Abstract
Objectives
Diabetes mellitus and obesity are prevalent in the Hispanic community. This group has not benefited greatly from diabetes interventions due to cultural, language and financial constraints. We designed a prospective cohort study to determine the clinical impact on adiposity and glycemic control in Hispanics with type 2 diabetes.
Research design and methods
The program conducted in Spanish by a multidisciplinary team of health care providers focused on improving glycemic control and complications through cultural lifestyle changes. Outcomes were changes in glycemic control by fasting insulin, glucose and HbA1c, body composition and selected adipokines, adiponectin, leptin and ghrelin. Body composition was measured by dual energy x-ray absorptiometry. Changes from baseline at three months were compared using paired t-tests and with Spearman’s correlations.
Results
Glycemic control improved by HbA1c (7.9% ± 2.0% vs 7.1% ± 1.7%; P = <0.001), and fasting glucose (166.4 ± 66.0 mg/dl vs 143.2 ± 57.9 mg/dl; P = 0.003). Body weight (81.3 ± 17.9 kg vs 80.3 ± 18.0 kg; P = 0.002), waist circumference (101.6 ± 13.4 cm vs 99.1 ± 12.7 cm; P = 0.015), and truncal fat (16.5 ± 5.7 kg vs 15.9 ± 5.6 kg; P = 0.001) decreased. Only leptin (19.6 ± 15.0 ng/ml vs 16.3 ± 12.7 ng/ml; P = 0.002) was reduced and related to change in body weight (r = 0.392; P = 0.022).
Conclusions
Our program significantly improved glycemic control and decreased obesity in diabetic Hispanic subjects. The early benefits on glycemic control may be related to reductions in leptin through loss of adipose tissue. Success in impacting diabetes and related complications can occur in a culturally focused and multidisciplinary context.
Keywords: glycemic control, obesity, leptin, culture
Introduction
Previous studies have not evaluated the effects of diabetes education programs on the complex body composition changes, changes in adipokines and changes in glycemic control. The purpose of this study was to determine if a culturally sensitive program for Hispanic diabetics could improve glycemic control and obesity. Our program is unique because we assessed the role of body composition changes and the adipokine environment on short term changes in glycemic control and obesity.
By ‘culturally sensitive’, we mean that in addition to providing the education in their native Spanish language, we emphasized the foods that are part of their specific culture. For example, when their diet included tortillas, instead of eating six or more tortillas per meal, they were advised to eat only 1 or 2 per meal. Also, we included foods like nopales which are a vegetable made from the young pads of the prickly pear cactus. Nopales are very rich in insoluble and soluble dietary fiber. They are also rich in vitamins (especially A, C, K, riboflavin and B6) and minerals (especially magnesium, potassium, manganese, iron and copper). Nopales are low in carbohydrates and may help in the treatment of diabetes. [1]
The Hispanic population in the United States is particularly burdened with diabetes and its complications [2]. Approximately 10% of Hispanics aged 20 years or older have been diagnosed with diabetes as compared with 7.8% of non-Hispanic Whites [2]. Hispanics tend to have more complications and worse outcomes than non-Hispanic Whites [3–5]. Several studies show Hispanics also have rates of secondary complications (nephropathy, renal failure, neuropathy, retinopathy, and peripheral vascular disease) more than twice those reported for non- Hispanic Whites [6–8].
Hispanics with type 2 diabetes often do not have access to regular medical care, cannot afford medication, and generally do not have the community infrastructure to support self-management practices [9–10]. In addition, diabetes education and self-management programs that address the cultural and language issues in the Hispanic population are not widely available [11–18]. Diabetes education and support have the potential to impact diabetes control [19]. In recent years diabetes education and self-management have gained a significant foothold in the treatment of type 2 diabetes as studies have documented the benefits of diabetes education and self-management in improving glucose control [20]. It is imperative that diabetes programs in the Hispanic community address the cultural and language differences to succeed.
Hispanic populations are particularly prone to develop obesity. Obesity, and in particular abdominal obesity, is clearly associated with risk of cardiovascular disease and hyperglycemia in patients with type 2 diabetes. One of the major factors that may improve glucose control in persons with type 2 diabetes is weight loss. Interventions in the Hispanic community must be focused on successful weight loss strategies. A recent meta-analysis Whittemore [21] identified 11 published reports that provided information on culturally competent diabetes interventions to promote self-management in Hispanic adults with type 2 diabetes. These studies encompassed seven randomized controlled trials, one study used a matched control group design, and three were pre- /post-test studies. Eight studies reported improvements in HbA1c concentrations, but none of the four studies that reported body mass index (BMI) values showed a significant reduction in BMI or assessed the effect of the intervention on abdominal obesity.
There are clear links between abdominal obesity, insulin resistance and cardiovascular disease. Adipose tissue produces several soluble hormone-like substances called adipokines which regulate glucose metabolism and have effects on blood vessel biology [22]. Specifically, adiponectin and leptin are involved in balancing energy use as well as influencing a wide range of metabolic effects. Multiple studies have suggested that adiponectin may both decreases insulin resistance and may have an anti-atherosclerotic action [23]. Adiponectin is decreased in obesity [24] suggesting that the adverse health consequences of obesity may be related to reductions in adiponectin. Abdominal body fat distribution is negatively associated with plasma adiponectin [25].
Leptin is a satiety hormone that acts on the hypothalamus of the central nervous system to suppress food intake and stimulate energy expenditure to regulate appetite and energy balance. Recent studies have demonstrated that leptin has a direct effect on insulin release through effects on β-cell function. Obesity is generally characterized by increased leptin concentration suggesting that obese subjects are leptin resistant through a chronic low-grade pro-inflammatory state [26]. The β-cell may be adversely affected by chronic increased leptin levels eventually leading to diabetes. Weight loss reduces circulating leptin levels while simultaneously lowering plasma levels of inflammation markers associated with obesity and therefore decrease the risk of both diabetes and cardiovascular events [27].
Ghrelin is a peptide synthesized predominantly in the epithelial cells lining the stomach fundus and involved in the stimulation of short-term regulation of feeding and long-term energy metabolism and hence involved in the regulation of energy balance [28–29]. Low circulating concentrations of ghrelin are associated with obesity, insulin resistance and type 2 diabetes [30].
Specifically, we investigated the interrelationships of glycemic and adipose parameters with body weight, abdominal body fat, body composition at baseline and after three months and examined whether changes in adiponectin, leptin and ghrelin were correlated with changes in anthropometric measurements, insulin concentrations and glycemic control. There is clear evidence that Hispanic patients have an increased risk for obesity and diabetes. This risk is often attributed to changes in the cultural and lifestyle environment.
Research design and methods
This was a prospective three-month uncontrolled intervention pilot study conducted by Loma Linda University Center for Health Care Disparities and Molecular Medicine, School of Medicine and the School of Public Health from February through May 2005. The protocol was approved by the Loma Linda University Institutional Review Board. The study was designed to determine the feasibility and short-term benefits of a culturally-sensitive program conducted by a multidisciplinary team in Spanish.
Participants were primary Spanish speaking Hispanic women and men aged 20–75 years diagnosed previously with type 2 diabetes. Flyers developed specifically for the study and contact telephone numbers were placed in markets, ambulatory care clinics preferred by Hispanics, and medical offices serving the San Bernardino/Inland Empire area Hispanic population. Respondents were initially screened via telephone for inclusion/exclusion criteria. At personal interviews qualified potential subjects provided brief medical histories, listing of medications, physical activity, and diet histories. Fifty-nine (n=59) respondents met the criteria to participate in the study. Forty-four (n=44) subjects were enrolled and signed consent forms to participate. Ten subjects were lost through attrition.
Exclusion criteria were pregnancy, lactation, history of drug or alcohol abuse, impaired mental condition, clinically-relevant history of cardiovascular disease, cardiac pacemaker, hepatic, neurologic, endocrine, or major systemic disease. Written informed consent in Spanish was obtained by Spanish-speaking study personnel
The education team consisted of Spanish-speaking registered dietitians, registered nurses, physicians and nutrition students. The classes were conducted twice a week during the first month for three weeks. Each session lasted for three hours including a question and answer period. Study participants’ family members were encouraged to attend the classes. Instruction focused on maintaining glycemic control and general aspects of managing diabetes and complications. Topics included: What is Diabetes?, Complications of Diabetes, Blood Glucose Control, Hyperglycemia, How to Choose Healthier Foods, Hypoglycemia, How to Use the Glucose Monitor, The Role of Nutrition in Diabetes Care, Taking Care of Your Feet, What to Do When You Get Sick, Nutrition Labeling, Weight Control Principles, Dining Out, Principles of Exercise and Weight Control, Food Portions Control, and Food Pyramid. During months 2 and 3 participants who had missed sessions had the opportunity to make up classes. Each participant received a glucose monitor (glucometer) and was taught to use it for their daily blood glucose levels checks and values in a log record.
Baseline and three-month data collection included blood analyses, anthropometrics and body composition assessment. Subjects were asked to fast for 14 hours prior to blood draws which were performed the following morning between 8 and 9 am. Blood parameters were drawn in duplicate and samples were analyzed for serum glucose and insulin and HbA1c concentrations (at Loma Linda University Medical Center Clinical Laboratory). Samples for analysis of serum adiponectin, serum leptin, and plasma ghrelin concentrations were stored at −80 C°, then packaged and shipped by express delivery to Linco Laboratories, St. Charles, MO for analysis. Published intra- and inter-assay coefficients of variation (%) for adiponectin were 1.6 and 1.5, for leptin 3.0 and 2.7, for insulin 2.3 and 4.0, and for ghrelin 3.4 and 3.1, respectively [31].
Measurements of height, weight, waist and hip circumferences were done according to Lohman [32] and BMI and waist-to-hip ratios were calculated. The fan beam DXA with Hologic QDR 4500-A software version 8.1A (Hologic Inc., Waltham, MA) was used to determine total and regional body composition. Variables analyzed included total fat, the percentage of fat in relation to body mass (total % fat), trunk fat, and trunk fat as a percentage of total fat (trunk % fat).
Statistical analysis
The primary outcome measure was the change in fasting blood glucose. Power for this study was based on a predicted reduction in fasting blood glucose over a 3 month follow-up period of 13% from baseline. In order to have 80% power (1-β) and allowing for a type I (α) error of 5%, 44 subjects were required. A total of 34 subjects completed the study. Power and Precision software version 1.02 with type I error set at α = 0.05 was used to calculate the new power for the study based on the 34 participants who completed the program. These calculations showed power in the study was reduced to 60%.
This was a pretest-posttest study where the subjects served as their own controls, thus baseline values were compared to values at three months follow-up. Data are presented as mean ± SD or median with percentiles if skewed. Log transformation was used to improve the normality of skewed variables. These were BMI, glucose, HbA1c, insulin, adiponectin, leptin, ghrelin, DXA trunk fat, DXA total fat, and DXA total % fat. Spearman’s product-moment correlations were performed to relate baseline and three month values and changes in adiponectin, leptin and ghrelin concentrations to clinical and other laboratory parameters.
Multiple linear regression was used to evaluate the independent predictors of the change in the dependent variables (adiponectin, leptin and ghrelin). Statistical analyses were done with SPSS for Windows version 15.0 (SPSS Inc, Chicago, IL) with type I error set at α = 0.05.
Results
Table 1 presents baseline characteristics of participants (n=34). Participants ranged from 30 to 69 years in age, with the mean age of 51.9 ± 9.3 years. Nearly three-fourths of the participants were female. BMI ranged from 19 to 47 kg/m2, with a mean BMI of 32.18 ± 7.02 kg/m2. The participants had diabetes for a range of 1–20 years, with a mean of 6.6 ± 5.5 years.
Table 1.
Baseline Characteristics of Study Participants in the Hispanic Diabetes Education Study (n=34).
| Age in years (mean ± SD) | 51.9 ± 9.25 |
| Gender shown as n (percentage) | |
| Female | 25 (73.5%) |
| Male | 9 (26.5%) |
| Educational level (percentage) | |
| Up to elementary school | 55% |
| Junior high to high school | 30% |
| Some college or higher | 15% |
| BMI (percentage) | |
| < 25 kg/m2 | 15% |
| 25 – 29.9 kg/m2 | 29% |
| ≥ 30 kg/m2 | 56% |
| Number of years with diabetes (percentage) | |
| 1–4 | 47% |
| 5–9 | 24% |
| 10.+ | 29% |
| Use of medication (percentage) | |
| Oral hypoglycemics | 74% |
| Insulin | 5% |
| None or missing | 21% |
Table 2 shows that with the exception of BMI and hip circumference, there were significant changes in all anthropometric and body composition measurements between baseline and three months.
Table 2.
Baseline and 3-month changes in anthropometric parameters of Hispanic diabetes education program participants (n=34). Values are shown as means ± standard deviations.
| Variables | Baseline | Three Months | Change | P-value† |
|---|---|---|---|---|
| Body weight | 81.22 ± 17.90 | 80.31 ± 17.95 | 1.16% | 0.01 |
| BMI (kg/m2) | 32.18 ± 7.02 | 31.73 ± 6.72 | 1.40% | 0.132 |
| Waist circumference | 101.57 ± 13.37 | 99.12 ± 12.70 | 2.41% | 0.015 |
| Hip circumference | 109.10 ± 14.56 | 109.04 ± 13.00 | 0.05% | 0.921 |
| Waist-to-hip ratio | 0.93 ± 0.08 | 0.91 ± 0.07 | 2.15% | 0.03 |
| DXA trunk fat (kg) | 16.54 ± 5.73 | 15.93 ± 5.60 | 3.69% | 0.001 |
| DXA trunk % fat | 36.37 ± 7.50 | 35.77 ± 7.53 | 1.65 | 0.003 |
| DXA total fat (kg) | 30.40 ± 10.68 | 29.50 ± 10.52 | 2.96% | 0.001 |
| DXA total % fat | 39.60 ± 10.61 | 35.77 ± 7.52 | 9.08% | 0.001 |
p-values are calculated based on the log transformation for BMI, DXA trunk fat, DXA total fat and DXA total % fat.
Table 3 shows the baseline and three month values of glucose, HbA1c, insulin, adiponectin, leptin and glucose concentrations. Concentrations of glucose, HbA1c, and leptin decreased significantly after three months (P = 0.003, <0.001, and 0.002 respectively).
Table 3.
Baseline and 3-month changes in blood parameters of Hispanic diabetes education program participants (n=34). Values are shown as means ± standard deviations.
| Variables value† | Baseline | 3 Months | Change | P-value† |
|---|---|---|---|---|
| Glucose (mg/dL) | 166.41 ± 65.98 | 143.21 ± 57.89 | 13.94% | 0.003 |
| HbA1c (%) | 7.90 ± 2.02 | 7.08 ± 1.66 | 10.40% | <0.001 |
| Insulin (uIU/L) | 12.89 ± 9.60 | 12.55 ± 12.41 | 2.64% | 0.753 |
| Adiponectin (mg/ml) | 8.28 ± 5.45 | 8.38 ± 6.04 | 4.77% | 0.832 |
| Leptin (ng/ml) | 19.55 ± 14.98 | 16.34 ± 12.66 | 16.42% | 0.002 |
| Ghrelin (pg/ml) | 6.62 ± 0.33 | 6.63 ± 0.43 | <0.001% | 0.432 |
P-values are calculated based on the log transformation for glucose, HbA1c, adiponectin, leptin and ghrelin.
Table 4 presents Spearman’s correlations coefficients between age, anthropometric characteristics, and blood parameters at baseline. Consistent with numerous previous studies, body weight was inversely correlated with adiponectin (r = −0.420, P = 0.013) and ghrelin (r = −0.727, P <0.001), and positively correlated with leptin (r = 0.433, P = 0.011). Numerous other measures of body composition revealed a similar trend. BMI was inversely correlated with ghrelin (r = −0.507, P = 0.002) and positively correlated with leptin (r = 0.583, P <0.001). Waist circumference was inversely correlated with adiponectin (r = −0.452, P = 0.007) and ghrelin (r = −0.629), but positively correlated with leptin (r = 0.418, P = 0.014). Waist-to-hip ratio was inversely correlated with adiponectin (r = −0.469, P = 0.005) and leptin, (r = −0.476, P = 0.004).
Table 4.
Spearman’s correlation coefficients between age, anthropometric parameters, glycemic control and insulin with concentrations of adiponectin, leptin and ghrelin at baseline (n=34). P-values are shown under each correlation coefficient.
| Adiponectin | Leptin | Ghrelin | |
|---|---|---|---|
| Age | 0.243 (.165) | −0.079 (.658) | 0.283 (.105) |
| Body weight | −0.420 (.013) | 0.433 (.011) | −0.727 (.001) |
| BMI | −0.235 (.181) | 0.583 (.001) | −0.507 (.002) |
| Waist | −0.452 (.007) | 0.418 (.014) | −0.629 (.001) |
| Hip | −0.193 (.274) | 0.710 (.001) | − 0.554 (.001) |
| Waist-to-hip ratio | −0.469 (.005) | −0.476 (.004) | −0.218 (.216) |
| DXA trunk fat | −0.259 (.139) | 0.670 (.001) | −0.558 (.001) |
| DXA trunk % fat | −0.082 (.644) | 0.840 (.001) | −0.158 (.373) |
| DXA total fat | −0.166 (.347) | 0.797 (.001) | −0.415 (.015) |
| DXA total % fat | 0.064 (.720) | 0.852 (.001) | −0.144 (.417) |
| Glucose | 0.072 (.686) | −0.099 (.579) | 0.124 (.483) |
| HBA1c | 0.092 (.603) | −0.124 (.483) | 0.181 (.307) |
| Insulin | −0.332 (.055) | 0.569 (.001) | −0.587 (.001) |
Correlation coefficients are calculated based on the log transformation for BMI, DXA trunk fat, DXA total fat, DXA total % fat, glucose and insulin.
Trunk fat measured by DXA was positively correlated with leptin (r = 0.670, P <0.001), and negatively correlated with ghrelin (r = −0.558, P <0.001). DXA % trunk fat showed a positive correlation with leptin (r = 0.840, P <0.001). Total body fat was positively correlated with leptin (r = 0.797, P <0.001) and negatively correlated with ghrelin (r = −0.415, P = 0.015). DXA total % fat was positively correlated with leptin (r = 0.852, P <0.001). Insulin was positively correlated with leptin (r = 0.569, P <0.001) and inversely with ghrelin (r = −0.587, P <0.001). Our baseline assessment is consistent with a positive relationship of leptin and body fat and a negative relationship with body fat and adiponectin and ghrelin.
Table 5 shows the changes at three months using Spearman correlation coefficients between anthropometric characteristics and blood parameters. Adiponectin was negatively correlated with body weight (r = −0.443, P = 0.009), BMI (r = −0.364, P = 0.034) waist (−0.381, P = 0.026), DXA trunk fat (−0.344, P = 0.046) and insulin (r = −0.472, P = 0.005).
Table 5.
Spearman’s correlation coefficients between age, anthropometric parameters, glycemic control and insulin with concentrations of adiponectin, leptin and ghrelin at three months (n=34). P-values are shown under each correlation coefficient.
| Adiponectin | Leptin | Ghrelin | |
|---|---|---|---|
| Age | 0.205 (.246) | −0.126 (.479) | 0.384 (.025) |
| Body weight | −0.443 (.009) | 0.538 (.001) | −0.673 (.001) |
| BMI | −0.364 (.034) | 0.764 (.001) | −0.559 (.001) |
| Waist | −0.381 (.026) | 0.561 (.001) | −0.620 (.001) |
| Hip | −0.210 (.234) | 0.844 (.001) | −0.480 (.004) |
| Waist-to-hip ratio | −0.325 (.061) | −0.283 (.104) | −0.247 (.159) |
| DXA trunk fat | −0.344 (.046) | 0.787 (.001) | −0.576 (.001) |
| DXA trunk % fat | −0.128 (.471) | 0.895 (.001) | −0.239 (.173) |
| DXA total fat | −0.284 (.104) | 0.879 (.001) | −0.457 (.001) |
| DXA total % fat | −0.027 (.881) | 0.882 (.001) | −0.121 (.494) |
| Glucose | −0.043 (.808) | −0.102 (.568) | −0.067 (.707) |
| HBA1c | 0.076 (.669) | 0.049 (.782) | 0.102 (.568) |
| Insulin | −0.472 (.005) | 0.566 (.001) | −0.666 (.001) |
Correlation coefficients are calculated based on the log transformation for BMI, DXA trunk fat, DXA total fat, DXA total % fat, glucose and insulin.
Leptin was positively correlated with body weight, BMI, waist circumference, hip circumference, DXA trunk fat, DXA% trunk fat, DXA total fat, DXA total % fat and insulin (r = 0.538, 0.764, 0.561, 0.844, 0.787, 0.895, 0.879, 0.882 and 0.566 respectively; P <0.001).
Ghrelin was positively correlated with age (r = 0.384, P = 0.025) and negatively correlated with hip circumference (r = −0.480, P = 0.004), body weight, BMI, waist circumference, DXA trunk fat, DXA total fat and insulin, respectively (r = −0.673, −0.559, −0.620, 0.576, −0.457, and −0.666; P <0.001).
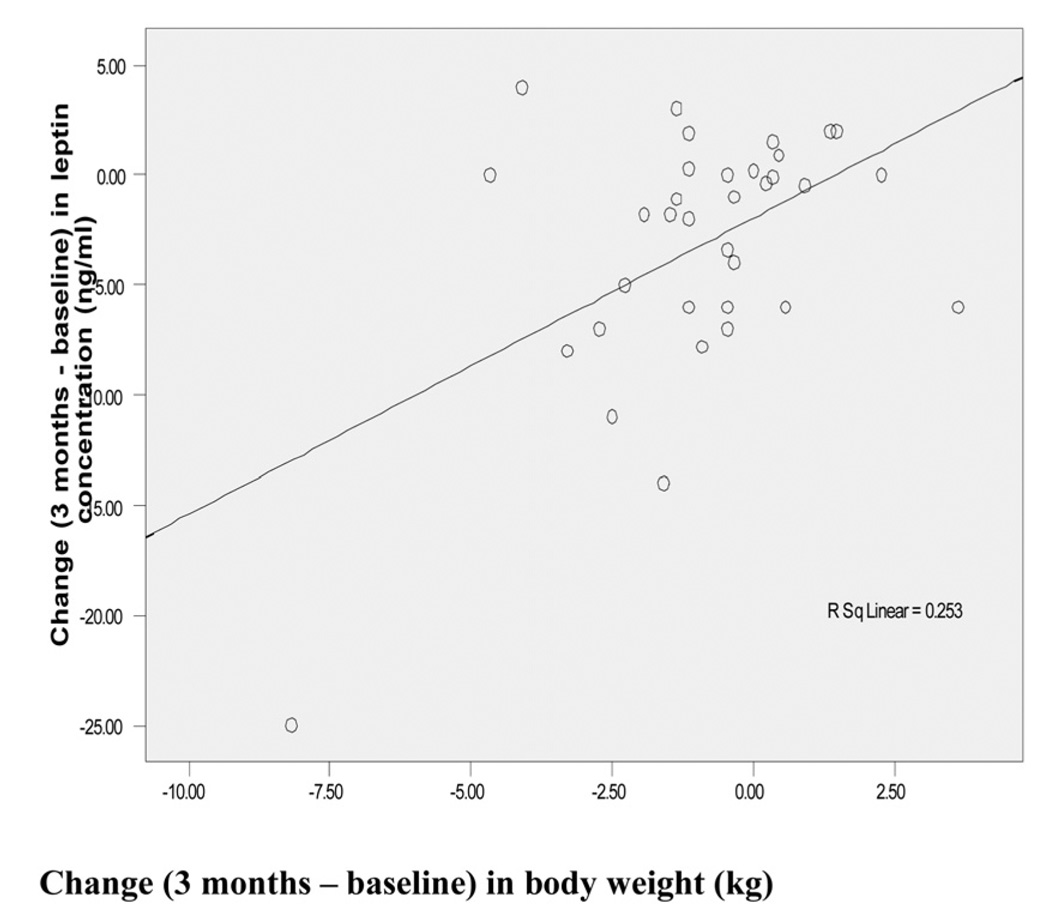
Table 6 shows Spearman’s correlation coefficients between changes in leptin concentration (at three months minus baseline) versus change in anthropometric characteristics. Change in body weight was significantly negatively correlated with change in leptin concentration (r = −0.392, P = 0.022) as also demonstrated by the scatterplot in Figure 1.
Table 6.
Spearman’s correlation coefficients between change in anthropometric parameters and change in concentrations of leptin between baseline and three months (n=34). P-values are shown to the right of each correlation coefficient.
| Leptin | |
|---|---|
| Body weight | −0.392 (.022) |
| BMI | 0.126 (.478) |
| Waist circumference | −0.159 (.368) |
| Hip circumference | −0.203 (.250) |
| Waist-to-hip ratio | −0.203 (.250) |
| DXA trunk fat | −0.075 (.674) |
| DXA trunk % fat | 0.055 (.757) |
| DXA total fat | −0.027 (.879) |
| DXA total % fat | −0.170 (.336) |
| Glucose | −0.038 (.831) |
| HBA1c | −0.190 (.283) |
| Insulin | 0.134 (.451) |
Correlation coefficients are calculated based on the log transformation for BMI, DXA trunk fat, DXA total fat, DXA total % fat, glucose and insulin.
Figure 1.
Change in body weight (kg) vs change in leptin concentration (ng/ml) between baseline and 3 months.
Table 7 shows the change in leptin concentration as related to change in body weight after adjusting for age and gender in a multiple linear regression model equation. In these calculations, the relationship lost statistical significance.
Table 7.
Multiple linear regression results to evaluate the change in leptin concentration (at three months minus baseline) in relationship to body weight change with adjustment for age and sex.
| B | Std. Error | βeta | P-values | 95% C I for β | ||
|---|---|---|---|---|---|---|
| (Constant) | −1.362 | 1.680 | 0.424 | −4.793 | 2.070 | |
| Age (continuous) | 0.002 | 0.006 | 0.054 | 0.784 | −0.011 | 0.015 |
| Sex (1=M; 2=F) | −0.101 | 0.129 | −0.148 | 0.440 | −0.363 | 0.162 |
| Body weight (kg) | 0.246 | 0.275 | 0.180 | 0.378 | −0.315 | 0.807 |
R2 = 0.067; CI=confidence interval
Conclusions
We have demonstrated that a culturally sensitive program directed at Hispanic subjects with diabetes results in improved short term glycemic control and reduction in obesity. The clinically significant results were seen within a 3-month period and were modest but based on recent outcome studies such as the United Kingdom Prospective Diabetes Study (UKPDS) [33], they are clinically significant. The changes were accompanied by a reduction in leptin concentration of ~ 16%. The reduction in leptin was related to the reduction in body weight, but not to changes in glycemic control or central adiposity. Concentrations of insulin, adiponectin and ghrelin were unchanged, possibly because the amount of weight loss was insufficient to show such changes. All of the so called appetitive hormones, adiponectin, leptin and ghrelin, were related to central fat as measured by DXA and by waist circumference, similar findings to those of Ritland [31].
Our findings were consistent with previous studies demonstrating the effectiveness of diabetes lifestyle interventions in improving glycemic control in type 2 diabetes patients [21]. However, due to the pre-posttest design of the study it is not possible to demonstrate causality. Thus, factors other than the educational program may have been associated with the improvement in glycemic control. For example, any change in anti-diabetes medication during the three months of the study was not recorded. However, the participants were required to be on stable medication for three months prior to start of the study and thus less likely to require adjustment of medication while taking part in the program. Due to the accompanying weight and central fat loss, which are strong determinants of glycemic control, it is plausible that the intervention contributed to the improvement in glycemia.
The mean reduction in HbA1c of about 0.8% in this study was of a magnitude that could lead to clinical benefits if sustained over time. In 2001 the Diabetes Control and Complications Trial demonstrated that a 0.5% reduction in HbA1c resulted in a significant diminution in complications of diabetes. In the UKPDS trial conducted in type 2 diabetics a reduction in HbA1c of 0.9% was related to a reduction in all diabetes endpoints [33].
In a recent systematic review of diabetes education programs among Hispanic population samples, Whittemore [21] found eight studies that reported improvement in glycemia following the intervention. Duraski [34] reported that a culturally sensitive stroke prevention education program in the rapid growing Hispanic community with a high prevalence of hypertension, diabetes, physical inactivity and alcohol use might help decrease risk of stroke.
The mean body weight loss by subjects in the study was small, but weight loss was not the primary focus of the education program. Individuals with type 2 diabetes may require more intensive intervention to achieve weight loss than other overweight or obese individuals [35–36]. The weight loss of about 1 kg was confirmed by the DXA measurement of total fat, which showed a reduction of about 0.9 kg. Even small weight losses may have substantial clinical benefits. For example, Valsamakis [37] reported that modest weight loss weight loss of <5% may improve insulin sensitivity and have potentially favorable effects on adipokine concentrations.
A strength of this study is the use of DXA to assess body composition. According to Nelson [38], measures of trunk fat may contribute more information on the development of diabetes than waist circumference. DXA appears to be most useful in providing an accurate and reliable measure of overall adiposity and regional fat distribution [31]. Notably the associations we observed between these hormones and central fat as measured by DXA did not seem substantially different than those measured by waist girth. In terms of regional adiposity, waist circumference was reduced, as was waist-hip ratio, and the absolute and relative amounts of truncal fat were reduced, as shown by DXA measurements. Excess abdominal tissue has a greater impact on whole body insulin sensitivity than peripheral distribution of fat deposits around the hip and thighs [39]. Reducing central adiposity may be of substantial benefit for Hispanic patients, who have been shown to have increased central adiposity contributing to their increased risk of insulin resistance and type 2 diabetes [40].
High leptin concentrations are associated with inflammatory effects and increased risk of cardiovascular disease [27]. The decrease in leptin concentration was correlated with the reduction in body weight as demonstrated by the scatterplot in Figure 1, a finding that agrees with Hukshorn [27]. These authors demonstrated that weight loss reduces circulating leptin levels and simultaneously lowers plasma levels of inflammation markers associated with obesity. In a recently published study by De Luis [41], the effects of a lifestyle modification weight reduction program on adipokine levels was studied. Mean weight loss was about 4% after three months. Leptin was the only adipokine that decreased significantly, by about 12%. The decrease in leptin in the present study appears to be larger, compared to the greater decrease in body weight achieved by De Luis [41]. Our results suggest that Hispanic subjects may be more sensitive to the effects of weight loss on serum leptin concentrations than others. However, this conclusion is limited due to the short duration and small number of subjects in our study. While the idea that the improvement in glycemic control beyond weight loss may have contributed to the decrease in leptin, the lack of correlation between leptin concentrations and glycemia (Tables 4–6) speaks against this notion.
Serum insulin concentration was reduced by < 3%, which was not statistically significant, in the present study, despite the decrease in leptin. However, insulin concentrations show great variability. Leptin and insulin actions are closely related. Individuals with diabetes may have impaired leptin response to glucocorticoid administration because of a simultaneous defect in insulin secretion and a worsening of insulin resistance [42]. However, other underlying factors, including central obesity may cause impairments in both. According to Montez [26], leptin and insulin signaling pathways may overlap or share signal transduction components, and weight loss improves both hyperinsulinemia and hyperleptinemia associated with obesity.
Clearly larger and longer studies must be conducted to determine the relationship of leptin, insulin and diabetes outcome and the specific role in the Hispanic population. Adiponectin concentrations were not changed between baseline and three months, probably because the amount of weight loss was insufficient consistent with previous studies. Madsen [43] assessed the amount of weight loss necessary for long-term improvement of plasma levels of adiponectin in abdominally obese subjects. Weight loss ≥ 13 kg significantly elevated adiponectin levels by about 22%. A smaller weight loss of 5–7 kg did not produce a significant short or long term increase in adiponectin levels. Our observation of a lack of correlation between glycemia and adiponectin supports the idea that improvement in glycemic control may have lesser effects than weight loss on adiponectin concentrations. On the other hand adiponectin is closely related to insulin concentrations as shown in tables 4 (not significant) and 5. Thus, the lack of improvement in insulin concentrations may be an additional explanation of the lack of change in adiponectin. Another factor may be that the relative frequency of isoforms of adiponectin may change [44], but this may not be reflected in total adiponectin quantifications.
Ghrelin concentrations at baseline and three months were inversely correlated to body weight, BMI, and deposition of fat in the waist and hips, as well as to truncal fat and insulin concentrations. These findings are in line with other findings in previous observations in which ghrelin concentration were found to be negatively correlated with body fat and BMI [31]. Decreased ghrelin is also shown to be associated with insulin resistance; however, it is not known what is cause and effect, ie whether decreased ghrelin in insulin resistance and type 2 diabetes is due to compensatory mechanisms against diminished insulin action or against hyperglycemia [45].
We observed similar plasma ghrelin concentrations at baseline and after three months. Garcia [46] reported that plasma ghrelin concentrations rise in response to acute weight loss, as a likely counterregulatory action stimulating hunger and food intake, after which ghrelin then returns to baseline with sustained weight maintenance. There was no relation between ghrelin and glycemia.
This was a pilot study designed to understand the feasibility and barriers to the program. Further groups are currently being studied (Cordero-MacIntyre, personal communication). While achieved study power was less than planned, we did observe biologically plausible associations that may be useful for planning future studies. Data regarding compliance to the program and physical activity and dietary data were collected and will be reported in future publication.
Our study clearly has achieved the primary objective. We have demonstrated the feasibility of a multidisciplinary team using a culturally sensitive program to improve short term glycemic control and obesity in Hispanic diabetic subjects. If the changes are sustained or increased we would predict complications would be decreased in this high risk population. Long term studies with larger number of subjects are obviously needed to validate our exciting preliminary findings.
We have also discovered that in Hispanic subjects there is a reduction in body weight and central adiposity associated with a decrease in leptin concentrations. These findings suggest important early changes in adipokines may be important in glycemic control and reduction in risk for complications. Future studies now may build on these findings, to better understand the potential of diabetes education programs to directly improve biological determinants of risk for adverse outcomes.
Acknowledgements
Funded by: Grants CMS 03-00335 from Centers for Medicare and Medicaid Services, and NIH award 5P20MD001632.
References
- 1.Use of the Latin food staple nopales: The prickly pear cactus. Available at: http://repositories.cdlib.org/cgi/viewcontent.cgi?article=1038&context=uclabiolchem/nutritionbytes.
- 2.Thompson JR, Horton C, Flores C. Advancing diabetes self-management in the Mexican American population. Diabetes Educ. 2007;33 Supp 6:159S–165S. doi: 10.1177/0145721707304077. [DOI] [PubMed] [Google Scholar]
- 3.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999;22(3):403–408. doi: 10.2337/diacare.22.3.403. [DOI] [PubMed] [Google Scholar]
- 4.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287(19):2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 5.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association Clinical Practice Recommendations among U.S. adults with diabetes, 1999–2002: The National Health and Nutrition Examination Survey. Diabetes Care. 2006;29(3):531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 6.Harris MI, Flegal K, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey. Diabetes Care. 1998;21(4):518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 7.National Institute of Diabetes and Digestive and Kidney Disease. Diabetes in Hispanic Americans [Internet] Bethesda, MD: The Institute, National Diabetes Information Clearinghouse; [Accessed Oct. 18, 2004]. [Google Scholar]
- 8.American Diabetes Association. Complications of Diabetes in the United States. [Accessed Jan. 22, 2007]; [ http://www.diabetes.org/diabetes-statistics/complications.jsp]
- 9.Fox HB, McManus MA, Zarit M, Cassedy AE, Fairbrother G. Racial and ethnic disparities in health and access to care among older adolescents. [Accessed: Feb 10, 2007]; [ www.intercenterstrategies.org]
- 10.Mainous AG, Diaz VA, Koopman RJ, Everett CJ. Quality of care for Hispanic adults with diabetes. Health Services Research. 2007;39(5):351–356. [PubMed] [Google Scholar]
- 11.Castillo A, Giachello A, Bates R, Concha J, Ramirez V, Sanchez C, Pinsker E, Arrom J. Community-based diabetes education for Latinos: The diabetes empowerment education program. Diabetes Educ. 2010 Jun 10; doi: 10.1177/0145721710371524. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Deitrick LM, Paxton HD, Rivera A, Gertner EJ, Biery N, Letcher AS, Lahoz LM, Maldonado E, Salas-Lopez D. Understanding the role of the promotora in the Latino diabetes education program. Qual Health Res. 2010;20(3):386–399. doi: 10.1177/1049732309354281. [DOI] [PubMed] [Google Scholar]
- 13.Voda SC. Improving diabetes education for minority-group members. Nursing. 2008;38(7):12–13. doi: 10.1097/01.NURSE.0000325314.03395.12. [DOI] [PubMed] [Google Scholar]
- 14.Gold R, Yu K, Liang LJ, Adler F, Balingit P, Luc P, Hernandez J, Toro Y, Modilevsky T. Synchronous provider visit and self-management education improves glycemic control in Hispanic patients with long-standing type 2 diabetes. Diabetes Educ. 2008;34(6):990–995. doi: 10.1177/0145721708323744. [DOI] [PubMed] [Google Scholar]
- 15.Levine DA, Allison JJ, Cherrington A, Richman J, Scarinci IC, Houston TK. Disparities in self-monitoring of blood glucose among low-income ethnic minority populations with diabetes, United States. Ethn Dis. 2009;19(2):97–103. [PubMed] [Google Scholar]
- 16.Porter SJ, Chapman-Novakofski KM, Scherer JA. Your guide to diet and diatetes: web-based diabetes education tailored to Hispanics. J Nutr Educ Behav. 2009;41(5):374–376. doi: 10.1016/j.jneb.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 17.de Peralta E, Patel K, Wan C, Fuentes B, Baldwin D. Culturally competent diabetic education to lower hemoglobin A1c levels of diabetic patients: Está major. Tex Med. 2005;101(6):54–56. [PubMed] [Google Scholar]
- 18.Brown SA, Hanis CL. Culturally competent diabetes education for Mexican Americans: the Starr County Study. Diabetes Educ. 1999;25(2):226–236. doi: 10.1177/014572179902500208. [DOI] [PubMed] [Google Scholar]
- 19.Ingram M, Gallegos G, Elenes J. Diabetes is a community issue: the critical elements of a successful outreach and education model on the U.S.-Mexico border. Prev Chronic Dis. 2005;2(1):A15. [PMC free article] [PubMed] [Google Scholar]
- 20.Brown SA, Garcia AA, Winchell M. Reaching underserved populations and cultural competence in diabetes education. Curr Diab Rep. 2002;2(2):166–176. doi: 10.1007/s11892-002-0077-3. [DOI] [PubMed] [Google Scholar]
- 21.Whittemore R. Culturally competent interventions for Hispanic adults with type 2 diabetes: a systemic review. J Transcult Nurs. 2007;12(2):157–166. doi: 10.1177/1043659606298615. [DOI] [PubMed] [Google Scholar]
- 22.Chan DC, Watts GF, Ng TWK, Uchida YU, Sakai N, Yamashita S, Barrett PHR. Adiponectin and other adipocytokines as predictors of markers of triglyceride-rich lipoprotein metabolism. Clin Chem. 2005;51(3):578–585. doi: 10.1373/clinchem.2004.045120. [DOI] [PubMed] [Google Scholar]
- 23.Trijillo ME, Scherer PE. Adiponectin – journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257(2):167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 24.Motoshima H, Wu X, Sinha MK. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab. 2002;87(12):5662–5667. doi: 10.1210/jc.2002-020635. [DOI] [PubMed] [Google Scholar]
- 25.Buemann B, Astrup A, Pedersen O, Black E, Holst C, Toubro S, et al. Possible role of adiponectin and insulin sensitivity in mediating the favorable effects of lower body fat mass on blood lipids. J Clin Endrocrinol Metab. 2006;91(5):1698–1704. doi: 10.1210/jc.2005-1062. [DOI] [PubMed] [Google Scholar]
- 26.Montez JM, Saukas A, Asilmaz E, Fayzikhodjaeva G, Fantuzzi G, Friedman JM. Acute leptin deficiency, leptin resistance, and the physiologic response to leptin withdrawal. Proc Natl Acad Sci USA. 2005;102(7):2537–2542. doi: 10.1073/pnas.0409530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hukshorn CJ, Lindeman JHN, Toet KH, Saris WHM, Eilers PHC, Westerterp-Plantenga MS, Kooistra T j. Leptin and the pro-inflammatory state associated with human obesity. J Clin Endrocrinol Metab. 2004;89(4):1773–1778. doi: 10.1210/jc.2003-030803. [DOI] [PubMed] [Google Scholar]
- 28.Casanueva FF, Dieguez C. Ghrelin: the link connecting growth with metabolism and energy homeostasis. Rev Endocr Metab Discord. 2002;3(4):325–338. doi: 10.1023/a:1020901624103. [DOI] [PubMed] [Google Scholar]
- 29.Meier U, Gressner AM. Endocrine regulation of energy metabolism: Review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50(9):1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 30.Ingelsson E, Larson MG, Yin X, Wang TJ, Meigs JB, Lipinska I, et al. Circulating ghrelin, leptin, and soluble leptin receptor concentrations and cardiometabolic risk factors in a community-based sample. J Clin Endocrinol Met. 2008;93(8):3149–3157. doi: 10.1210/jc.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritland LM, Alekel DL, Matvienko OA, Hanson KB, Stewart JW, Hanson LN, et al. Centrally located body fat is related to appetitive hormones in healthy postmenopausal women. Eur J Endocrinol. 2008;158(6):889–897. doi: 10.1530/EJE-07-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign: Human Kinetics Books; 1991. (abridged ed.) [Google Scholar]
- 33.Murray P, Chune GW, Raghavan VA. Legacy effects from DCCT and UKPDS: What they mean and implications for future diabetes trials. Curr Atheroscler Rep. 2010 July 23; doi: 10.1007/s11883-010-0128-1. [DOI 10.1007/s11883-010-0128-1] [published online] [DOI] [PubMed] [Google Scholar]
- 34.Duraski SA. Stroke prevention education in the Hispanic community. Rehabil Nurs. 2006;31(1):5–9. doi: 10.1002/j.2048-7940.2006.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 35.Pi-Sunyer X. The obesity epidemic. Manag Care Interface. 2007;20(5):21–22. 37. [PubMed] [Google Scholar]
- 36.Pi-Sunyer FX. How effective are lifestyle changes in the prevention of type 2 diabetes mellitus? Nutr Rev. 2007;65(3):101–110. doi: 10.1111/j.1753-4887.2007.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 37.Valsamakis G, McTernan PG, Chetty R, Al Daghri N, Field A, Hanif W, Barnett AH, Kumar S. Modest weight loss and reduction in waist circumference after medical treatment are associated with favorable changes in serum adipocytokines. Metabolism. 2004;53(4):430–434. doi: 10.1016/j.metabol.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Nelson TL, Bessesen DH, Marshall JA. Relationship of abdominal obesity measured by DXA and waist circumference with insulin sensitivity in Hispanic and non-Hispanic white individuals: The San Luis Valley Diabetes Study. Diabetes Metab Res Rev. 2008;24(1):33–40. doi: 10.1002/dmrr.747. [DOI] [PubMed] [Google Scholar]
- 39.Staiger H, Tschritter O, Machann J, Thamer C, Fritsche A, Maerker E, et al. Relationship of serum adiponectin and leptin concentrations with body fat distribution in humans. Obes Res. 2003;11(3):368–372. doi: 10.1038/oby.2003.48. [DOI] [PubMed] [Google Scholar]
- 40.Dowling HJ, Pi-Sunyer FX. Race-dependent health risks of upper body obesity. Diabetes. 1993;42(4):537–543. [PubMed] [Google Scholar]
- 41.De Luis DA, Aller R, Izaola O, Gonzalez Sagrado M, Conde R, Perez Castrillon JL. Effects of lifestyle modification on adipocytokine levels in obese patients. Eur Rev Med Pharmacol Sci. 2008;12(1):33–39. [PubMed] [Google Scholar]
- 42.Liu J, Askari H, Dagogo-Jack S. Basal and stimulated plasma leptin in diabetic subjects. Obes Res. 1999;7(6):537–544. doi: 10.1002/j.1550-8528.1999.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 43.Madsen EL, Rissanen A, Bruun JM, Skogstrand K, Tonstad S, Hougaard DM, Richelsen B. Weight loss larger than 10% is needed for general improvement of levels of circulating adiponectin and markers of inflammation in obese subjects: a 3-year weight loss study. Eur J Endocrinol. 2008;158(2):179–187. doi: 10.1530/EJE-07-0721. [DOI] [PubMed] [Google Scholar]
- 44.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 45.Pusztai P, Sarman B, Ruzicska E, Toke J, Racz K, Somogyi A, et al. Ghrelin: a new peptide regulating the neurohormonal system, energy homeostatis and glucose metabolism. Diabetes Metab Res Rev. 2008;24(5):343–352. doi: 10.1002/dmrr.830. [DOI] [PubMed] [Google Scholar]
- 46.Garcia JM, Iyer D, Poston WS, Marcelli M, Reeves R, Foreyt J, et al. Rise of plasma ghrelin with weight loss is not sustained during weight maintenance. Obesity (Silver Spring) 2006;14(10):1716–1723. doi: 10.1038/oby.2006.197. [DOI] [PubMed] [Google Scholar]