vein against the spine and pelvic brim is known to cause iliofemoral deep venous thrombosis (May–Thurner syndrome) as well as the pelvic varices of PCS.
CLINICAL PRESENTATION
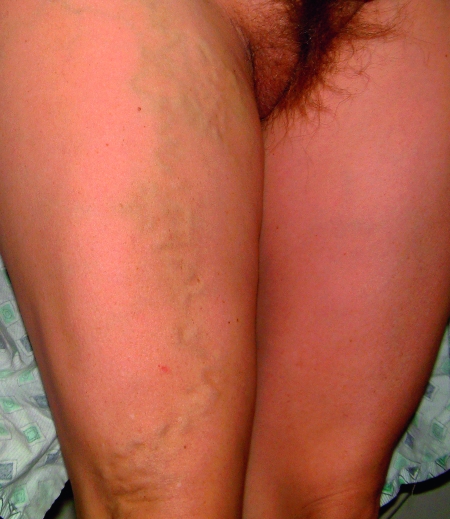
Most women with PCS (Figs. 1 and 2) present with a noncyclical pain lasting more than 6 months in duration. This pain may be worsened by the following: sitting, standing, at the end of the day, during or after intercourse (dyspareunia), or just before the onset of menses. Other symptoms of pelvic congestion are nonspecific and variable in intensity. Affected women may have generalized lethargy, depression, abdominal or pelvic tenderness, vaginal discharge, dysmenorrhea, swollen vulva, lumbosacral neuropathy, rectal discomfort, or urinary frequency. On examination, patients can have cervical motion tenderness or point tenderness over the ovaries or uterus on bimanual exam. One may note hemorrhoids, varicose veins of the perineum, buttocks, or lower extremities. Additionally, these problems may overlap with concomitant pathology, making diagnosis and treatment even more difficult.
Figure 1.
Vulvar varices under soft tissue; note asymmetry.
Figure 2.
Varices extending into leg.
DIAGNOSIS
For many women with PCS, the road toward a definitive diagnosis has been long and laborious. Certainly the diagnosis of PCS continues to challenge all physicians involved. However, a heightened awareness and clinical suspicion for the specific symptomatology and associated findings may bring about a more rapid progression to the much anticipated treatment.
When other pelvic pathology has been ruled out, an interventional radiologist may be consulted for additional evaluation and treatment of PCS. Several imaging modalities may already have been used by the time the patient presents to an interventional radiologist. However, the diagnosis of PCS is often missed, presumably because most imaging is done in the supine position, and venous distension may be underappreciated or even absent in this position.
Pelvic ultrasound (US) and/or computed tomography (CT) scan are usually the first imaging modalities in the evaluation of patients with chronic pelvic pain. Both provide excellent resolution of the uterus. Although a CT scan has greater sensitivity for showing varicosities throughout the lower pelvis, US with Doppler examination provides dynamic information about visualized venous blood flow.4 Criteria for the sonographic diagnosis of varices includes (1) the visualization of dilated ovarian veins greater than 4 mm in diameter, (2) dilated tortuous arcuate veins in the myometrium that communicate with bilateral pelvic varicose veins, (3) slow blood flow (less than 3 cm/s), and reversed caudal or retrograde venous blood flow particularly in the left ovarian vein.5 Interestingly, more than 50% of women with PCS have associated cystic ovaries as well. The US appearance may range from classic polycystic ovarian syndrome to clusters of cysts in bilaterally enlarged ovaries (4 to 6 cysts of 5 to 15 mm in diameter).6 The significance of these cystic changes in the ovary are unclear, particularly because most patients with PCS are not hirsute or amenorrheic. However, there is the repeated suggestion of estrogen overstimulation in women with PCS.
Either a CT scan or US may take only 20 to 30 minutes for most outpatients, and both modalities are readily accessible at most imaging centers. These studies have a relatively lower sensitivity for PCS compared with magnetic resonance imaging/MR venogram (MRI/ MRV) or diagnostic venogram; nevertheless, they are quite valuable in ruling out other pathologies especially underlying malignancies.7,8
For most interventional radiologists who see PCS patients for treatment, MRI/MRV is the best primary imaging modality for this problem. The study is done as an outpatient, is noninvasive, requires no radiation, and is highly sensitive to the findings of pelvic varices. Typical findings of PCS on MRI include dilated, tortuous, enhancing tubular structures near the uterus and ovary that may extend to the broad ligament and pelvic sidewall. Imaging includes the following characteristics: On T1-weighted images, varices appear as flow voids; gradient-echo (GRE) shows varices have high signal intensity. On T2-weighted images, varices usually appear low in signal intensity. On 3-dimensional T1-weighted gradient-echo sequences with gadolinium, varices have high signal intensity.6,7,8 Contrast enhancement with gadolinium improves visualization, and may even increase sensitivity if sequences are obtained with the patient during a Valsalva maneuver. MRV images are excellent for demonstrating the complete network of pelvic venous anatomy as well as the extent of pathology.
Laparoscopy is often used in patients with chronic pelvic pain in search of a specific diagnosis. This direct visualization is excellent for ruling out other etiologies distinct from PCS such as endometriosis. However, because the examination is done supine and requires insufflation of CO2 gas, there may be compression of varices if present, thereby masking the diagnosis of PCS.9 Many Ob/Gyn physicians now opt to do the full laparoscopic view of the pelvis before insufflating with CO2; pelvic varices can then occasionally be seen filling at this point. Despite these efforts, laparoscopy can still be negative in 80 to 90% of patients who do have PCS.
Certainly, the diagnostic venogram continues to provide physicians with a reliable minimally invasive gold standard tool in patients with PCS.3 Using fluoroscopy, access is obtained through the common femoral vein. A catheter is then used to select the ovarian veins and pelvic veins respectively for contrast injection at each site. The diagnosis of PCS is confirmed with the following venographic findings: ovarian vein diameter > 6 mm in diameter, retrograde ovarian or pelvic venous flow, presence of several tortuous collateral pelvic venous pathways, and delayed or stagnant clearance of contrast at the end of injection. Although the venogram study does require radiation, the use of contrast, and is invasive, it has several advantages over other imaging. The diagnostic venogram gives immediate dynamic flow information and measurements of ovarian and pelvic veins with the option of changing patient position (e.g., on a tilt table).10 Furthermore, the diagnostic venogram may be performed with balloon occlusion in the pelvis to further delineate venous reflux. And finally, once completed, the diagnostic venogram enables the treating interventional radiologist to immediately perform embolotherapy.10
A transfundal pelvic venogram is an alternative diagnostic technique that has been performed by some physicians.5 Briefly, the catheter is placed directly 0.5 to 1 cm into the myometrium under fluoroscopic guidance with injection of contrast. This imaging will show venous abnormalities associated with the uterus, but there is incomplete evaluation of the ovarian veins, and other causes of pelvic varices.
TREATMENT
Treatment options for PCS remained elusive until recently, due to controversial diagnostic methods and poor understanding of its etiology ranging from psychosomatic origin to vascular causes. Since Topolanski-Sierra first noted an association in the 1950s between chronic pelvic pain and ovarian and pelvic varices,11 many treatment modalities have been proposed. Medical management with hormone analogues and analgesics, surgical ligation of ovarian veins, hysterectomy with or without bilateral salpingo-oophorectomy and transcatheter embolization have been described in the literature as treatment options for patients with PCS today.
Medical treatment of PCS includes psychotherapy, progestins, danazol, phlebotonics, gonadotropins receptor agonists (GnRH) with hormone replacement therapy (HRT), dihydroergotamine, and nonsteroidal antiinflammatory drugs (NSAIDS). Specifically, the literature supports use of medroxyprogesterone acetate (MPA), or the GnRH analogue goserelin in an effort to suppress ovarian function and/or increase venous contraction. MPA may be given orally 30 mg/day for 6 months. Goserelin acetate is dosed as an injection of 3.6 mg monthly over a 6-month period. As chemical ovarian ligation has numerous side effects, estrogen replacement or “add-back” therapy is frequently required as well.12
In the 1980s, surgical treatment was described by Rundqvist et al, which consisted of extraperitoneal resection of the left ovarian vein, which proved to be useful in relieving symptoms of PCS.13 Subsequent studies described anatomic abnormalities with the proposed etiology being incompetent ovarian veins. This notion was supported by the fact that surgical treatment such as ventrosuspension of the retroverted uterus and hysterectomies proved to be of little benefit. Despite the curative intent of hysterectomy, studies reported residual pain in 33% of patients and a 20% recurrence rate,14,15 which led to the advent of surgical ligation or resection of ovarian veins. More recently, laparoscopic ligation bilateral ovarian veins has been gaining popularity among laparoscopic gynecologists, but surgical experience of ovarian vein ligation is anecdotal with only a few available case studies.3,16 The procedure is performed in a supine position with insufflation of pressurized carbon dioxide into the peritoneal cavity, forcing venous effacement and decompression, which potentially underestimates the number of actual varices thereby decreasing procedural efficacy.9 In addition, laparoscopy is an invasive procedure that generally requires anesthesia and may be associated with significant morbidity, poor cosmesis, and a hospital stay of at least 2 days.9
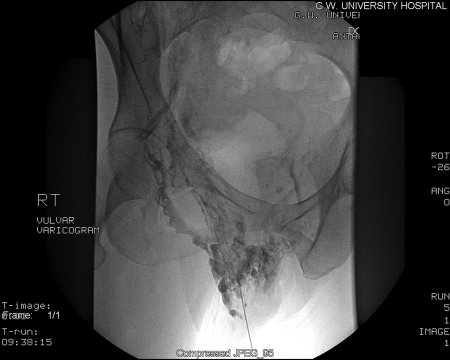
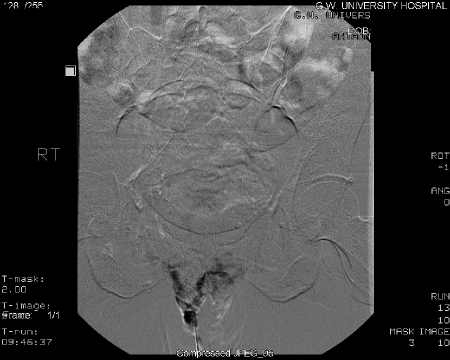
To improve clinical efficacy and reduce perioperative and postoperative morbidity, percutaneous pelvic vein embolization therapy has been utilized (Figs. 3 and 4). Since its introduction in 1993 by Edwards et al, this modality has revolutionized the treatment of PCS.17 The procedure is usually performed at the time of diagnostic venography using a variety of embolic agents, including sclerosant foam and coils. In several published series in the 1990s, success rates for reduction of chronic pelvic pain ranged from 50 to 80%. With advancements in technique, clinical success is achieved in 70 to 85% of treated patients.9,18,19 Kim et al found significant improvement in 83% of women in their overall pain perception levels with a mean of 45 months of long-term follow up.18
Figure 3.
Vulvar varices venogram.
Figure 4.
Vulvar varices post embolization.
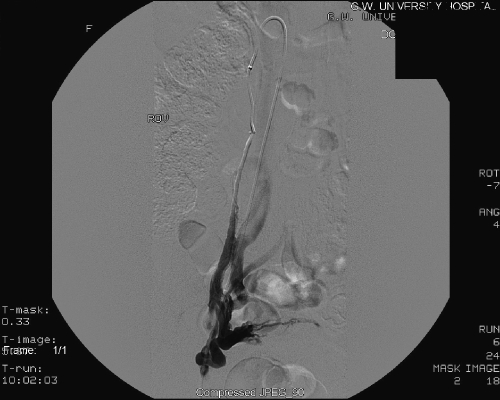
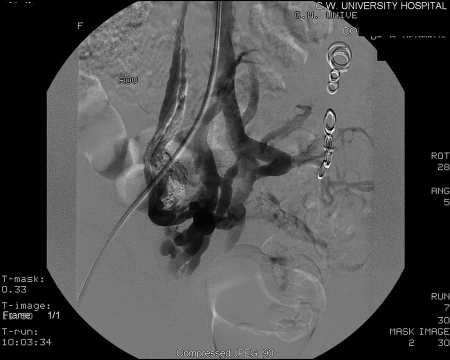
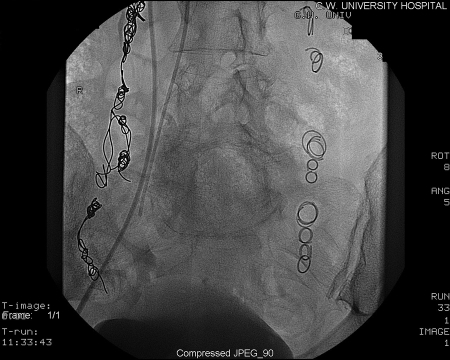
The technique of transcatheter embolotherapy for ovarian and pelvic varices is straightforward, and a brief summary of the technique is presented here. The ovarian and internal iliac veins may be approached from a jugular or femoral approach; we prefer the latter. A 7 French (7 F) femoral sheath is placed and a 7 F guiding catheter with a “Hopkins curve” shape (Cordis J&J, Miami Lakes, FL) is used to select the left renal vein. Once the catheter is “seated,” a 5 F coaxially directed catheter is advanced over a guide wire into the ovarian vein plexus (Figs. 5 and 6). A slurry of Gelfoam (Pfizer, Inc., New York, NY) and 5% sodium morrhuate (American Regent Laboratories Inc., Shirley, NY) is injected. After an interval of 3 to 5 minutes, the main left ovarian vein is occluded using coils (Fig. 7). Other embolic agents including sclerosants and glue have also been detailed in the literature. From the inferior vena cava, the right ovarian vein is then selectively catheterized using a Simmons I or II guiding catheter (Cordis Endovascular, Warren, NJ). A second catheter is then advanced coaxially over a guide wire down into the right pelvic varices. The embolization procedure is then repeated.9
Figure 5.
Right ovarian venogram.
Figure 6.
Right ovarian varices.
Figure 7.
Post coil embolization. No variceal filling.
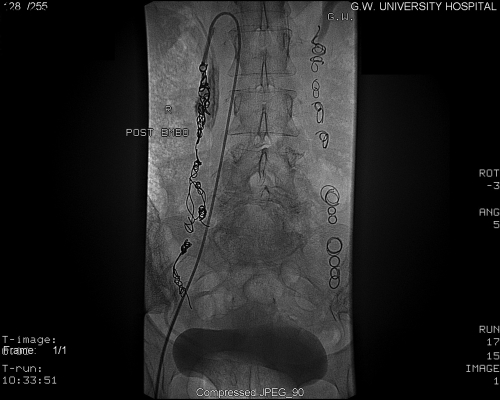
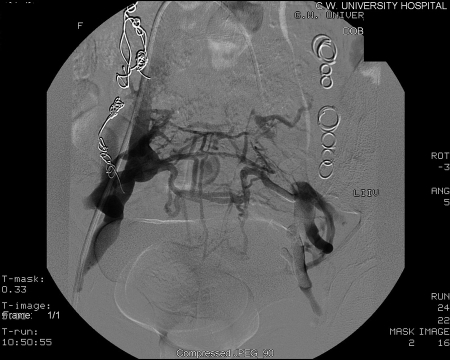
Given the communications that exist between ovarian veins and internal iliac veins, bilateral venography and embolization of both ovarian and internal iliac veins is required to reduce the theoretical chance of recurrence (Figs. 8 and 9). To evaluate the internal iliac veins, it may be helpful to advance a balloon catheter. At our institution we use an 11.5 French compliant (soft and malleable) balloon catheter. Briefly, the balloon is inflated at the proximal vein, and venogram performed through the end hole of the catheter. This allows for improved visualization of the vein's course and caliber. The balloon occlusion technique should be used to evaluate the internal iliac veins. If pelvic varices or communications with the ovarian varices are identified with the internal iliac veins, then transcatheter embolotherapy should be performed in these veins as well. To embolize the pelvic varices, balloon occlusion venography is followed by injection of the embolization/sclerosant mixture described above. The balloon remains inflated for ~5 to 10 minutes to reduce the dilutional effects of returning blood flow.9,18 Coils should be avoided in the internal iliac veins because of the difficulty in delivering these devices in capacious veins and the inherent risk of their embolization to the lungs.
Figure 8.
Left internal iliac venogram; pelvic varices with filling to the right side.
Figure 9.
Post embolization with sodium morrhuate; no variceal filling.
We often perform a staged procedure, by embolizing the right and left ovarian varices, allowing the patient to recover for 3 to 6 weeks, then embolizing the pelvic varices. This sequential approach is governed by factors such as time constraints and pain tolerance. In general, patients experience moderate pain after the procedure, although this pain is usually less than that experienced by patients after arterial embolization. Access to oral, intramuscular, or intravenous pain medications may be required. If pain is severe, access to a patient-controlled analgesia pump is advisable. The embolization procedures can be performed on an outpatient basis offering rapid recovery time for patients along with elimination of postoperative morbidities associated with surgeries. Procedural technical success rates have been shown to reach as high as 99%.9,18
CLINICAL OUTCOMES/RESULTS
Pelvic pain is a common and debilitating condition in women of reproductive age and may account for ~10 to 15% of outpatient gynecologic visits.1 It can negatively affect the quality of life and personal relationships of women, and result in physical and psychological suffering. Patients with chronic pelvic pain report a high incidence of anxiety, depression, and physical worries.20 Pain due to an elusive cause is frustrating both for the physician and patient, especially when treatments are less than satisfactory. Fortunately, with recent scientific advances, more options are becoming available for PCS patients.
The final evaluation of patients with chronic pelvic pain from pelvic congestion is difficult, as in any study where the severity and temporal extent of pain must be assessed. For many of these patients who are premenopausal, it is also important to evaluate the neuroendocrine axis for any changes following treatment. Additionally, a thorough review of patients' subsequent psychosocial issues as manifested in sexual function, employment, and general quality of life are essential considerations for treatment results to be interpreted in the proper light. Unfortunately, detailed studies that encapsulate the analysis of multiple such variables have yet to be completed. Only a modest amount of published data on the outcomes of treatment for PCS exists, but the results are still quite encouraging for these patients with chronic pelvic pain.
Medical therapy has been shown to give patients relief, but the results may be short lived.21 Many studies find goserelin, an injectable gonadotropin releasing hormone super-agonist, which stops production of testosterone and estrogen, to be superior to MPA in treating pure PCS. It showed a decrease in variceal evidence on venography and reduced pelvic pain.12 However, several meta-analyses reported both MPA and goserelin to be equally effective. Farquhar et al found that after 9 months there was no overall significant effect of MPA or psychotherapy, but there was a combined interaction between MPA and psychotherapy, with 71% of the women in this group showing ≥ 50% reduction in pain score.22 An exhaustive review of the literature for follow-up of medical therapy revealed only one article with a follow-up term of 1 year—the longest documented.12 Therefore, it is unclear if the benefits of contemporary chemical ligation for pelvic varices are truly sustained long term. Moreover, side effects of GnRH agonists and hormonal treatments including weight gain, hot flashes, bone loss, and mood changes, which might be offset by estrogen “add-back” therapy cannot be overlooked.21 Further trials are needed to establish the efficacy of the GnRH/estrogen combination. Even though relief from pain is not sustained in the long term with hormonal therapy, it may be most acceptable for those looking for a nonsurgical/interventional treatment.
As mentioned above, dihydroergotamine is also reported in the literature as a viable medical treatment option for PCS. Pain scores after dihydroergotamine were reduced for up to 48 hours postinjection.23 The mean difference in pain score at this time point was 4.1 cm on a 10 cm visual analog scale; however, 16 out of 22 women experienced amenorrhea as a complication. This single study which employed intravenous dihydroergotamine was very small and should not be taken as a basis for current therapy as the systemic vasoconstrictor properties of dihydroergotamine have led to its withdrawal from the market.21
Nonmedical therapy in the treatment of PCS has evolved dramatically since the 1980s. Previous studies had shown that hysterectomy with oophorectomy gave moderate relief to patients with PCS, but a recurrence rate or residual pain persisted in 30% of patients at one year follow-up.15 Ten years ago, surgical ligation and embolization were also shown to be nearly equal in efficacy in reducing patient symptomatology for ~80% of PCS patients treated. Experience continues to be quite limited for outcome with surgical/laparoscopic ligation of ovarian veins, with only small investigative cohorts involved.24,25 Moreover, surgical treatments including hysterectomy with or without bilateral salpingo-oophorectomy, laparoscopic ligation of bilateral ovarian veins present with their own complications. Hysterectomy is associated with longer hospital stay, and delayed restoration to normal daily life. Moreover, if performed with bilateral oophorectomy, it prematurely induces a menopausal state and ends the reproductive potential of a premenopausal woman. Even with laparoscopic procedures employed, 20% of the patients experience unsatisfactory results.15 Small series of bilateral laparoscopic ligation boast absence of complications, but also acknowledge that the same may not hold in future studies with a larger sample size.16 The procedure is also technically challenging. Multiple main trunks off the ovarian vein exist in as many as 40% of cases on the left and 25% of cases on the right. This can make laparoscopic procedures difficult, with a high potential of recurrence resulting from inadequate obliteration of all channels.
In a series by Gargiulo et al, complete remission of pain and absence of pelvic varicosities in patients who underwent surgical ligation lasted up to 12 months.24 The authors were unsure in their conclusion whether transcatheter embolization was better, as this was not a randomized trial and patient numbers were small. Surgical management may also add the risk of abdominal and pelvic adhesion formation, ultimately increasing pain and significant patient morbidity.
Embolotherapy for PCS is an exciting therapy that has proven to be safer over the past 2 decades. A more recent article by Chung et al examined the effect of patient stress level on treatment efficacy, directly comparing hysterectomy with oophorectomy versus venous embolization for the treatment of PCS. Using both the social readjustment rating scale and visual analog pain scale, patients were divided into subsets. Following directed comparison of the subgroups after treatment, analysis of pain scores showed that venous embolization was more effective than hysterectomy, especially for patients who are “typically or moderately highly stressed.”25 Kim et al has demonstrated that PCS patients who underwent ovarian and pelvic venous embolization have a more durable result in reduction of their pelvic pain.18 In this study, 83% of patients had a positive treatment response clinically at long-term follow-up with the average duration of follow-up being ~48 months. Further, patients who had had venous embolotherapy showed no significant change in menses, fertility, or hormone levels. Finally, a subset of patients who had previously undergone hysterectomy before embolization still achieved significant improvement based on numeric pain perception scores. In their long-term results, Kim et al reported no major complications and also did not find any significant changes in the basal follicle-stimulating hormone, luteinizing hormone, or estradiol levels.18
One study reported complications such as gonadal vein perforation, nontarget embolization including pulmonary coil embolization, and cardiac arrhythmias in 8% of their participants.19 Other reported complications of embolotherapy are rare (< 4%) and include ovarian vein thrombophlebitis, recurrence of varices, migration of embolic material, and radiation exposure to ovaries.26 Long-term data shows no demonstrable negative effects on menstrual cycle or fertility from transcatheter embolotherapy.18 It has proven to be a safe and effective nonsurgical approach in reducing chronic pelvic pain associated with pelvic venous incompetence. Moreover, the authors reported a 50% pregnancy rate in premenopausal women who would otherwise become infertile in exchange for pain relief with either medical or surgical therapies.9,18
CONCLUSION
There is no panacea for patients with chronic pelvic pain and PCS. Fortunately, as medical and surgical tools have advanced, options for evaluation and treatment are indeed more available for this debilitating problem. Although more investigation is needed to discern the best combination of therapies for PCS, the formidable obstacles of diagnosis and management of PCS patients can certainly be overcome with the methods that exist today. Interventional Radiology in particular is quite promising for offering definitive diagnosis and symptomatic relief to PCS patients.
REFERENCES
- Robinson J C. Chronic pelvic pain. Curr Opin Obstet Gynecol. 1993;5:740–743. [PubMed] [Google Scholar]
- Taylor H C. Vascular congestion and hyperemia: their effects on structure and function in the female reproductive system. Am J Obstet Gynecol. 1949;57:637–653. doi: 10.1016/0002-9378(49)90422-6. [DOI] [PubMed] [Google Scholar]
- Stones R W. Pelvic vascular congestion: half a century later. Clin Obstet Gynecol. 2003;46(4):831–836. doi: 10.1097/00003081-200312000-00013. [DOI] [PubMed] [Google Scholar]
- Park S J, Lim J W, Ko Y T, et al. Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. AJR Am J Roentgenol. 2004;182(3):683–688. doi: 10.2214/ajr.182.3.1820683. [DOI] [PubMed] [Google Scholar]
- Beard R W, Highman J H, Pearce S, et al. Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet. 1984;2:946–949. doi: 10.1016/s0140-6736(84)91165-6. [DOI] [PubMed] [Google Scholar]
- Coakley F V, Varghese S L, Hricak H. CT and MRI of pelvic varices in women. J Comput Assist Tomogr. 1999;23:429–434. doi: 10.1097/00004728-199905000-00018. [DOI] [PubMed] [Google Scholar]
- Umeoka S, Koyama T, Togashi K, Kobayashi H, Akuta K. Vascular dilatation in the pelvis: identification with CT and MR imaging. Radiographics. 2004;24:193–208. doi: 10.1148/rg.241035061. [DOI] [PubMed] [Google Scholar]
- Kuligowska E, Deeds L, Kang L. Pelvic pain: overlooked and underdiagnosed gynecologic conditions. Radiographics. 2005;25(1):3–20. doi: 10.1148/rg.251045511. [DOI] [PubMed] [Google Scholar]
- Venbrux A C, Chang A H, Kim H S, et al. Pelvic congestion syndrome (pelvic venous incompetence): impact of ovarian and internal iliac vein embolotherapy on menstrual cycle and chronic pelvic pain. J Vasc Interv Radiol. 2002;13(2):171–178. doi: 10.1016/s1051-0443(07)61935-6. [DOI] [PubMed] [Google Scholar]
- Venbrux A C, Lambert D L. Embolization of the ovarian veins as a treatment for patients with chronic pelvic pain caused by pelvic venous incompetence (pelvic congestion syndrome) Curr Opin Obstet Gynecol. 1999;11:395–399. doi: 10.1097/00001703-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Topolanski-Sierra R. Pelvic phlebography. Am J Obstet Gynecol. 1958;76:44–45. doi: 10.1016/s0002-9378(16)36864-8. [DOI] [PubMed] [Google Scholar]
- Soysal M E, Soysal S, Vidcan K, Ozer S. A randomized controlled trial of goserelin and medroxyprogesterone acetate I in the treatment of pelvic congestion. Hum Reprod. 2001;16(5):931–939. doi: 10.1093/humrep/16.5.931. [DOI] [PubMed] [Google Scholar]
- Rundqvist E, Sandholm L E, Larsson G. Treatment of pelvic varicosities causing lower abdominal pain with extraperitoneal resolution of left ovarian vein. Ann Chir Gynaecol. 1984;73:339–341. [PubMed] [Google Scholar]
- Beard R W, Kennedy R G, Gangar K F, et al. Bilateral oophorectomy and hysterectomy in the treatment of intractable pelvic pain associated with pelvic congestion. Br J Obstet Gynaecol. 1991;98(10):988–992. doi: 10.1111/j.1471-0528.1991.tb15336.x. [DOI] [PubMed] [Google Scholar]
- Carter J E. Surgical treatment for chronic pelvic pain. JSLS. 1998;2(2):129–139. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Mochizuki M, Kitagaki S. Laparoscopic varicocele ligation for pelvic congestion syndrome. Int J Gynaecol Obstet. 1996;55:177–178. doi: 10.1016/s0020-7292(96)02733-6. [DOI] [PubMed] [Google Scholar]
- Edwards R D, Robertson J R, MacLean A B, Hemmingway A P. Case report: pelvic pain syndrome – successful treatment of a case by ovarian vein embolization. Clin Radiol. 1993;47:429–431. doi: 10.1016/s0009-9260(05)81067-0. [DOI] [PubMed] [Google Scholar]
- Kim H S, Malhotra A D, Rowe P C, Lee J M, Venbrux A C. Embolotherapy for pelvic congestion syndrome: long-term results. J Vasc Interv Radiol. 2006;17:289–297. doi: 10.1097/01.RVI.0000194870.11980.F8. [DOI] [PubMed] [Google Scholar]
- Maleux G, Stockx L, Wilms G, et al. Ovarian vein embolization for the treatment of pelvic congestion syndrome: long term technical and clinical results. J Vasc Interv Radiol. 2000;11:859–864. doi: 10.1016/s1051-0443(07)61801-6. [DOI] [PubMed] [Google Scholar]
- Walling M K, Reiter R C, O'Hara M W, et al. Abuse history and chronic pain in women: prevalences of sexual abuse and physical abuse. Obstet Gynecol. 1994;84:193–199. [PubMed] [Google Scholar]
- Stones W, Cheong Y C, Howard F M. Interventions for treating chronic pelvic pain in women. Cochrane Database Syst Rev. 2005;(2) doi: 10.1002/14651858.CD000387.pub2. CD000387. DOI:10.1002/14651858. CD000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar C M, Rogers V, Franks S, et al. A randomized controlled trial of medroxyprogesterone acetate and psychotherapy for the treatment of pelvic congestion. Br J Obstet Gynaecol. 1989;96(10):1153–1162. doi: 10.1111/j.1471-0528.1989.tb03190.x. [DOI] [PubMed] [Google Scholar]
- Reginald P W, Beard R W, Kooner J S. Intravenous dihydroergotamine to relieve pelvic congestion with pain in young women. Lancet. 1987;2:351–353. doi: 10.1016/s0140-6736(87)92380-4. [DOI] [PubMed] [Google Scholar]
- Gargiulo T, Mais V, Brokaj L, Cossu E, Melis G B. Bilateral laparoscopic transperitoneal ligation of ovarian veins for treatment of pelvic congestion syndrome. J Am Assoc Gynecol Laparosc. 2003;10(4):501–504. doi: 10.1016/s1074-3804(05)60156-9. [DOI] [PubMed] [Google Scholar]
- Chung M-H, Huh C-Y. Comparison of treatments for pelvic congestion syndrome. Tohoku J Exp Med. 2003;201:131–138. doi: 10.1620/tjem.201.131. [DOI] [PubMed] [Google Scholar]
- Ganeshan A, Upponi S, Hon L, Uthappa M C, Warakaulle D R, Uberoi R. Chronic pelvic pain due to pelvic congestion syndrome: the role of diagnostic and interventional radiology. Cardiovasc Intervent Radiol. 2007;30:1105–1111. doi: 10.1007/s00270-007-9160-0. [DOI] [PubMed] [Google Scholar]