Abstract
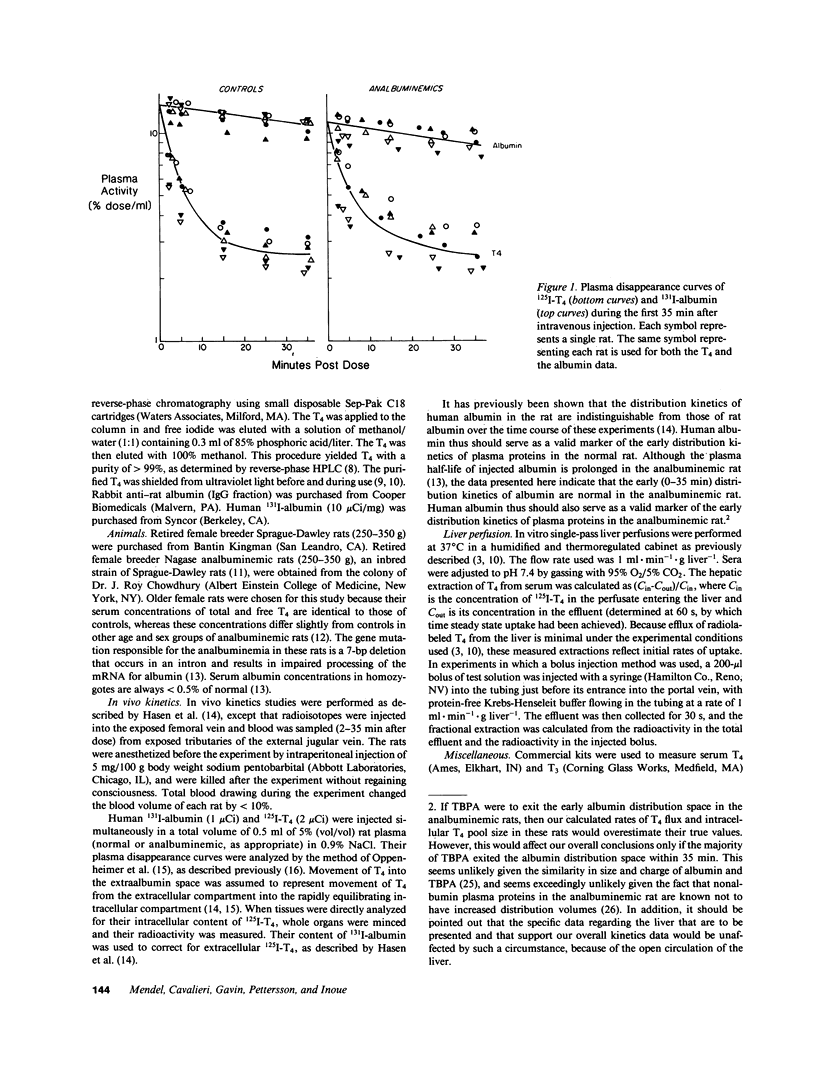
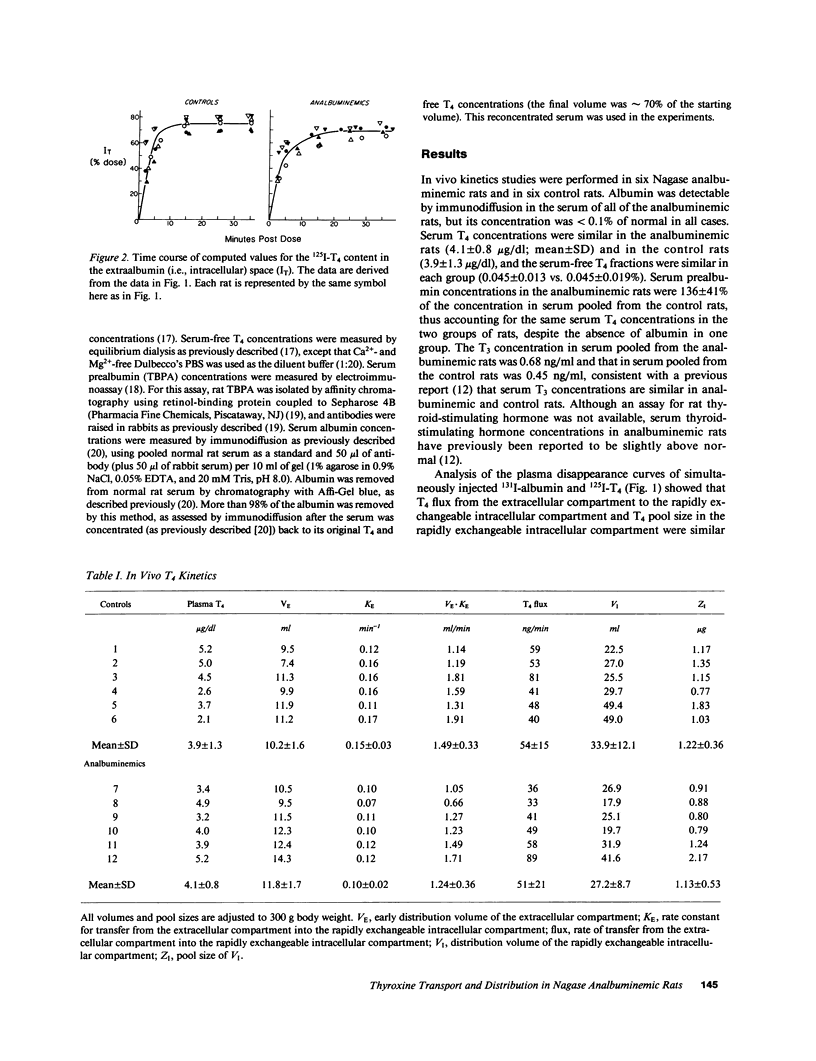
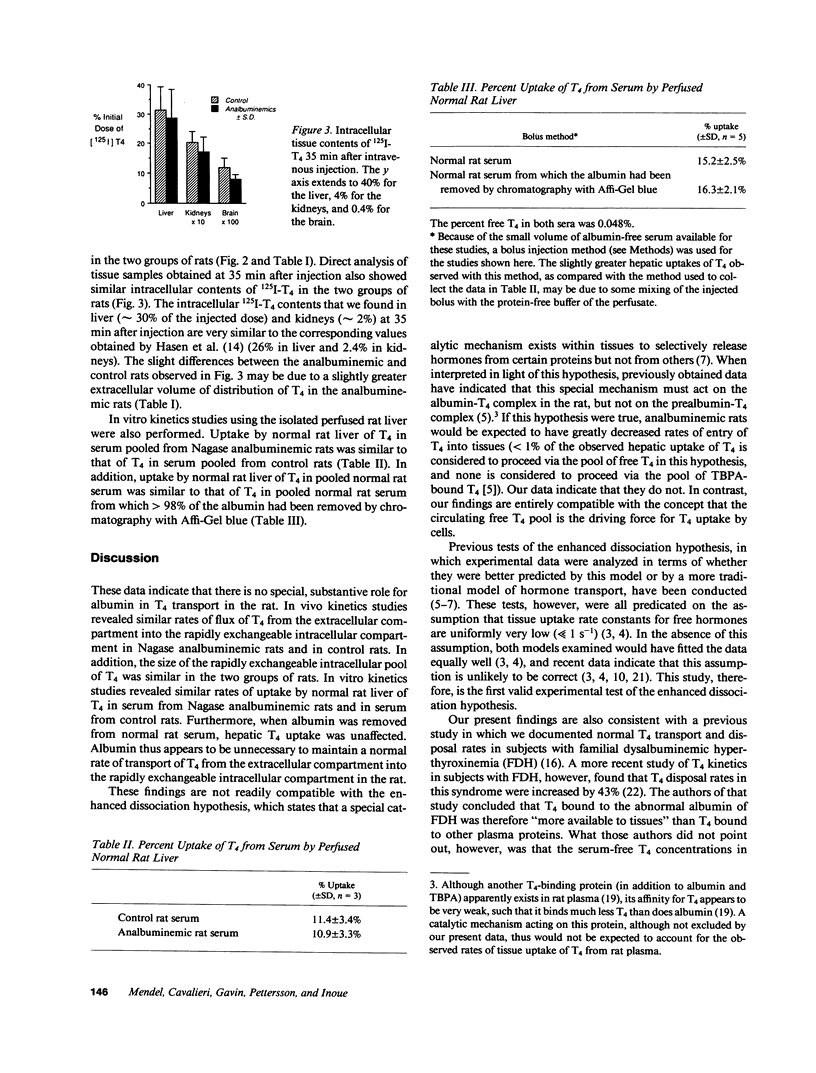
The postulate that thyroxine (T4) in plasma enters tissues by protein-mediated transport or enhanced dissociation from plasma-binding proteins leads to the conclusion that almost all T4 uptake by tissues in the rat occurs via the pool of albumin-bound T4 (Pardridge, W. M., B. N. Premachandra, and G. Fierer. 1985. Am. J. Physiol. 248:G545-G550). To directly test this postulate, and to test more generally whether albumin might play a special role in T4 transport in the rat, we performed in vivo kinetics studies in six Nagase analbuminemic rats and in six control rats, all of whom had similar serum T4 concentrations and percent free T4 values. Evaluation of the plasma disappearance curves of simultaneously injected 125I-T4 and 131I-albumin indicated that the flux of T4 from the extracellular compartment into the rapidly exchangeable intracellular compartment was similar in the analbuminemic rats (51 +/- 21 ng/min, mean +/- SD) and in the control rats (54 +/- 15 ng/min), as was the size of the rapidly exchangeable intracellular pool of T4 (1.13 +/- 0.53 vs. 1.22 +/- 0.36 micrograms). This latter finding was confirmed by direct analysis of tissue samples (liver, kidney, and brain). We also performed in vitro kinetics studies using the isolated perfused rat liver. The single-pass fractional extraction by normal rat liver of T4 in pooled analbuminemic rat serum was indistinguishable from that of T4 in pooled control rat serum (10.9 +/- 3.3%, n = 3, vs. 11.4 +/- 3.4%). When greater than 98% of the albumin was removed from normal rat serum by chromatography with Affi-Gel blue, the single-pass fractional extraction of T4 (measured by a bolus injection method) did not change (16.3 +/- 2.1%, n = 5, vs. 15.2 +/- 2.5%). These data provide the first valid experimental test of the enhanced dissociation hypothesis and indicate that there is no special, substantive role for albumin in T4 transport in the rat.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchi R., Iervasi G., Pilo A., Vitek F., Ferdeghini M., Cazzuola F., Giraudi G. Role of serum carrier proteins in the peripheral metabolism and tissue distribution of thyroid hormones in familial dysalbuminemic hyperthyroxinemia and congenital elevation of thyroxine-binding globulin. J Clin Invest. 1987 Aug;80(2):522–534. doi: 10.1172/JCI113101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri R. R., Castle J. N., McMahon F. A. Effects of dexamethasone on kinetics and distribution of triiodothyronine in the rat. Endocrinology. 1984 Jan;114(1):215–221. doi: 10.1210/endo-114-1-215. [DOI] [PubMed] [Google Scholar]
- DiStefano J. J., 3rd, Malone T. K., Jang M. Comprehensive kinetics of thyroxine distribution and metabolism in blood and tissue pools of the rat from only six blood samples: dominance of large, slowly exchanging tissue pools. Endocrinology. 1982 Jul;111(1):108–117. doi: 10.1210/endo-111-1-108. [DOI] [PubMed] [Google Scholar]
- Esumi H., Sato S., Okui M., Sugimura T., Nagase S. Turnover of serum proteins in rats with analbuminemia. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1191–1199. doi: 10.1016/s0006-291x(79)80033-9. [DOI] [PubMed] [Google Scholar]
- Hasen J., Bernstein G., Volpert E., Oppenheimer J. H. Analysis of the rapid interchange of thyroxine between plasma and liver and plasma and kidney in the intact rat. Endocrinology. 1968 Jan;82(1):37–46. doi: 10.1210/endo-82-1-37. [DOI] [PubMed] [Google Scholar]
- Hillier A. P. The mechanism of thyroxine transfer from plasma to tissue binding sites. J Physiol. 1971 Sep;217(3):635–639. doi: 10.1113/jphysiol.1971.sp009590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M. Metabolism and transport of amphipathic molecules in analbuminemic rats and human subjects. Hepatology. 1985 Sep-Oct;5(5):892–898. doi: 10.1002/hep.1840050531. [DOI] [PubMed] [Google Scholar]
- Irvine C. H. Concentration of thyroxine in cellular and extracellular tissues of the sheep and the rate of equilibration of labeled thyroxine. Endocrinology. 1974 Apr;94(4):1060–1071. doi: 10.1210/endo-94-4-1060. [DOI] [PubMed] [Google Scholar]
- Larsson M., Pettersson T., Carlström A. Thyroid hormone binding in serum of 15 vertebrate species: isolation of thyroxine-binding globulin and prealbumin analogs. Gen Comp Endocrinol. 1985 Jun;58(3):360–375. doi: 10.1016/0016-6480(85)90108-x. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Mendel C. M., Cavalieri R. R. Red blood cell thyroxine in nonthyroid illness and in heparin-treated patients. J Clin Endocrinol Metab. 1984 Jun;58(6):1117–1124. doi: 10.1210/jcem-58-6-1117. [DOI] [PubMed] [Google Scholar]
- Mendel C. M., Cavalieri R. R. Thyroxine distribution and metabolism in familial dysalbuminemic hyperthyroxinemia. J Clin Endocrinol Metab. 1984 Sep;59(3):499–504. doi: 10.1210/jcem-59-3-499. [DOI] [PubMed] [Google Scholar]
- Mendel C. M., Cavalieri R. R., Weisiger R. A. On plasma protein-mediated transport of steroid and thyroid hormones. Am J Physiol. 1988 Aug;255(2 Pt 1):E221–E227. doi: 10.1152/ajpendo.1988.255.2.E221. [DOI] [PubMed] [Google Scholar]
- Mendel C. M., Frost P. H., Cavalieri R. R. Effect of free fatty acids on the concentration of free thyroxine in human serum: the role of albumin. J Clin Endocrinol Metab. 1986 Dec;63(6):1394–1399. doi: 10.1210/jcem-63-6-1394. [DOI] [PubMed] [Google Scholar]
- Mendel C. M., Weisiger R. A., Cavalieri R. R. Uptake of 3,5,3'-triiodothyronine by the perfused rat liver: return to the free hormone hypothesis. Endocrinology. 1988 Oct;123(4):1817–1824. doi: 10.1210/endo-123-4-1817. [DOI] [PubMed] [Google Scholar]
- Mendel C. M., Weisiger R. A., Jones A. L., Cavalieri R. R. Thyroid hormone-binding proteins in plasma facilitate uniform distribution of thyroxine within tissues: a perfused rat liver study. Endocrinology. 1987 May;120(5):1742–1749. doi: 10.1210/endo-120-5-1742. [DOI] [PubMed] [Google Scholar]
- Nagase S., Shimamune K., Shumiya S. Albumin-deficient rat mutant. Science. 1979 Aug 10;205(4406):590–591. doi: 10.1126/science.451621. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Bernstein G., Hasen J. Estimation of rapidly exchangeable cellular thyroxine from the plasma disappearance curves of simultaneously administered thyroxine-131-I and albumin-125-I. J Clin Invest. 1967 May;46(5):762–777. doi: 10.1172/JCI105577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Landaw E. M. Tracer kinetic model of blood-brain barrier transport of plasma protein-bound ligands. Empiric testing of the free hormone hypothesis. J Clin Invest. 1984 Sep;74(3):745–752. doi: 10.1172/JCI111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M. Plasma protein-mediated transport of steroid and thyroid hormones. Am J Physiol. 1987 Feb;252(2 Pt 1):E157–E164. doi: 10.1152/ajpendo.1987.252.2.E157. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Premachandra B. N., Fierer G. Transport of thyroxine bound to human prealbumin into rat liver. Am J Physiol. 1985 May;248(5 Pt 1):G545–G550. doi: 10.1152/ajpgi.1985.248.5.G545. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Transport of protein-bound hormones into tissues in vivo. Endocr Rev. 1981 Winter;2(1):103–123. doi: 10.1210/edrv-2-1-103. [DOI] [PubMed] [Google Scholar]
- Sutherland R. L., Brandon M. R. The thyroxine-binding properties of rat and rabbit serum proteins. Endocrinology. 1976 Jan;98(1):91–98. doi: 10.1210/endo-98-1-91. [DOI] [PubMed] [Google Scholar]
- TATA J. R. Biochemical applications of a newly discovered property of throxine. Ann N Y Acad Sci. 1960 Apr 23;86:469–483. doi: 10.1111/j.1749-6632.1960.tb42823.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Wakabayashi K., Nagase S. Hormone levels of anterior pituitary gland and serum in Nagase analbuminemia rats. Endocrinol Jpn. 1984 Apr;31(2):185–193. doi: 10.1507/endocrj1954.31.185. [DOI] [PubMed] [Google Scholar]



