ABSTRACT
The American Cancer Society estimates that in 2007, 178,000 women in the United States will be diagnosed, and that 40,000 women will die from breast cancer. Metastatic breast cancer is a systemic disease, uncommonly involving an isolated organ. Liver metastases from breast cancer occur in ~50% of the patients who develop breast cancer metastases and are associated with a poor outcome. Hepatic metastasectomy as an adjuvant treatment even in patients with stable extrahepatic disease has been shown to impart a significant survival advantage over chemotherapy alone. In the treatment of hepatocellular carcinoma (HCC) and colorectal liver metastases (CRLM), radiofrequency ablation (RFA) has been shown to be a safe, minimally invasive treatment option with low morbidity and short hospital stay that is more readily repeatable than resection. The data supporting RFA of breast cancer liver metastases (BCLM) is currently limited to small, retrospective series that, like hepatic resection, have demonstrated adjuvant RFA improves survival compared with chemotherapy alone. This review will examine the rationale, indications, supportive data, and complications of RFA in the treatment of BCLM.
Keywords: Ablation, radiofrequency ablation, breast cancer, liver metastases
The incidence of breast cancer in the United States has decreased slightly in the last 5 years. The American Cancer Society estimates that 178,000 new cases of invasive breast cancer were diagnosed in 2000 and that over 40,000 women in the United States died from breast cancer making it second only to lung cancer as the leading cause of cancer-related death in women.1 Ultimately, survival is heavily dependent on the stage at time of diagnosis. Women diagnosed with localized disease can expect 5-year survival over 95%, whereas the 5-year survival in patients with distant metastases decreases to just over 50%.2
Complicating the treatment of metastatic breast cancer is the fact that breast cancer has a tendency to metastasize systemically. This is in contrast, for example, to colon cancer in which the liver is the most common site of distant metastases; subsequent liver failure due to replacement of hepatic parenchyma accounts for most of these metastatic colon cancer deaths. Only 5 to 8% of patients with metastatic breast cancer have metastases limited to the liver,3,4 and it has been reported that ~20% of deaths are attributable to liver involvement. Historically, patients with liver metastases from breast cancer have had a poor prognosis, with median survival of 6 months.5 More recently, advances in imaging techniques, and polychemotherapy including aromatase inhibitors, taxanes, and trastuzumab have improved survival to 16 months.3,6
There has been an interest in local therapy of liver metastases from breast cancer based on several reports in the surgical literature describing a survival advantage of hepatic metastasectomy dating back to 1991.7 Since this early report, the morbidity and mortality of hepatic resection have decreased significantly, giving further rationale to hepatic metastasectomy for breast cancer liver metastases (BCLM). In addition, in the last decade we have come to understand that cytoreduction in many tumors allows an improved response to chemotherapy based on the “log-kill hypothesis.” This theory proposes that chemotherapy kills a constant fraction of viable tumor cells and if the number of viable cells is reduced, then the likelihood of a complete response after several cycles is improved.8 Lastly, emerging data suggests that cytoreduction or debulking may promote an enhanced immune response to residual microscopic tumor.
In 2006, Adam et al reported on 85 patients with BCLM that underwent hepatic resection with no subsequent mortality, and these patients had a median survival rate of 32 months and a 5-year survival rate of 37%.9 It is notable that almost a third of patients in this series had evidence of extrahepatic disease. Independent predictors of poor survival in this cohort included a lack of response to preoperative chemotherapy, macroscopically incomplete (R2) resection and lack of potential for repeat hepatic resection. Patients with active extrahepatic disease at the time of hepatic resection had a 5-year survival of 16%, compared with 25% for patients with extrahepatic disease in remission (or resected) and 43% for patients with liver-only metastases. Although clearly patients with liver-only metastases had the best prognosis in this series, even patients with metastatic disease enjoyed a survival benefit compared with patients who received chemotherapy alone.
In a series of 54 breast cancer patients, Elias et al found that hormone receptor status significantly impact survival.5 Patients with hormone receptors had a median survival of 44 months, compared with 19 months in those who were receptor negative. Together, these studies validate an aggressive approach to the treatment of both isolated and liver dominant breast cancer metastases in well-selected patients.
In the era of minimally invasive interventions, surgical resection may not, however, be the ideal treatment of BCLM. Arguably, percutaneous ablation is less invasive, less expensive, less morbid, and repeatable in a larger proportion of patients than resection. Although posthepatectomy mortality is quite low today, in fact zero in many series, 12 to 20% patients do experience complications including biliary leaks, infection, and hemorrhage that prolong recovery.5,9 The mean hospital stay following liver resection is, at minimum, 5 days. Another factor to consider when contemplating resection versus ablation is the fact that more than 50% of patients who undergo “curative resection” will develop additional hepatic and/or extrahepatic metastases with disease-free intervals in the 10 to 16 month range. In our opinion, these data highlight the need for minimally invasive, parenchyma-sparing treatment options.
ABLATION STRATEGIES
Today, there are several options in addition to surgical resection to affect local control of liver metastases, including hepatic artery chemoinfusion, chemoembolization, bland embolization, and ablation. In this article, we will focus on ablation of BCLM. Ablation refers to the application of extreme temperature or direct injection of chemical agents to effect tumor cell death. The concept of medicinal thermal ablation is by no means a novel idea; ancient Egyptian papyri describe the use of heat to minimize operative bleeding and to cauterize superficial tumors over 3500 years ago. Modern ablation methods include the application of heat using radiofrequency ablation (RFA), laser interstitial thermotherapy (LITT), and microwave ablation (MWA). On the opposite end of the spectrum, cryotherapy causes tumor necrosis by alternating cycles of freezing and thawing. High-intensity focused ultrasound (HIFU), the only extracorporeal method, uses mechanical agitation delivered transcutaneously to cause heat and cavitation leading to cell death. Chemical ablation with absolute ethanol or acetic acid is commonly used to treat hepatocellular carcinoma (HCC). Chemical ablation is successful in HCC, a relatively soft tumor occurring most often in the setting of a cirrhotic (hard) liver. Most metastases are hard lesions in the setting of a normal (soft) liver, making direct injection difficult and adequate dispersion of the agent within the tumor poor.
The choice of modality depends on several factors, including tumor histology, the location, number and geometry of lesion(s), and perhaps most importantly, institutional and regional preference. Modality and indications are in evolution, and differ from country to country, institution to institution, and practitioner to practitioner. Whereas RFA is the most widespread modality in use in the United States, LITT is common in Europe, MWA in Japan, and HIFU in China; each using varying frequencies along the electromagnetic spectrum to affect tumor kill. Compared with surgical resection, these techniques are all in their relative infancy and the bulk of the literature involves either HCC or colorectal metastases. Since RFA is the most commonly used method in the United States and because most of the small number of reports on ablation of BCLM involve RFA, the remainder of this review will focus on RFA.
RADIOFREQUENCY ABLATION
In 1891 d'Arsonval notes that radiofrequency waves passing through tissue caused an elevation in temperature. Ultimately, this led to the development of an alternating electric current generator in the range of radiofrequency (200 to 1200 MHz)—the Bovie electrocautery device. Current RFA uses a high energy alternating current applied via an exposed electrode placed directly into a target lesion. This alternating current causes agitation of intracellular ions that are constantly trying to align with the direction of current. The frictional heat generated results in desiccation of cells, protein denaturation, microvascular thrombosis and ultimately coagulative necrosis. Malignant cells are more resistant to freezing than normal cells, but more sensitive to hyperthermic damage. Thermal injury begins at 42°C, and as the temperature is increased, the time needed to cause cell death decreases. At lethal temperatures of 60°C and over, protein denaturation, tissue coagulation, and vascular thrombosis result in a zone of complete ablation. On the periphery of the ablation, a rim of partial tissue destruction up to 8 mm in diameter can be seen surrounding the zone of coagulation.10
In clinical practice, there are three manufacturers of RFA devices on the market in the United States. The Rita device (Rita Medical Systems, Inc., Freemont, CA) uses temperature as a surrogate marker for cell death. If exceedingly high temperatures are reached (> 110°C) in the tissue surrounding the electrode, tissue charring results. Charring increases the local impedence and therefore decreases energy transfer to surrounding tissues, ultimately resulting in smaller zone of ablation. The LeVeen RF ablation system (Boston Scientific, Natick, MA) uses impedence-based feedback in the tissue near the tip of the electrode to adjust the delivered power. When the tissue is completely coagulated, the impedence rises exponentially and the current stops, a phenomenon known as “roll-off,” the end point of the ablation with this device. The third device is the “Cool-tip” electrode (Covidien, Boulder, CO), an insulated hollow needle or cluster of three needles for larger lesions with two channels to allow for internal circulation of cool water to minimize charring. Similar to the Boston Scientific device, there is a feedback loop to adjust power when impedence at the tip rises. With this device, energy is delivered for a set time of 12 minutes.
Early studies using RFA in the liver, dating back to 1990, reported solely on the treatment of hepatocellular carcinoma, and not metastases. It should be noted that the ability to extrapolate the experience in treating HCC to liver metastases is limited for at least two reasons. First, in HCC the cirrhotic liver acts as an insulator, potentiating the heat deposited within the tumor—a phenomenon known as the “oven effect” that is not relevant to ablation in normal liver parenchyma. Second, metastases are more likely to have microscopic tumor surrounding the imaged tumor thereby increasing the risk of treatment failure and recurrence at the periphery of a lesion.
In vitro, the size of the ablation zone is dependent on both radiant and conductive properties, and is proportional to the square of the RF current. In vivo, however, other factors come in to play, including the presence of large vessels adjacent to the target ablation zone. Flowing blood, though protective of the vascular endothelium, limits the amount of heat deposited in portions of tumor abutting large vessels—a phenomenon known as “heat sink.” It has been suggested that RF combined with temporary hepatic inflow occlusion, in surgery with a Pringle maneuver11,12 or by percutaneous balloon occlusion,13 can enlarge the ablation zone and increase the likelihood of ablating tumor on the vascular margin.
Additionally, large bile ducts do not have the same protection from thermal injury that blood vessels do because the slow flow of bile does not remove the applied heat in the same way that flowing blood does. Therefore, lesions at the hilar plate (where the common bile duct enters the liver) and adjacent to the falciform ligament where the left hepatic duct lies should be treated with caution, as ablation of these ducts can lead to biliary fistula or stricture. Placement of biliary catheters for cool saline infusion during RFA may decrease the incidence of biliary complications,14,15 but this has been attempted in few patients and further studies are needed. For lesions adjacent to the gallbladder or bowel, carbon dioxide, sterile water, or a balloon catheter can be injected or inserted to provide a safe window. Saline as an injectable solution should be avoided because it is an ionic fluid and can counterproductively conduct RF energy to the structure intended to be protected.
RADIOFREQUENCY ABLATION AND BREAST CANCER METASTASES
There are no prospective studies and only a small number of retrospective reports on RFA for BCLM. In 2001 Livraghi et al reported on 24 patients with 64 hepatic breast metastatic foci treated with RFA.16 Complete necrosis by imaging was found in 92%, and 58% went on to develop new sites of metastases during follow-up. Since then, there have been a handful of small retrospective studies reporting survival data. In 2006 we reported our experience treating BCLM with RFA of 14 lesions in 12 patients, 10 of whom had stable extrahepatic disease at the time of ablation.17 With a median follow up of 22.5 months, actuarial 3- and 5-year survival rates were 70% and 30%, respectively.
Similar results were reported by Gunabushanam et al in 2007,18 who used RF to treat 14 patients with 16 BCLM. In this series, 28% had limited bone metastases and 72% had only BCLM. They found a 1-year survival rate of 64% and the median survival was not reached in the median follow-up of 19 months. Local tumor progression was seen in 2 BCLM, and new metastases were found in 50%. In 2006, Lawes et al reported 19 patients with BCLM treated with RFA, 11 of whom had stable extrahepatic disease.8 They found a 42% actual survival rate at 30 months. Three patients had local treatment failure due to the size (7.3 cm) or number (7) of metastases.
The reason most reported series on ablation of BCLM are relatively small and represent a diverse population (size and number of lesions, presence or absence of metastatic disease, variable chemotherapeutic regimens) is that metastatic breast cancer is a systemic disease. For this reason, in these series describing hepatic resection or ablation for BCLM, most of the patients are receiving concurrent systemic chemotherapy. In other words, both ablation and resection for BCLM should be considered an adjuvant therapy rather than a substitute for systemic therapy. In fact, it has been proposed that the mechanism by which surgical metastasectomy of BCLM improves survival is by cytoreduction, thus allowing systemic chemotherapy to be more effective.8,19 Based on this theory, RFA may ultimately prove to be as effective a strategy as surgical resection for debulking of BCLM, and prospective randomized trials would be needed to determine whether one technique offers a survival advantage.
Another rationale to support RFA in the treatment of BCLM is to afford patients with limited disease a “test of time.”20 In this approach, initially described for patients with isolated colorectal liver metastases, patients with potentially resectable disease instead undergo ablation and short interval follow-up. If there is no disease progression (i.e., new lesions) within 3 to 6 months, resection is then performed. If new lesions are detected, the patient is spared a surgery that would not have been curative. In this way, the test of time or wait and see approach can be an additional selection criteria to help determine which patients are most likely to benefit from resection.
Although the arguments above could support RFA as a first-line treatment for amenable BCLM, generally accepted inclusion criteria for ablation are patients who are not surgical candidates based on either lesion location (adjacent vital structures, precluding margin negative resection, and bilobar disease), hepatic reserve or comorbid disease. Intraoperative ablation may also be performed in concert with hepatic resection, broadening the criteria for resectability to include more patients with bilobar disease. Patients should have liver only or liver dominant metastases amenable to ablation based on location, number (< 4) and size (< 5 cm).
Although acceptable size criteria vary greatly, it is known that local recurrence and treatment failure are higher with larger lesions due to incomplete ablation at the periphery. The largest device currently available is the StarBurst XLi from Rita Medical Systems that creates a 7 cm ablation using saline dripped through each of 9 electrodes. If perfectly centered, the largest tumor that the StarBurst XLi can treat with a single ablation is 5 cm, allowing for a 1 cm margin of normal liver. Based on computer modeling, with a 5 cm ablation, 6 optimally positioned overlapping ablations are required to adequately treat a 4.25 cm tumor.21 In 2001 Solbiati et al treated 172 colorectal metastases in 109 patients and achieved local control in 70% of lesions.22 In their series, local recurrence was seen in only 16.5% of patients with lesions under 3 cm and 56% in patients with lesions ≥ 3 cm.
Percutaneous ablation may be performed using ultrasound (US), computed tomography (CT) or magnetic resonance imaging (MRI) guidance. In most centers, US is the modality of choice because it is relatively inexpensive, readily available, does not use ionizing radiation, and allows for real-time visualization of probe placement and ablation. CT is useful in targeting lesions difficult to see with US. Most commonly, it is used for lesions in the dome because air in the base of the lung reflects the US beam, limiting the utility of US to see this area. CT also offers superior visualization of the ablation zone during and immediately following ablation. Ultrasound during ablation shows a hyperechoic ball that makes assessment of the ablation zone and the need to reposition the probe to treat large lesions difficult because of shadowing.
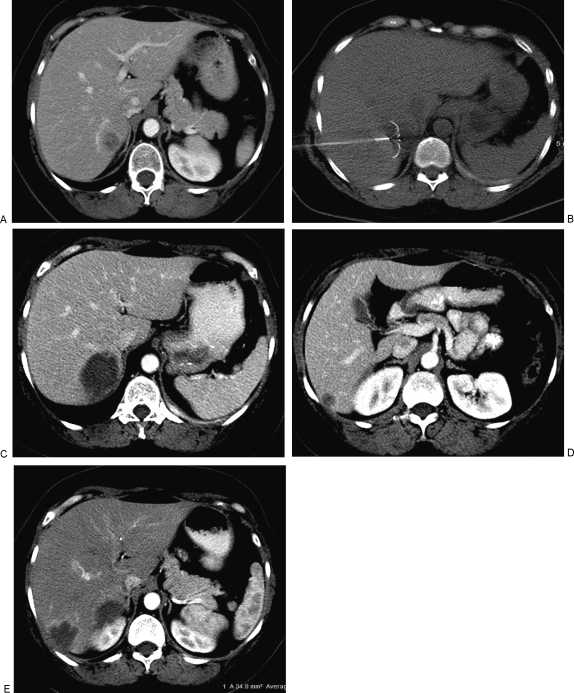
We routinely evaluate patients following RFA with CT (or MRI) at 4 to 6 weeks for evidence of complete ablation (Fig. 1).23 The normal appearance at this time is an ablation zone larger than the treated lesion, with a smooth enhancing hypervascular rim of inflammatory tissue. Any nodularity or asymmetry of the hyperemia should raise the suspicion of tumor. Successful retreatment of incompletely ablated or recurrent lesions has been shown to provide similar survival benefit than if the lesion is completely treated in one session. The hyperemic rim usually resolves by the next imaging study at 3 or 6 months. Imaging is obtained every 3 months for 1 year, every 6 months for the next 2 years, and yearly thereafter. Other modalities, including dynamic MRI, microbubble US, and positron emission tomography (PET) have also been used to evaluate for recurrence and progression.
Figure 1.
(A) Contrast enhanced computed tomography (CT) image of a patient with metastatic breast cancer to bone and pleura that were well controlled; however, biopsy proved solitary metastasis in segment VI of the liver. (B) CT guided radiofrequency ablation (RFA) was performed with a Radio Therapeutics device shown with the tines deployed in the lesion. (C) CT scan 6 weeks after RFA demonstrates complete ablation of the target lesion with no residual hypervascular nodularity. (D) Three months after ablation of the lesion in (A), the patient had a second liver metastases, also in segment VI of the liver, which was treated with RFA. (E) Contrast enhanced CT 4 weeks after ablation of the second lesion demonstrates the typical smooth hypervascular rim of inflammatory tissue. Note the absence of hypervascular nodularity which would indicate residual viable tumor. Seven months after the first RFA, there is no evidence of viable liver metastases and the extrahepatic metastases to bone and pleura remain stable.
Complications of RFA include bleeding, tract seeding, sepsis, and intestinal perforation. In a multicenter study, Livraghi et al reported complications following RFA in 3,554 lesions.24 There were 6 deaths (0.3%), including 2 caused by multiorgan failure following intestinal perforation; one case each of sepsis, massive hemorrhage, and liver failure, and one case of sudden death of uncertain cause 3 days after the procedure. Fifty (2.2%) patients had additional major complications including intraabdominal hemorrhage requiring treatment (12), tract seeding (12), liver abscess (6), intestinal perforation, cardiac arrest, pulmonary embolism, pneumothorax, biloma, and cholecystitis. An increased number of RF sessions were related to a higher rate of major complications (p < .01), whereas the number of complications was not significantly different when tumor size or electrode type were compared. Minor complications were observed in less than 5% of patients and included skin burn, self-limited bleeding, arterioportal and bilioportal fistulas, biloma, pain, and biliary stricture.
CONCLUSION
Unlike HCC and colon cancer metastases that usually involve only the liver, metastatic breast cancer is a systemic disease. Surgical metastasectomy for BCLM has been shown to impart a survival advantage for patients with liver-only disease as well as for patients with well-controlled extrahepatic metastases, but most patients will develop disease progression within 10 to 16 months. RFA has been shown to be a safe, minimally invasive treatment option with low morbidity and short hospital stay that is more readily repeatable than resection. Several small, retrospective series have demonstrated adjuvant RFA to improve survival compared with chemotherapy alone. Ultimately, to determine the best option for these patients, prospective studies with long-term follow-up to compare resection and RFA with respect to recurrence, disease-free and overall survival rates are needed.
REFERENCES
- American Cancer Society Breast Cancer Facts & Figures 2007–2008. Atlanta, GA: American Cancer Society, Inc.; 2008.
- Surveillance E, Results E. (SEER) Program Registries, Division of Cancer Control and Population Science. Bethesda, MD: National Cancer Institute; 2007.
- Atalay G, Biganzoli L, Renard F, et al. EORTC Breast Cancer and Early Clinical Studies Groups. Clinical outcome of breast cancer patients with liver metastases alone in the anthracycline-taxane era: a retrospective analysis of two prospective, randomised metastatic breast cancer trials. Eur J Cancer. 2003;39(17):2439–2449. doi: 10.1016/s0959-8049(03)00601-4. [DOI] [PubMed] [Google Scholar]
- Jardines L, Callans L S, Torosian M G. Recurrent breast cancer: Presentation, diagnosis and treatment. Semin Oncol. 1993;20:538–547. [PubMed] [Google Scholar]
- Elias D, Maisonnette F, Druet-Cabanac M, et al. An attempt to clarify indications for hepatectomy for liver metastases from breast cancer. Am J Surg. 2003;185:158–164. doi: 10.1016/s0002-9610(02)01204-7. [DOI] [PubMed] [Google Scholar]
- Pentheroudakis G, Fountzilas G, Bafaloukos D, et al. Metastatic breast cancer with liver metastases: A registry analysis of clinicopathologic, management and outcome characteristics of 500 women. Breast Cancer Res Treat. 2005;97:237–244. doi: 10.1007/s10549-005-9117-4. [DOI] [PubMed] [Google Scholar]
- Elias D, Lasser P H, Spielmann M, et al. Surgical and chemotherapeutic treatment of hepatic metastases from carcinoma of the breast. Surg Gynecol Obstet. 1991;172:461–464. [PubMed] [Google Scholar]
- Lawes D, Chopada A, Gillams A, et al. Radiofrequency ablation as a cytoreductive strategy for hepatic metastasis from breast cancer. Ann R Coll Surg Engl. 2006;88:639–642. doi: 10.1308/003588406X149129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R, Aloia T, Krissat J, et al. Is liver resection justified for patents with hepatic metastases from breast cancer? Ann Surg. 2006;244:897–908. doi: 10.1097/01.sla.0000246847.02058.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley S A, Cusack J C, Jr, Tanabe K K, Stoelzing O, Ellis L M. Advances in the treatment of liver tumors. Curr Probl Surg. 2002;39:449–571. doi: 10.1067/msg.2002.122810. [DOI] [PubMed] [Google Scholar]
- Denys A L, De Baere T, Mahe C, et al. Radio-frequency tissue ablation of the liver: effects of vascular occlusion on lesion diameter and biliary and portal damages in a pig model. Eur Radiol. 2001;11(10):2102–2108. doi: 10.1007/s003300100973. [DOI] [PubMed] [Google Scholar]
- Patterson E J, Scudamore C H, Owen D A, et al. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227(4):559–565. doi: 10.1097/00000658-199804000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, Kurokawa F, Shirahashi H, et al. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2002;95(11):2353–2360. doi: 10.1002/cncr.10966. [DOI] [PubMed] [Google Scholar]
- Elias D, Sideris L, Pocard M, et al. Intraductal cooling of the main bile ducts during radiofrequency ablation prevents biliary stenosis. J Am Coll Surg. 2004;198(5):717–721. doi: 10.1016/j.jamcollsurg.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Stippel D L, Bangard C, Kasper H U, et al. Experimental bile duct protection by intraductal cooling during radiofrequency ablation. Br J Surg. 2005;92(7):849–855. doi: 10.1002/bjs.5002. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Goldberg S N, Solbiati L, et al. Percutaneous radio-frequency ablation of liver metastases from breast cancer: initial experience in 24 patients. Radiology. 2001;220(1):145–149. doi: 10.1148/radiology.220.1.r01jl01145. [DOI] [PubMed] [Google Scholar]
- Sofocleous C T, Nascimento R G, Gonen M, et al. Radiofrequency ablation in the management of liver metastases from breast cancer. AJR Am J Roentgenol. 2007;189:883–889. doi: 10.2214/AJR.07.2198. [DOI] [PubMed] [Google Scholar]
- Gunabushanam G, Sharma S, Thulkar S, et al. Radiofrequency ablation of liver metastases from breast cancer: results in 14 patients. J Vasc Interv Radiol. 2007;18:67–72. doi: 10.1016/j.jvir.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Pocard M, Pouillart P, Asselain B, Salmon R. Hepatic resection in metastatic breast cancer: results and prognostic factors. Eur J Surg Oncol. 2000;26:155–159. doi: 10.1053/ejso.1999.0761. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg S N, Gazelle G S. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach”. Cancer. 2003;97(12):3027–3035. doi: 10.1002/cncr.11426. [DOI] [PubMed] [Google Scholar]
- Dodd G D, III, Frank M S, Aribandi M, et al. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001;177:777–782. doi: 10.2214/ajr.177.4.1770777. [DOI] [PubMed] [Google Scholar]
- Solbiati L, Livraghi T, Goldberg S N, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- Goldberg S N, Grassi C J, Cardella J F, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728–739. doi: 10.1148/radiol.2353042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livraghi T, Solbiati L, Meloni M F, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]