ABSTRACT
Vertebral fractures account for ~27% of all osteoporotic fractures in both men and women. The economic burden is substantial and growing: osteoporosis is expected to affect 14 million people by the year 2020. There is substantial morbidity associated with osteoporotic vertebral compression fractures (VCFs) including decreased quality of life, reduced pulmonary function, and increased mortality. Relatively recent additions to the treatment armamentarium include vertebral augmentation using vertebroplasty and kyphoplasty. Numerous retrospective and case studies demonstrate short-term efficacy and low complication rates of vertebroplasty and kyphoplasty in the treatment of osteoporotic VCFs, but controlled trials are needed for validation. The pathophysiology, risk factors, consequences, characteristics, and imaging of osteoporotic VCFs are presented in detail along with a discussion of treatment options and patient selection. Vertebral augmentation is comprehensively reviewed, including the technical aspects of the procedures, contraindications, complications, and clinical outcomes.
Keywords: Vertebral compression fracture, vertebroplasty, kyphoplasty, osteoporosis, vertebral augmentation
Osteoporosis is a systemic skeletal disorder characterized by compromised bone strength, low bone mass, and disruption of bone architecture, predisposing to an increased risk of fracture. The World Health Organization (WHO) defines osteoporosis as having a bone mineral density (BMD) at the hip or lumbar spine greater than 2.5 standard deviations below the young normal adult reference population. It is the most common bone disease in the United States and represents a major public health problem. More than 10 million Americans (8 million women and 2 million men) have osteoporosis and an additional 33.6 million individuals have low BMD.1 It is estimated that the prevalence of osteoporosis will increase to 14 million people by 2020.2
Vertebral fractures are the most common osteoporotic fracture in postmenopausal women. It is estimated that there are 550,000 to 700,000 osteoporotic vertebral compression fractures (VCFs) annually, which account for ~27% of all osteoporotic fractures in both men and women.2,3 The incidence is difficult to quantify accurately as only 23 to 33% of these fractures are clinically evident.4
The economic burden is substantial and growing. Estimates of direct medical costs attributed to osteoporosis in the United States are between 13.7 to 20.3 billion dollars with osteoporotic VCFs accounting for ~1.1 billion dollars. By 2025, analysts project the number of annual overall fractures and costs to rise nearly 50%.2 Invariably, the actual costs are much higher because it is difficult to account for indirect costs of disability such as time off work, pain, diminished mobility, insomnia, and depression. Vertebral compression fractures in people over 45 years of age account for 150,000 hospital admissions, 161,000 physician office visits, and over 5 million restricted activity days annually in the United States.5
PATHOGENESIS OF OSTEOPOROTIC VERTEBRAL COMPRESSION FRACTURE
Bone is composed of a cortical component and metabolically active trabecular component. Remodeling of bone is a continuous process and a balance between bone formation and resorption. Peak bone mass is achieved by age 25 to 30.1 After this age, there is a steady 3 to 5% rate of bone loss per decade.6 Bone loss results in a decreased number of trabecular plates, leaving a weakened architectural structure and increased bone fragility and risk of fracture.7 Trabecular thinning and bone loss occurs with advancing age in both men and women, but to a greater extent in women.8 Within the first decade after menopause, bone loss affecting the lumbar spine nearly triples in women.9 Bone loss can also occur secondary to other causes, such as long-term steroid use, which accelerates bone resorption by osteoclasts. Osteoporotic VCFs occur when the combined axial and bending loads on the spine exceed the strength of the vertebral body.10
RISK FACTORS FOR OSTEOPOROTIC VERTEBRAL COMPRESSION FRACTURE
Low BMD is associated with an increased risk of vertebral fracture.11 Risk for vertebral fracture increases greater than 4-fold with each standard deviation decrease from the mean BMD.12 The risk of osteoporotic VCF also increases with age. Ten percent of white women 50 to 54 years old and 50% of women 80 to 84 years old have at least one vertebral fracture.13 With advancing age, each 5-year increment increases the risk of vertebral fracture up to 2.0 times.12
A prevalent or preexisting fracture is a predictor of a future fracture, independent of BMD, and is associated with a 5-fold increased risk.13 The combination of prevalent fractures and low BMD is an even stronger predictor of risk. For each standard deviation decrease in baseline BMD value below the mean for a young healthy population, there is a 60% increase in risk for fracture within the first year following the incident fracture.4 The absolute risk of vertebral fractures is greater than 50% in women with both a previous fracture and BMD in the osteoporotic range.11
The prevalence and incidence of fractures are highest at T7–8 in the upper thoracolumbar spine, and at T12-L1 in the lower spine.13 Other factors such as smoking, low body mass index (BMI), low levels of daily physical activity, falls, and low calcium uptake during periods of high calcium needs (pregnancy or during teenage years) are also associated with increased risk of a first vertebral fracture.14
OSTEOPOROTIC VERTEBRAL COMPRESSION FRACTURE CHARACTERISTICS
Osteoporotic VCFs may be an incidental radiographic finding or a symptomatic clinical event. VCFs associated with severe trauma, including falls from greater than standing height, are nearly always symptomatic, but not all VCFs that result from minimal to moderate trauma cause back pain.15 Many osteoporotic VCFs occur spontaneously or with usually innocuous activities such as sneezing or twisting.
Just as not all osteoporotic VCFs that occur spontaneously or with minimal to moderate trauma are symptomatic, the intensity and duration of pain vary from patient to patient. Lyritis et al studied the natural history of VCF in 210 postmenopausal women with painful osteoporotic VCFs and identified two groups.16 In individuals with type I fractures, the osteoporotic VCF was radiographically evident and a single episode of pain was severe and acute, persisting for 4 to 8 weeks. In type II fractures, the fracture was not clear radiographically, but a wedge deformity gradually developed over the next few months. The pain in type II fractures was less severe and of shorter duration than type I, but a new attack of pain occurred after 6 to 16 weeks and often recurred over a period of 6 to 18 months. In this study, the women with type I fractures had a lower BMD than type II.16
Acute osteoporotic VCF pain is usually associated with intense, deep pain, tenderness to palpation at the site of the fracture, and often lasts from 2 weeks to 3 months. Prolonged sitting, standing, bending, and motion exacerbate the pain. Rest, recumbency, heat, and diversion may produce symptomatic relief. Paraspinal muscle spasm and ligament tenderness are common and can extend several levels up or down from the site of fracture. Irritation or compression of nerve roots can result from the fracture, with pain radiating anteriorly along the rib cage with thoracic osteoporotic VCFs or down into the buttocks or legs with lumbar osteoporotic VCFs. Spinal cord compression and myelopathy are rare with osteoporotic VCFs, but are more commonly reported in patients with vertebral metastases.17,18
Chronic pain can develop following VCF and may occur after an asymptomatic period of variable length. The etiology of chronic pain is unknown, but likely results from multiple sources including accentuated kyphosis, paraspinal muscle spasm and fatigue, neural irritation, facet joint arthroses, and physical deconditioning. The risk of chronic pain increases with the number of fractured vertebrae.6
CONSEQUENCES OF OSTEOPOROTIC VERTEBRAL COMPRESSION FRACTURE
There is substantial morbidity with osteoporotic VCFs. Patients experience reduced quality of life, difficulties with activities of daily living, loss of independence, depression or low self-esteem, impaired gait, poor balance, and higher mortality rates.15,19,20,21,22 Vertebral body height loss and progressive kyphosis, especially in patients with multiple osteoporotic VCFs, result in reduction in volume of the thoracic and abdominal cavities leading to reduced pulmonary function and early satiety, respectively.15,19 Even patients with asymptomatic osteoporotic VCF or those who are nonsmokers may have a decline in pulmonary function tests due to the increased kyphotic deformity.23,24,25 Patients with asymptomatic osteoporotic VCF also experience decreased quality of life, increased hospitalization, and mortality.26,27
Multiple studies suggest increased mortality with osteoporotic VCFs.20,26,28,29 In a study of 6,459 women with low bone mass aged 55 to 81 years, there was a 1.5-fold increased risk of death in women with prevalent osteoporotic VCFs compared with women without existing vertebral deformities.26 In a European cohort study of 6,480 men and women aged 50 to 79 years, adjusted mortality rate ratios were 1.6 for women and 1.2 for men in those with vertebral deformities compared with those without deformities.29 The risk was reduced after adjusting for adverse health and lifestyle factors including smoking, BMI, alcohol consumption, and general health. The rate of mortality also increased with increasing number of osteoporotic VCFs; women with 3 or more vertebral deformities were 4 times as likely to die when compared with women without deformities.26
IMAGING
For the evaluation of back pain, patients often undergo plain radiographs of the spine in the lateral and anteroposterior (AP) projections. Plain radiographs can be used to evaluate the degree of height loss and progression of deformity on sequential images. Prior comparison films can help determine if an event is acute, but the age of the fracture can be difficult to determine accurately. Osteopenic or osteoporotic bone makes subtle fractures difficult to detect.
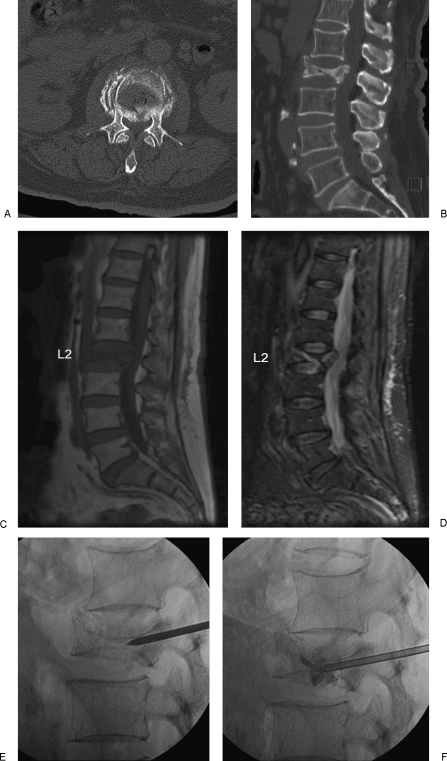
Magnetic resonance imaging (MRI) can differentiate between acute, subacute, and healed osteoporotic VCFs and allows assessment of the spinal canal, retropulsed fragments, and presence or absence of spinal cord compression. MRI can also uncover other causes for back pain such as malignancy or spinal stenosis. Acute fractures exhibit low signal intensity on T1-weighted sequences and high signal intensity on heavily T2-weighted sequences such as short tau inversion recovery (STIR) sequences (Fig. 1). Complete replacement of the marrow, involvement of the posterior elements, and/or an associated epidural or paraspinal mass suggest malignancy, but these findings are not specific and may occur with benign fractures. In cases suspicious for tumor, a biopsy can be performed at the time of vertebral augmentation.
Figure 1.
A 71-year-old female with osteoporosis and intractable back pain after a fall. (A) Axial CT image and sagittally reconstructed image (B) demonstrates a severe L2 vertebral compression fracture with retropulsion into the spinal canal. Note that the fracture does not extend into the pedicles. Abnormal marrow signal representing edema is hypointense on the T1-weighted (C) and hyperintense on the short tau inversion recovery (STIR) (D) MR images. (E,F) Unipedicular approach with careful placement of the vertebroplasty needle and injection of polymethylmethacrylate (PMMA) under biplane fluoroscopic guidance.
Computed tomography (CT) and bone scan are alternative imaging modalities in patients who cannot undergo MRI scanning. CT is ideal to evaluate the integrity of the posterior wall of the vertebral body and to evaluate suspected pedicle or posterior element fractures (Fig. 1). This information is useful to determine the appropriate needle path. Bone scans have high sensitivity, but low specificity for vertebral fractures and can remain positive for over one year after substantial healing has occurred (Fig. 2).
Figure 2.
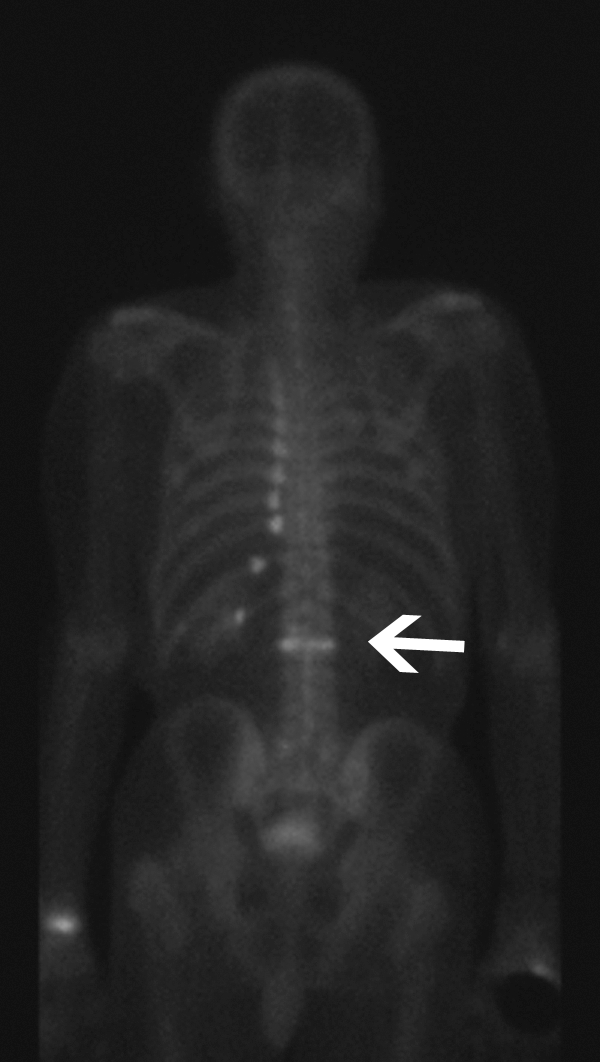
Tc-99m-labeled radionuclide bone scan (posterior view) shows increased radiotracer uptake secondary to an upper lumbar vertebral compression fracture (white arrow) and multiple left rib fractures.
CONSERVATIVE THERAPY
Conservative therapy of osteoporotic VCFs includes narcotics, nonsteroidal antiinflammatory drugs (NSAIDs), bed rest, external bracing, physical therapy/exercise, and medical treatment of osteoporosis. If radicular pain is present, nerve root blocks or epidural injections with steroids and/or anesthetics may be beneficial. Medications that address neurogenic pain including antidepressants, anticonvulsants, and α − 2 agonists may be useful to treat pain that is chronic in nature.30 Treatments of osteoporosis such as hormone replacement therapy, calcitonin, bisphosphonates, raloxifene, and Teriparatide (Eli Lilly & Co., Indianapolis, IN) may decrease pain by reducing the risk of fractures.31 Additionally calcitonin, intravenous bisphosphonates, and Teriparatide may directly relieve bone pain.17
Spinal orthoses or braces can reduce pain by reducing motion, decreasing postural flexion, and providing axial support in patients with muscle fatigue and spasm. Physical therapy improves body mechanics and posture, which may reduce pain. Exercise may provide benefit by increasing muscle strength, improving flexibility and balance, maintaining BMD, and reducing the incidence of additional osteoporotic VCFs.30,32
COMPLICATIONS OF CONSERVATIVE THERAPY
Although narcotics may be necessary to treat pain induced by VCF, adverse drug reactions such as cognitive impairment, sedation, and constipation can occur, which may be especially debilitating in elderly patients. Additionally, NSAIDs can cause gastrointestinal problems such as gastritis and ulcers.
Bed rest and immobilization aggravates bone loss, which is reported to occur at a rate of BMD losses of 0.25 to 1% per week.6 Muscle strength decreases 10 to 15% per week, with almost half of normal strength lost within 3 to 5 weeks of immobilization.33 Immobility can also lead to decreases in cardiac function, endurance, and respiratory capacity in addition to predisposing individuals to deep venous thrombosis, pulmonary embolism, pressure sores, glucose intolerance, poor appetite, and ligament contractures.33
Adverse effects of braces include noncompliance, poor fit in obese patients, expense, and difficulty in putting on and removing the device. Atrophy of supported back muscles can also occur with ongoing use of the rigid braces.
VERTABRAL AUGMENTATION
Percutaneous vertebroplasty was the first image-guided percutaneous vertebral augmentation performed. In 1984, Deramond and Galibert injected polymethylmethacrylate (PMMA) into a C2 vertebral body to treat pain caused by an aggressive hemangioma.34 Vertebroplasty was used to treat osteoporotic VCFs shortly afterwards and was introduced in the United States at the University of Virginia.35 Kyphoplasty was first performed in 1998. The technique was developed by Dr. Mark Reiley, an orthopedic surgeon, and designed to not only stabilize VCFs, but also to restore vertebral body height and minimize the associated kyphotic deformity with the use of an inflatable bone tamp.36 Since 1993, the number of inpatient vertebroplasty and kyphoplasty procedures in the United States have increased from a combined total of 182 to 23,691 in 2004 (a nearly 130-fold increase).37 The overall number of vertebral augmentation procedures for the treatment of osteoporotic VCFs is substantially higher because many procedures occur in the outpatient setting.
PATIENT SELECTION/INDICATIONS
Vertebral augmentation with either vertebroplasty or kyphoplasty is indicated for treatment of painful primary and secondary osteoporotic VCFs refractory to medical therapy. Ideal patients have pain corresponding to the level of the compression fracture, which worsens with bending, prolonged standing or sitting, and improves with rest and recumbency. There is usually focal tenderness over the site of the fractured vertebrae, but this is not an absolute indication for intervention. In one study, 9 out of 100 patients who had back pain, but tenderness either distant or lateral to the fracture had significant pain improvement following vertebroplasty.38 An acute or subacute vertebral fracture should be verified by the presence of a fracture and edema on MRI or increased uptake on bone scan. Some radicular pain can be present that may require adjuvant therapy, but it should not be the primary component of the patient's pain.
Timing of the intervention is controversial in terms of what defines “refractory to medical therapy” or failure of medical management. Some practitioners will wait to intervene until after 2 to 6 weeks of nonoperative treatment, whereas others treat patients within days after the occurrence. Early intervention may be helpful if the pain is severe enough to require hospitalization and parenteral narcotics. Earlier vertebral augmentation has also been performed in those patients who have complications of nonoperative treatment, are nonambulatory because of refractory pain, at significant risk for functional decline, or have symptomatic progressive vertebral collapse on imaging. Other investigators advocate immediate treatment because they believe that an additional goal besides pain relief should be to correct the kyphotic deformity, which is thought to increase the risks of future fractures due to alterations in spinal load distributions.39
PRECAUTIONS/CONTRAINDICATIONS
Vertebral augmentation with either vertebroplasty or kyphoplasty is not indicated or approved for asymptomatic osteoporotic VCFs or for prophylactic treatment of osteoporotic VCFs. Active infection, sepsis, cord compression, allergy to PMMA, and uncorrectable coagulopathy are contraindications for vertebral augmentation. Myelopathy with neurologic signs or deficits should be evaluated for surgical decompression.
Radiculopathy is not an absolute contraindication for vertebral augmentation, but the procedure may not improve and could possibly exacerbate symptoms. This is especially true if the radicular pain is in excess of the vertebral pain. Imaging of patients with congenital or acquired spinal stenosis should be carefully reviewed for retropulsion of the posterior cortex or retropulsed bone fragments, as further retropulsion and canal narrowing can occur following the PMMA injection. Retropulsion with spinal canal compromise is no longer an absolute contraindication to augmentation, as vertebroplasty and kyphoplasty have been safely performed in this clinical setting.40 These cases, however, should be approached with caution and performed by experienced operators.
Height loss greater than 70% or vertebra plana is technically challenging for needle placement, but procedures in many of these cases have been successfully performed with good clinical results.41 The lateral portions of the vertebral body are usually not as compressed as the center, and the needles can be positioned via a posterolateral or extrapedicular lateral approach. With kyphoplasty, advancement and inflation of the bone tamps can be done gradually to allow advancement of the system with access into the anterior portion of the vertebral body.
Fractures above T5 are technically difficult to treat due to the small size and parallel orientation of the pedicles. Fluoroscopic visualization, even with high quality equipment is poor due to the osteoporotic bone and obscuration by the shoulders. If the fracture involves the pedicle, an extrapedicular approach may prove to be a safer alternative to the standard transpedicular approach.
TECHNICAL ASPECTS OF VERTEBROPLASTY AND KYPHOPLASTY
High quality fluoroscopic equipment is essential to the performance of safe and successful vertebroplasty and kyphoplasty. Biplane fluoroscopy is not a requirement but may decrease procedure time, which is advantageous when treating frail patients with numerous comorbidities. Several commercial self-contained vertebroplasty kits contain access needles, radiopaque cement, and various cement delivery systems. Kyphoplasty differs from vertebroplasty by the addition of an inflatable bone tamp that is used to create a cavity prior to cement deposition.
Sedation and analgesia, monitored anesthesia, and general anesthesia have all been used during vertebral augmentation procedures. There are advantages and disadvantages for each type of sedation. General anesthesia is used most often when the procedure is performed in the operating room or when treating multiple levels. The type of sedation provided is often operator and institution dependent.
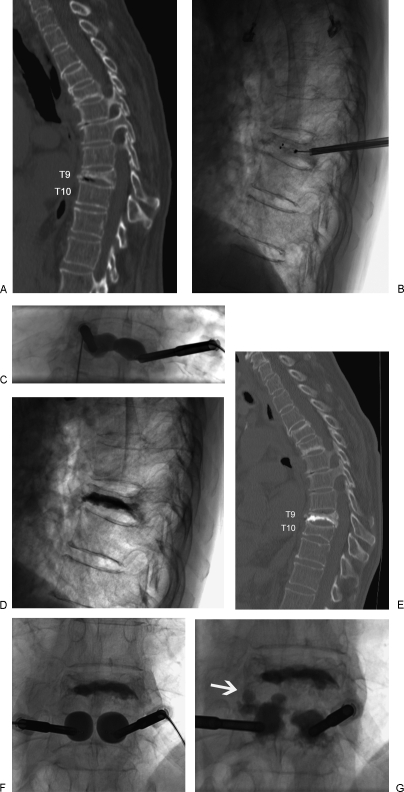
Both unipedicular (Fig. 1) and bipedicular (Fig. 3) vertebral body access have been described and reported for vertebroplasty and kyphoplasty.42,43 Advantages of unipedicular access are decreased procedure time, cost, and risk from the single needle placement. However, conversion to a bipedicular approach is needed if PMMA does not cross the midline and achieve contralateral filling of the vertebral body. Early on, many practitioners performed antecedent interosseous venograms prior to cement injection to assess the risk of cement leakage and identify direct venous connections, but the venograms had questionable efficacy and are no longer routinely used.44,45
Figure 3.
A 82-year-old female with osteoporosis and intractable back pain after a fall. (A) Sagittally reconstructed CT image demonstrates a severe T9 VCF. (B) Lateral radiograph with uninflated bone tamps placed via bipedicular approach. (C) Anteroposterior radiograph of inflated bone tamps. (D) Post-kyphoplasty lateral radiograph. One-month post-kyphoplasty sagittally reconstructed CT image (E) obtained for new back pain demonstrates an adjacent T10 VCF. (F,G) Anteroposterior radiographs of inflated bone tamps and subsequent PMMA deposition with extension of PMMA into the T9-T10 disc space (white arrow).
PMMA is injected into the vertebral body after appropriate positioning of the needles or trocars under fluoroscopy. Adequate cement opacification is essential to being able to identify a small leak; most commercially available cement in the United States adds barium sulfate (up to 30% by weight). The consistency of the cement is also important because there is an inverse relationship between viscosity and working time. The cement delivered during vertebroplasty tends to be thinner in consistency to fill the interstices of cancellous bone compared with that used in kyphoplasty, where the cement fills the cavities created by the bone tamps. In either case, the cement should not be injected in a runny, liquid-like state. Additionally, to prevent nontarget deposition, monitoring with continuous fluoroscopy should be used. With single plane equipment, fluoroscopic monitoring in the lateral projection with periodic checks in the anteroposterior projection is used to evaluate for lateral cement extravasation. Cement deposition should be terminated if flow into a blood vessel or toward the posterior cortical margin is noted.
POSSIBLE MECHANISMS OF PAIN RELIEF
The exact mechanism of pain relief is unknown. One theory is related to mechanical stabilization of the vertebral body and improved load-bearing ability.46,47 In this theory, mechanical stabilization prevents further collapse and painful micro motion of the vertebral fracture.47 Fracture reduction may allow the anterior and posterior longitudinal ligaments to realign into a more anatomical position with a resultant decrease in pain from pain fibers. Pain relief may result from damage to local pain receptors due to exposure to unreacted cytotoxic methacrylate monomer.47 Additionally, the exothermic polymerization of PMMA generates a significant amount of heat that may result in thermal injury to free nerve endings.47
CLINICAL OUTCOMES
Over 100 studies on vertebroplasty and kyphoplasty assess clinical outcomes, but the majority of these investigations are case study or retrospective cohort designs. A meta-analysis of 14 vertebroplasty and 7 kyphoplasty studies that used the visual analog scale (VAS) to measure pain relief found that greater than 90% of patients had an immediate improvement in their symptoms, and ~50% of individuals reported a reduction of the amount of pain in the immediate postoperative period.48 There was no significant difference between vertebroplasty and kyphoplasty. In another literature review of 32 vertebroplasty studies and 7 kyphoplasty studies, 87% and 92% of patients had pain relief following vertebroplasty and kyphoplasty, respectively.49
In three studies comparing kyphoplasty with conventional medical treatment, kyphoplasty resulted in a significant reduction in pain as measured by VAS. The pain improvement was greater than medical care treatment at 3, 6, 12, and 36 months follow-up. Functional capacity improved after kyphoplasty and exceeded conventional medical care at 6 months follow-up.50 In a study comparing vertebroplasty and conservative therapy, Diamond et al demonstrated a 53% improvement in pain scores and a 29% improvement in physical functioning 24 hours after vertebroplasty. This compared with no improvement in the patients treated conservatively. There was, however, no significant long-term improvement, with similar outcomes noted at 6 weeks, 6 months, and 12 months.51 In a prospective, randomized study comparing vertebroplasty with optimal pain medication treatment (VERTOS study), vertebroplasty patients used fewer analgesics and had significantly better VAS scores 24 hours after intervention. At 2 weeks, vertebroplasty patients used fewer analgesics and had significantly better quality of life and disability scores. In this particular prospective randomized study, 14 of the 16 patients in the optimal pain medication group crossed over into the vertebroplasty group.52
HEIGHT RESTORATION CONUNDRUM
The intent of kyphoplasty is to restore vertebral body height and correct the kyphotic deformity. In a kyphoplasty phase I efficacy study, the mean central vertebral height lost following fracture was 8.7 mm, and the mean percentage of height restoration was 35% (2.9 mm).36 A separate study reported an average kyphosis correction of 62.4 ± 16.7%.53 Proponents of kyphoplasty have touted height restoration and correction of kyphotic deformity as advantages of this procedure over vertebroplasty. As a result, there have been several vertebroplasty studies also reporting height restoration and correction of kyphosis.49,54,55 Factors such as age of the fracture, preoperative fracture mobility, and the presence of intravertebral clefts may influence the degree of possible height restoration. It is difficult to accurately compare and contrast results of the two procedures as calculation methods, reporting methodology, and confounding factors such as the age, severity, and mobility of the fractures are neither controlled for nor standardized. Although the theoretical benefits of height restoration and deformity correction are intuitive, the actual clinical significance is unknown. Height restoration and kyphosis correction has not been shown to translate into a similar correction in overall spinal sagittal alignment.56 In addition, studies have not found a significant relationship between pain relief and improvement in vertebral height.50
COMPLICATIONS OF VERTEBRAL AUGMENTATION
The complication rates of both vertebroplasty and kyphoplasty are low, ranging from 1 to 3% in osteoporotic VCFs to up to 10% when performed for malignant tumor-related VCFs.6,57 Complications include those related to needle placement, cement extravasation, infection, bleeding, and iatrogenic fractures. Iatrogenic pedicle fractures may result from excessive torque on the needle during placement. Due to fragility of osteoporotic bone, fractures of the ribs and hips can occur from transmitted forces during needle placement with the patient lying prone.
The cement can leak into the disk space (Fig. 3), paravertebral tissues, epidural space, neural foramina, or venous system. The majority of these cement leaks is asymptomatic, but a significant leak into the spinal canal or neural foramina can result in worsening pain, radiculopathy, or spinal cord compression. Fortunately, most of these symptoms can be managed with steroids, narcotics, nerve blocks, or epidural injections; intractable symptoms or neurologic compromise may require surgical intervention. Migration of PMMA through the epidural or paravertebral venous system may result in pulmonary embolism. Emboli are usually asymptomatic, but there have been at least 3 deaths reported in the literature.58,59 Other lethal consequences have been reported such as paradoxical cerebral embolism,60 renal artery embolism,61 cardiac perforation, and tricuspid regurgitation.62,63
Cement extrusion into the disk may be a risk factor for a VCF of an adjacent vertebra. In a retrospective study by Lin et al., fractures in 10 out of 14 patients during a one year follow-up period were associated with cement leakage into the disk.64 A separate study by Lazary et al suggests that vertebral filler materials such as PMMA can accelerate degeneration of nucleus pulposus cells, resulting in a less flexible disk and possibly an increased risk of new vertebral fractures.65
New adjacent and nonadjacent VCFs have been described following both vertebroplasty and kyphoplasty (Fig. 3).66,67,68,69,70,71,72 The rate of subsequent VCF may be higher in patients with secondary osteoporosis due to steroids as opposed to primary osteoporosis. In a study of 115 patients, the incidence of new fractures in patients with secondary osteoporosis and primary osteoporosis was 48.6% and 11.3%, respectively.70,71 The high incidence of new fractures within the first 2 to 3 months following vertebral augmentation may be the result of increased mobility or activity secondary to the relief of pain.49,68
Questions remain as to whether vertebral augmentation results in an increased number of new fractures or if this phenomenon is a result of the natural history of osteoporosis. Grados et al calculated the odds ratio to be 2.27 for a VCF in the vicinity of the cemented vertebra as opposed to 1.44 for a VCF in the vicinity of an uncemented fractured vertebra.66 In another study, 67% of subsequent VCF were adjacent to a previously treated level, compared with 33% of fractures that occurred in a nonadjacent vertebral body.68 In recent analyses of risk factors for new osteoporotic VCF after vertebral augmentation, a low BMI was a statistically significant factor between patients who did or did not sustain a new fracture.57,72 Other studies have confirmed this association between a low BMI and a higher prevalence of osteoporosis and VCF.14,57 In a study of 111 women with osteoporotic VCF treated with kyphoplasty, there was a lower incidence rate (15.5%) of new compression fractures than the rate (19.2%) due to the progression of osteoporosis, which the authors hypothesized may be due to the high percentage (93.7%) of patients taking antiosteoporotic medication.72 Only one study compares the new vertebral fracture rate after vertebral augmentation with a conservative treatment group.73 It found no significant difference but it had limited statistical power and a short follow-up period.49,73
FUTURE DIRECTION
Despite the hundreds of published articles on vertebroplasty and kyphoplasty for osteoporotic VCFs, there are still several unanswered questions and issues, including long-term clinical efficacy, economic impact, and cost effectiveness of the treatments. The clinical significance of height restoration is unclear. Optimal timing of vertebral augmentation is controversial as is the effect of vertebral augmentation on future fracture rate. More information is needed to determine whether these procedures decrease morbidity or mortality in the long-term, and what factors influence complication rates and successful outcomes. Currently, kyphoplasty is ~2.5 times more expensive than vertebroplasty and it is unknown whether the reported advantages justify the added cost, or if certain subgroups of patients may derive more benefit from one particular procedure.33,74
Randomized controlled trials are needed to answer these questions. Currently there are two randomized controlled trials underway designed to compare vertebroplasty with kyphoplasty in the treatment of osteoporotic VCFs. The purpose of KAVIAR (kyphoplasty and vertebroplasty in the augmentation and restoration of vertebral body compression fractures) is to evaluate short- and long-term safety and effectiveness in a head-to-head comparison of vertebroplasty and kyphoplasty.75 A second randomized trial, currently underway at the Mayo Clinic (Rochester, MN), is designed to compare the cost effectiveness and efficacy between the two procedures.76 Several other trials are registered and/or underway comparing vertebroplasty and kyphoplasty to conservative therapy, vertebroplasty against placebo treatment, and evaluating refracture rates after prophylactic vertebroplasty of adjacent vertebrae.
CONCLUSIONS
Osteoporosis is a common and widespread disease. As the population continues to grow and age, osteoporotic VCFs will likely become an even greater health care concern. Percutaneous vertebral augmentation in the form of vertebroplasty and kyphoplasty is an additional tool in our armamentarium for the treatment of symptomatic VCFs. Both procedures relieve pain, restore function, and have low complication rates; nevertheless, many questions and issues remain that hopefully will be answered with ongoing research and controlled clinical trails.
REFERENCES
- National Osteoporosis Foundation Clinician's Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2008; Accessed March 26, 2008. Available at: http://www.nof.org/professionals/NOF_Clinicians%20_Guide.pdf
- Burge R, Dawson-Hughes B, Solomon D H, Wong J B, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- Black D M, Arden N K, Palermo L, Pearson J, Cummings S R. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14:821–828. doi: 10.1359/jbmr.1999.14.5.821. [DOI] [PubMed] [Google Scholar]
- Lindsay R, Silverman S I, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- Riggs B L, Melton L J. Epidemiology of osteoporosis. Bone. 1995;17(suppl 1):S505–S511. doi: 10.1016/8756-3282(95)00258-4. [DOI] [PubMed] [Google Scholar]
- Rao R D, Singrakhia M D. Painful osteoporotic vertebral fracture. Pathogenesis, evaluation, and roles of vertebroplasty and kyphoplasty in its management. J Bone Joint Surg Am. 2003;85-A:2010–2022. [PubMed] [Google Scholar]
- Parfitt A M. Implications of architecture for the pathogenesis and prevention of vertebral fracture. Bone. 1992;13(suppl 2):S41–S47. doi: 10.1016/8756-3282(92)90196-4. [DOI] [PubMed] [Google Scholar]
- Mellish R W, Garrahan N J, Compston J E. Age-related changes in trabecular width and spacing in human iliac crest biopsies. Bone Miner. 1989;6:331–338. doi: 10.1016/0169-6009(89)90038-x. [DOI] [PubMed] [Google Scholar]
- Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int. 2002;13:105–112. doi: 10.1007/s001980200001. [DOI] [PubMed] [Google Scholar]
- Mathis J M, Barr J D, Belkoff S M, Barr M S, Jensen M E, Deramond H. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol. 2001;22:373–381. [PMC free article] [PubMed] [Google Scholar]
- Cauley J A, Hochberg M C, Lui L Y, et al. Long-term risk of incident vertebral fractures. JAMA. 2007;298:2761–2767. doi: 10.1001/jama.298.23.2761. [DOI] [PubMed] [Google Scholar]
- LaFleur J, McAdam-Marx C, Kirkness C, Brixner D I. Clinical risk factors for fracture in postmenopausal osteoporotic women: a review of the recent literature. Ann Pharmacother. 2008;42:375–386. doi: 10.1345/aph.1K203. [DOI] [PubMed] [Google Scholar]
- Nevitt M C, Ross P D, Palermo L, Musliner T, Genant H K, Thompson D E. Association of prevalent vertebral fractures, bone density, and alendronate treatment with incident vertebral fractures: effect of number and spinal location of fractures. Bone. 1999;25(5):613–619. doi: 10.1016/s8756-3282(99)00202-1. [DOI] [PubMed] [Google Scholar]
- Nevitt M C, Cummings S R, Stone K L, et al. Risk factors for a first-incident radiographic vertebral fracture in women > 65 years of age: the study of osteoporotic fractures. J Bone Miner Res. 2005;20:131–140. doi: 10.1359/JBMR.041003. [DOI] [PubMed] [Google Scholar]
- Silverman S L. The clinical consequences of vertebral compression fracture. Bone. 1992;13(suppl 2):S27–S31. doi: 10.1016/8756-3282(92)90193-z. [DOI] [PubMed] [Google Scholar]
- Lyritis G P, Mayasis B, Tsakalakos N, et al. The natural history of the osteoporotic vertebral fracture. Clin Rheumatol. 1989;8(suppl 2):66–69. doi: 10.1007/BF02207237. [DOI] [PubMed] [Google Scholar]
- Francis R M, Aspray T J, Hide G, Sutcliffe A M, Wilkinson P. Back pain in osteoporotic vertebral fractures. [published online ahead of print December 11, 2007] Available at: http:__www.springerlink.com_content_1m6l7g6l06584367_fulltext.html. Osteoporos Int. Accessed March 30, 2008 doi: 10.1007/s00198-007-0530-x. [DOI] [PubMed] [Google Scholar]
- Truumees E, Garfin S R. In: Resnick DK, Garfin SR, editor. Vertebroplasy and Kyphoplasty. New York: Thieme; 2005. Patient selection in the treatment of pathologic compression fractures. pp. 48–61.
- Gold D T. The clinical impact of vertebral fractures: quality of life in women with osteoporosis. Bone. 1996;18(3 suppl):S185–S189. doi: 10.1016/8756-3282(95)00500-5. [DOI] [PubMed] [Google Scholar]
- Kado D M, Browner W S, Palermo L, Nevitt M C, Genant H K, Cummings S R. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215–1220. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- Gold D T. The nonskeletal consequences of osteoporotic fractures. Psychologic and social outcomes. Rheum Dis Clin North Am. 2001;27:255–262. doi: 10.1016/s0889-857x(05)70197-6. [DOI] [PubMed] [Google Scholar]
- Lombardi I, Jr, Oliveira L M, Mayer A F, Jardim J R, Natour J. Evaluation of pulmonary function and quality of life in women with osteoporosis. Osteoporos Int. 2005;16:1247–1253. doi: 10.1007/s00198-005-1834-3. [DOI] [PubMed] [Google Scholar]
- Leech J A, Dulberg C, Kellie S, Pattee L, Gay J. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis. 1990;141:68–71. doi: 10.1164/ajrccm/141.1.68. [DOI] [PubMed] [Google Scholar]
- Culham E G, Jimenez H A, King C E. Thoracic kyphosis, rib mobility, and lung volumes in normal women and women with osteoporosis. Spine. 1994;19:1250–1255. doi: 10.1097/00007632-199405310-00010. [DOI] [PubMed] [Google Scholar]
- Schlaich C, Minne H W, Bruckner T, et al. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos Int. 1998;8:261–267. doi: 10.1007/s001980050063. [DOI] [PubMed] [Google Scholar]
- Ensrud K E, Thompson D E, Cauley J A, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass: Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48:241–249. doi: 10.1111/j.1532-5415.2000.tb02641.x. [DOI] [PubMed] [Google Scholar]
- Adachi J D, Ioannidis G, Berger C, et al. The influence of osteoporotic fractures on health-related quality of life in community-dwelling men and women across Canada. Osteoporos Int. 2001;12:903–908. doi: 10.1007/s001980170017. [DOI] [PubMed] [Google Scholar]
- Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med. 1997;103(2A):S12–S17. doi: 10.1016/s0002-9343(97)90022-x. [DOI] [PubMed] [Google Scholar]
- Ismail A A, O'Neill T W, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS) Osteoporos Int. 1998;8:291–297. doi: 10.1007/s001980050067. [DOI] [PubMed] [Google Scholar]
- Prather H, Watson J O, Gilula L A. Nonoperative management of osteoporotic vertebral compression fractures. Injury. 2007;38(suppl 3):S40–S48. doi: 10.1016/j.injury.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Agency for Healthcare Research and Quality U.S. Department of Health and Human Services. Comparative Effectiveness Review No. 12. Comparative Effectiveness of Treatments to Prevent Fractures in Men and Women with Low Bone Density or Osteoporosis. AHRQ Publication No. 08–EHC008-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2007. [PubMed]
- Sinaki M, Itoi E, Wahner H W, et al. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone. 2002;30:836–841. doi: 10.1016/s8756-3282(02)00739-1. [DOI] [PubMed] [Google Scholar]
- Jensen M E, McGraw J K, Cardella J F, Hirsch J A. Position statement on percutaneous vertebral augmentation: a consensus statement developed by the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, American Association of Neurological Surgeons/Congress of Neurological Surgeons, and American Society of Spine Radiology. AJNR Am J Neuroradiol. 2007;28:1439–1443. [PMC free article] [PubMed] [Google Scholar]
- Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty [in French] Neurochirurgie. 1987;33:166–168. [PubMed] [Google Scholar]
- Jensen M E, Evans A J, Mathis J M, Kallmes D F, Cloft H J, Dion J E. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol. 1997;18:1897–1904. [PMC free article] [PubMed] [Google Scholar]
- Lieberman I H, Dudeney S, Reinhardt M K, Bell G. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine. 2001;26:1631–1637. doi: 10.1097/00007632-200107150-00026. [DOI] [PubMed] [Google Scholar]
- Lad S P, Patil C G, Lad E M, Boakye M. Trends in pathological vertebral fractures in the United States: 1993–2004. J Neurosurg Spine. 2007;7:305–310. doi: 10.3171/SPI-07/09/305. [DOI] [PubMed] [Google Scholar]
- Gaughen J R, Jr, Jensen M E, Schweickert P A, Kaufmann T J, Marx W F, Kallmes D F. Lack of preoperative spinous process tenderness does not affect clinical success of percutaneous vertebroplasty. J Vasc Interv Radiol. 2002;13:1135–1138. doi: 10.1016/s1051-0443(07)61955-1. [DOI] [PubMed] [Google Scholar]
- Gaitanis I N, Carandang G, Phillips F M, et al. Restoring geometric and loading alignment of the thoracic spine with a vertebral compression fracture: effects of balloon (bone tamp) inflation and spinal extension. Spine J. 2005;5:45–54. doi: 10.1016/j.spinee.2004.05.248. [DOI] [PubMed] [Google Scholar]
- Hiwatashi A, Westesson P L. Vertebroplasty for osteoporotic fractures with spinal canal compromise. AJNR Am J Neuroradiol. 2007;28:690–692. [PMC free article] [PubMed] [Google Scholar]
- Peh W C, Gilula L A, Peck D D. Percutaneous vertebroplasty for severe osteoporotic vertebral body compression fractures. Radiology. 2002;223:121–126. doi: 10.1148/radiol.2231010234. [DOI] [PubMed] [Google Scholar]
- Chang W S, Lee S H, Choi W G, Choi G, Jo B J. Unipedicular vertebroplasty for osteoporotic compression fracture using an individualized needle insertion angle. Clin J Pain. 2007;23:767–773. doi: 10.1097/AJP.0b013e318154b6c3. [DOI] [PubMed] [Google Scholar]
- Chung H J, Chung K J, Yoon H S, Kwon I H. Comparative study of balloon kyphoplasty with unilateral versus bilateral approach in osteoporotic vertebral compression fractures. Int Orthop 2007; September 3. Available at: http://www.springerlink.com/content/y4138584r2622305/ Accessed April 4, 2008. Available at: http://www.springerlink.com/content/y4138584r2622305/ [DOI] [PMC free article] [PubMed]
- Wong W, Mathis J. Is intraosseous venography a significant safety measure in performance of vertebroplasty? J Vasc Interv Radiol. 2002;13:137–138. doi: 10.1016/s1051-0443(07)61929-0. [DOI] [PubMed] [Google Scholar]
- Gaughen J R, Jr, Jensen M E, Schweikert P A, et al. Relevance of antecedent venography in percutaneous vertebroplasty for the treatment of osteoporotic compression fractures. AJNR Am J Neuroradiol. 2002;23:594–600. [PMC free article] [PubMed] [Google Scholar]
- Belkoff S M, Mathis J M, Fenton D C, Scribner R M, Reiley M E, Talmadge K. An ex vivo biomechanical evaluation of an inflatable bone tamp used in the treatment of compression fracture. Spine. 2001;26:151–156. doi: 10.1097/00007632-200101150-00008. [DOI] [PubMed] [Google Scholar]
- Belkoff S M, Molloy S. Temperature measurement during polymerization of polymethylmethacrylate cement used for vertebroplasty. Spine. 2003;28:1555–1559. [PubMed] [Google Scholar]
- Gill J B, Kuper M, Chin P C, Zhang Y, Schutt R. Comparing pain reduction following kyphoplasty and vertebroplasty for osteoporotic vertebral compression fractures. Pain Physician. 2007;10:583–590. [PubMed] [Google Scholar]
- Hulme P A, Krebs J, Ferguson S J, Berlemann U. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine. 2006;31:1983–2001. doi: 10.1097/01.brs.0000229254.89952.6b. [DOI] [PubMed] [Google Scholar]
- Taylor R S, Fritzell P, Taylor R J. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J. 2007;16:1085–1100. doi: 10.1007/s00586-007-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond T H, Champion B, Clark W A. Management of acute osteoporotic vertebral fractures: a nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy. Am J Med. 2003;114:257–265. doi: 10.1016/s0002-9343(02)01524-3. [DOI] [PubMed] [Google Scholar]
- Voormolen M H, Mali W P, Lohle P N, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS Study. AJNR Am J Neuroradiol. 2007;28:555–560. [PMC free article] [PubMed] [Google Scholar]
- Theodorou D J, Theodorou S J, Duncan T D, Garfin S R, Wong W H. Percutaneous balloon kyphoplasty for the correction of spinal deformity in painful vertebral body compression fractures. Clin Imaging. 2002;26:1–5. doi: 10.1016/s0899-7071(01)00350-3. [DOI] [PubMed] [Google Scholar]
- Mathis J M, Ortiz O, Zoarski G H. Vertebroplasty versus kyphoplasty: a comparison and contrast. AJNR Am J Neuroradiol. 2004;25:840–845. [PMC free article] [PubMed] [Google Scholar]
- Teng M M, Wei C J, Wei L C, et al. Kyphosis correction and height restoration effects of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2003;24:1893–1900. [PMC free article] [PubMed] [Google Scholar]
- Pradhan B B, Bae H W, Kropf M A, Patel V V, Delamarter R B. Kyphoplasty reduction of osteoporotic vertebral compression fractures: correction of local kyphosis versus overall sagittal alignment. Spine. 2006;31:435–441. doi: 10.1097/01.brs.0000200036.08679.1e. [DOI] [PubMed] [Google Scholar]
- Lin W C, Cheng T T, Lee Y C, Wang T N, et al. New vertebral osteoporotic compression fractures after percutaneous vertebroplasty: retrospective analysis of risk factors. J Vasc Interv Radiol. 2008;19:225–231. doi: 10.1016/j.jvir.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Duran C, Sirvanci M, Aydoğan M, Ozturk E, Ozturk C, Akman C. Pulmonary cement embolism: a complication of percutaneous vertebroplasty. Acta Radiol. 2007;48:854–859. doi: 10.1080/02841850701422153. [DOI] [PubMed] [Google Scholar]
- Abdul-Jalil Y, Bartels J, Alberti O, Becker R. Delayed presentation of pulmonary polymethylmethacrylate emboli after percutaneous vertebroplasty. Spine. 2007;32:E589–E593. doi: 10.1097/BRS.0b013e31814b84ba. [DOI] [PubMed] [Google Scholar]
- Scroop R, Eskridge J, Britz G W. Paradoxical cerebral arterial embolization of cement during intraoperative vertebroplasty: case report. AJNR Am J Neuroradiol. 2002;23:868–870. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chung S E, Lee S H, Kim T H, Yoo K H, Jo B J. Renal cement embolism during percutaneous vertebroplasty. Eur Spine J. 2006;15(suppl 5):S590–S594. doi: 10.1007/s00586-005-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S Y, Seo J B, Do K H, Lee J S, Song K S, Lim T H. Cardiac perforation caused by acrylic cement: a rare complication of percutaneous vertebroplasty. AJR Am J Roentgenol. 2005;185:1245–1247. doi: 10.2214/AJR.04.1443. [DOI] [PubMed] [Google Scholar]
- Son K H, Chung J H, Sun K, Son H S. Cardiac perforation and tricuspid regurgitation as a complication of percutaneous vertebroplasty. Eur J Cardiothorac Surg. 2008;33:508–509. doi: 10.1016/j.ejcts.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Lin E P, Ekholm S, Hiwatashi A, Westesson P L. Vertebroplasty: cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. AJNR Am J Neuroradiol. 2004;25:175–180. [PMC free article] [PubMed] [Google Scholar]
- Lazáry Á, Speer G, Varga P P, et al. Effect of vertebroplasty filler materials on viability and gene expression of human nucleus pulposus cells. J Orthop Res. 2008;26:601–607. doi: 10.1002/jor.20532. [DOI] [PubMed] [Google Scholar]
- Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology. 2000;39:1410–1414. doi: 10.1093/rheumatology/39.12.1410. [DOI] [PubMed] [Google Scholar]
- Delmas P D, Genant H K, Crans G G, et al. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone. 2003;33:522–532. doi: 10.1016/s8756-3282(03)00241-2. [DOI] [PubMed] [Google Scholar]
- Uppin A A, Hirsch J A, Centenera L V, Pfiefer B A, Pazianos A G, Choi I S. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226:119–124. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- Fribourg D, Tang C, Sra P, Delamarter R, Bae H. Incidence of subsequent vertebral fracture after kyphoplasty. Spine. 2004;29:2270–2276. doi: 10.1097/01.brs.0000142469.41565.2a. [DOI] [PubMed] [Google Scholar]
- Harrop J S, Prpa B, Reinhardt M K, Lieberman I. Primary and secondary osteoporosis' incidence of subsequent vertebral compression fractures after kyphoplasty. Spine. 2004;29:2120–2125. doi: 10.1097/01.brs.0000141176.63158.8e. [DOI] [PubMed] [Google Scholar]
- Pflugmacher R, Schroeder R J, Klostermann C K. Incidence of adjacent vertebral fractures in patients treated with balloon kyphoplasty: two years' prospective follow-up. Acta Radiol. 2006;47:830–840. doi: 10.1080/02841850600854928. [DOI] [PubMed] [Google Scholar]
- Moon E S, Kim H S, Park J O, et al. The incidence of new vertebral compression fractures in women after kyphoplasty and factors involved. Yonsei Med J. 2007;48:645–652. doi: 10.3349/ymj.2007.48.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperk C, Hillmeier J, Nöldge G, et al. Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomized controlled study. J Bone Miner Res. 2005;20:604–612. doi: 10.1359/JBMR.041203. [DOI] [PubMed] [Google Scholar]
- Cloft H J, Jensen M E. Kyphoplasty: an assessment of a new technology. AJNR Am J Neuroradiol. 2007;28:200–203. [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health ClinicalTrials.gov Database. KAVIAR study: kyphoplasty and vertebroplasty in the augmentation and restoration of vertebral body compression fractures. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00323609?term=kyphoplasty&rank=1. Accessed April 4, 2008. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00323609?term=kyphoplasty&rank=1
- National Institutes of Health ClinicalTrials.gov Database. Cost effectiveness and efficacy of kyphoplasty and vertebroplasty trial. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00279877?term=kyphoplasty&rank=4. Accessed April 4, 2008. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00279877?term=kyphoplasty&rank=4