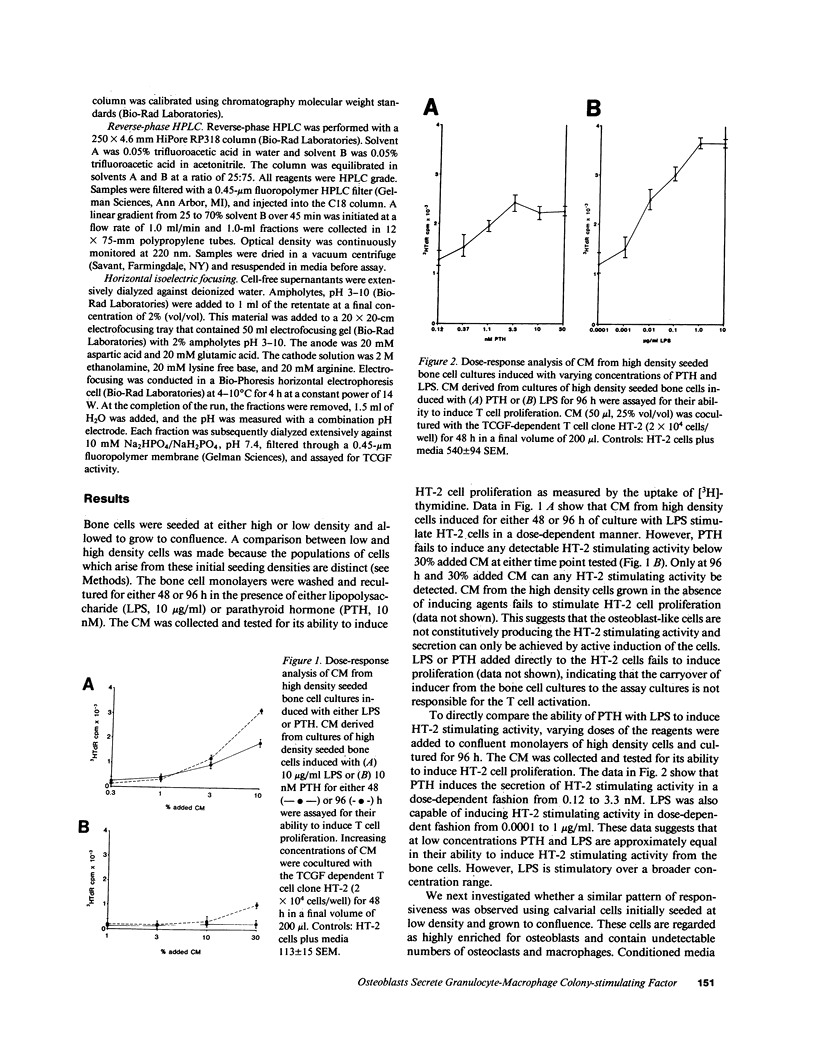
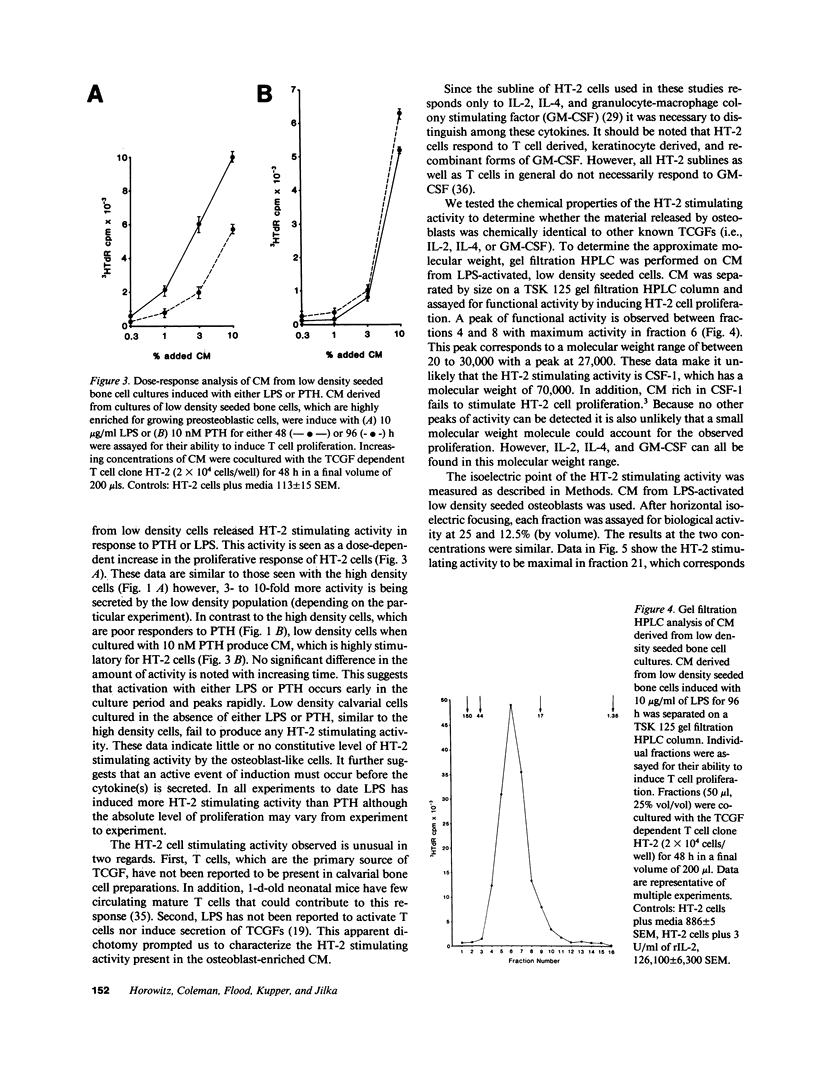
Abstract
Osteoblasts are the cells responsible for the secretion of collagen and ultimately the formation of new bone. These cells have also been shown to regulate osteoclast activity by the secretion of cytokines, which remain to be defined. In an attempt to identify these unknown cytokines, we have induced primary murine osteoblasts with two bone active agents, parathyroid hormone (PTH) and lipopolysaccharide (LPS) and analyzed the conditioned media (CM) for the presence of specific cytokines. Analysis of the CM was accomplished by functional, biochemical, and serological techniques. The data indicate that both PTH and LPS are capable of inducing the osteoblasts to secrete a cytokine, which by all of the techniques used, is indistinguishable from granulocyte-macrophage colony-stimulating factor (GM-CSF). Secretion of GM-CSF is not constitutive and requires active induction. Production of the cytokine is dependent on the dose of PTH or LPS added. It has been demonstrated that the addition of GM-CSF to bone marrow cultures results in the formation of increased numbers of osteoclasts. Therefore, these data suggest that osteoblasts not only participate in bone remodeling by formation of new matrix but may regulate osteoclast activity indirectly by their ability to regulate hematopoiesis.
Full text
PDF

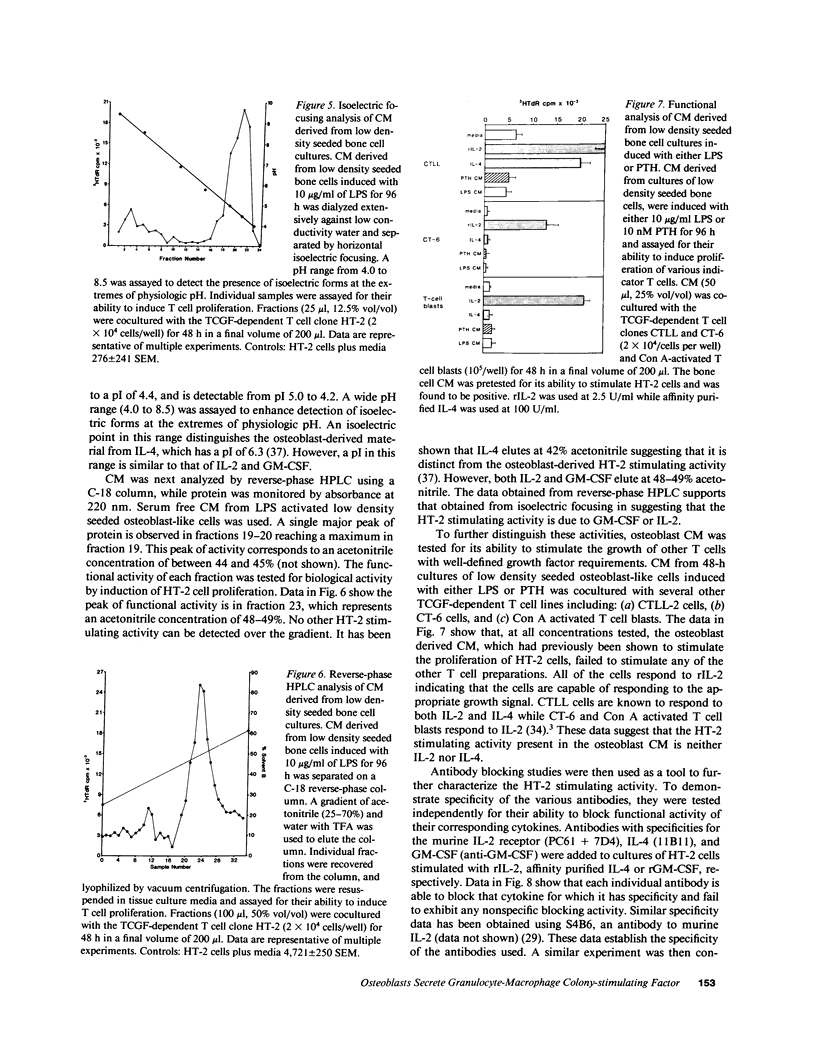
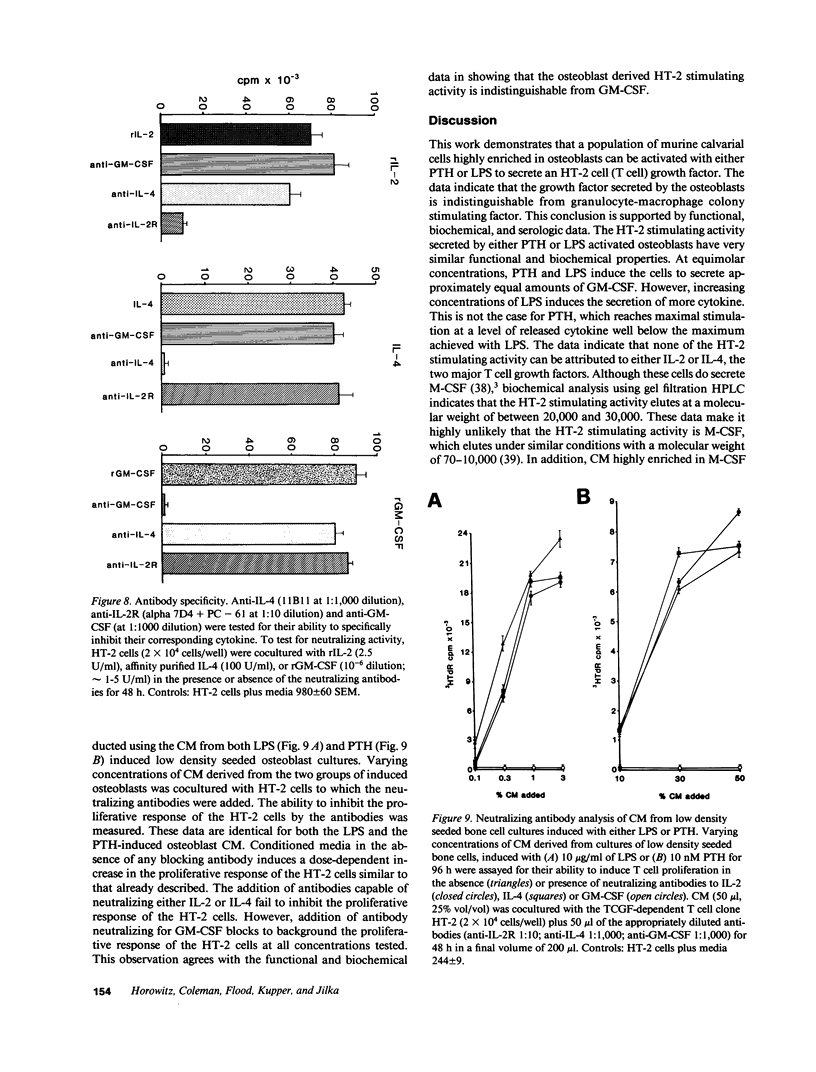



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987 Feb 25;262(6):2869–2874. [PubMed] [Google Scholar]
- Coleman D. L., Kupper T. S., Flood P. M., Fultz C. C., Horowitz M. C. Characterization of a keratinocyte-derived T cell growth factor distinct from interleukin 2 and B cell stimulatory factor 1. J Immunol. 1987 May 15;138(10):3314–3318. [PubMed] [Google Scholar]
- Elford P. R., Felix R., Cecchini M., Trechsel U., Fleisch H. Murine osteoblastlike cells and the osteogenic cell MC3T3-E1 release a macrophage colony-stimulating activity in culture. Calcif Tissue Int. 1987 Sep;41(3):151–156. doi: 10.1007/BF02563795. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Howard M., Fuller-Farrar J., Paul W. E. Biochemical and physicochemical characterization of mouse B cell growth factor: a lymphokine distinct from interleukin 2. J Immunol. 1983 Oct;131(4):1838–1842. [PubMed] [Google Scholar]
- Felix R., Elford P. R., Stoercklé C., Cecchini M., Wetterwald A., Trechsel U., Fleisch H., Stadler B. M. Production of hemopoietic growth factors by bone tissue and bone cells in culture. J Bone Miner Res. 1988 Feb;3(1):27–36. doi: 10.1002/jbmr.5650030106. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R., Sanders V. M., Oliver K. G., Chen Y. W., Krammer P. H., Uhr J. W., Vitetta E. S. Interleukin 4 mediates autocrine growth of helper T cells after antigenic stimulation. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9689–9693. doi: 10.1073/pnas.83.24.9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Abraham R. T., Gillis S., McKean D. J. Differential bioassay of interleukin 2 and interleukin 4. J Immunol Methods. 1987 Apr 2;98(1):99–104. doi: 10.1016/0022-1759(87)90441-8. [DOI] [PubMed] [Google Scholar]
- Horowitz M., Vignery A., Gershon R. K., Baron R. Thymus-derived lymphocytes and their interactions with macrophages are required for the production of osteoclast-activating factor in the mouse. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2181–2185. doi: 10.1073/pnas.81.7.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka Y., Noguchi K., Schuebel K. E., Miyake T., Murphy M. J., Jr A quantitative assay for mouse granulocyte (CFU-g) and macrophage (CFU-m) precursors using plasma clots. J Histochem Cytochem. 1985 Jul;33(7):617–623. doi: 10.1177/33.7.4008915. [DOI] [PubMed] [Google Scholar]
- Jilka R. L. Are osteoblastic cells required for the control of osteoclast activity by parathyroid hormone? Bone Miner. 1986 Sep;1(4):261–266. [PubMed] [Google Scholar]
- Jilka R. L., Cohn D. V. Role of phosphodiesterase in the parathormone-stimulated adenosine 3',5'-monophosphate response in bone cell populations enriched in osteoclasts and osteoblasts. Endocrinology. 1981 Sep;109(3):743–747. doi: 10.1210/endo-109-3-743. [DOI] [PubMed] [Google Scholar]
- Jilka R. L. Parathyroid hormone-stimulated development of osteoclasts in cultures of cells from neonatal murine calvaria. Bone. 1986;7(1):29–40. doi: 10.1016/8756-3282(86)90149-3. [DOI] [PubMed] [Google Scholar]
- Kahn A. J., Stewart C. C., Teitelbaum S. L. Contact-mediated bone resorption by human monocytes in vitro. Science. 1978 Mar 3;199(4332):988–990. doi: 10.1126/science.622581. [DOI] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S., Lee F., Coleman D., Chodakewitz J., Flood P., Horowitz M. Keratinocyte derived T-cell growth factor (KTGF) is identical to granulocyte macrophage colony stimulating factor (GM-CSF). J Invest Dermatol. 1988 Aug;91(2):185–188. doi: 10.1111/1523-1747.ep12464470. [DOI] [PubMed] [Google Scholar]
- Kupper T., Flood P., Coleman D., Horowitz M. Growth of an interleukin 2/interleukin 4-dependent T cell line induced by granulocyte-macrophage colony-stimulating factor (GM-CSF). J Immunol. 1987 Jun 15;138(12):4288–4292. [PubMed] [Google Scholar]
- Lanotte M., Metcalf D., Dexter T. M. Production of monocyte/macrophage colony-stimulating factor by preadipocyte cell lines derived from murine marrow stroma. J Cell Physiol. 1982 Jul;112(1):123–127. doi: 10.1002/jcp.1041120118. [DOI] [PubMed] [Google Scholar]
- Lorenzo J. A., Sousa S. L., Fonseca J. M., Hock J. M., Medlock E. S. Colony-stimulating factors regulate the development of multinucleated osteoclasts from recently replicated cells in vitro. J Clin Invest. 1987 Jul;80(1):160–164. doi: 10.1172/JCI113042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald B. R., Mundy G. R., Clark S., Wang E. A., Kuehl T. J., Stanley E. R., Roodman G. D. Effects of human recombinant CSF-GM and highly purified CSF-1 on the formation of multinucleated cells with osteoclast characteristics in long-term bone marrow cultures. J Bone Miner Res. 1986 Apr;1(2):227–233. doi: 10.1002/jbmr.5650010210. [DOI] [PubMed] [Google Scholar]
- McSheehy P. M., Chambers T. J. Osteoblast-like cells in the presence of parathyroid hormone release soluble factor that stimulates osteoclastic bone resorption. Endocrinology. 1986 Oct;119(4):1654–1659. doi: 10.1210/endo-119-4-1654. [DOI] [PubMed] [Google Scholar]
- McSheehy P. M., Chambers T. J. Osteoblastic cells mediate osteoclastic responsiveness to parathyroid hormone. Endocrinology. 1986 Feb;118(2):824–828. doi: 10.1210/endo-118-2-824. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Nicola N. A., Burgess A. W., Metcalf D. Similar molecular properties of granulocyte-macrophage colony-stimulating factors produced by different mouse organs in vitro and in vivo. J Biol Chem. 1979 Jun 25;254(12):5290–5299. [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Parker J. W., Metcalf D. Production of colony-stimulating factor in mitogen-stimulated lymphocyte cultures. J Immunol. 1974 Feb;112(2):502–510. [PubMed] [Google Scholar]
- Perry H. M., 3rd, Chappel J. C., Bellorin-Font E., Tamao J., Martin K. J., Teitelbaum S. L. Parathyroid hormone receptors in circulating human mononuclear leukocytes. J Biol Chem. 1984 May 10;259(9):5531–5535. [PubMed] [Google Scholar]
- Perry H. M., 3rd Parathyroid hormone-lymphocyte interactions modulate bone resorption. Endocrinology. 1986 Nov;119(5):2333–2339. doi: 10.1210/endo-119-5-2333. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Mundy G. R. Modulation of type beta transforming growth factor activity in bone cultures by osteotropic hormones. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2024–2028. doi: 10.1073/pnas.84.7.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliam N. B., Nyiredy K. O., Arnaud C. D. Parathyroid hormone receptors in avian bone cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2061–2063. doi: 10.1073/pnas.79.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry P. J., Gimbrone M. A., Jr Vascular endothelium as a regulator of granulopoiesis: production of colony-stimulating activity by cultured human endothelial cells. Blood. 1980 Dec;56(6):1060–1067. [PubMed] [Google Scholar]
- Roehm N., Herron L., Cambier J., DiGuisto D., Haskins K., Kappler J., Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells: distribution on thymus and peripheral T cells. Cell. 1984 Sep;38(2):577–584. doi: 10.1016/0092-8674(84)90512-9. [DOI] [PubMed] [Google Scholar]
- Rouleau M. F., Warshawsky H., Goltzman D. Parathyroid hormone binding in vivo to renal, hepatic, and skeletal tissues of the rat using a radioautographic approach. Endocrinology. 1986 Mar;118(3):919–931. doi: 10.1210/endo-118-3-919. [DOI] [PubMed] [Google Scholar]
- Schneider G. B., Relfson M. The effects of transplantation of granulocyte-macrophage progenitors on bone resorption in osteopetrotic rats. J Bone Miner Res. 1988 Apr;3(2):225–232. doi: 10.1002/jbmr.5650030216. [DOI] [PubMed] [Google Scholar]
- Sieff C. A. Hematopoietic growth factors. J Clin Invest. 1987 Jun;79(6):1549–1557. doi: 10.1172/JCI112988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. J., Kent G. N., Jilka R. L., Scott D. M., Fallon M., Cohn D. V. Formation of bone by isolated, cultured osteoblasts in millipore diffusion chambers. Calcif Tissue Int. 1982 May;34(3):291–294. doi: 10.1007/BF02411253. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Thomson B. M., Mundy G. R., Chambers T. J. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987 Feb 1;138(3):775–779. [PubMed] [Google Scholar]
- Thomson B. M., Saklatvala J., Chambers T. J. Osteoblasts mediate interleukin 1 stimulation of bone resorption by rat osteoclasts. J Exp Med. 1986 Jul 1;164(1):104–112. doi: 10.1084/jem.164.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Mermod J. J., Vassalli P. Phagocytosis and inflammatory stimuli induce GM-CSF mRNA in macrophages through posttranscriptional regulation. Cell. 1987 Feb 27;48(4):671–679. doi: 10.1016/0092-8674(87)90245-5. [DOI] [PubMed] [Google Scholar]
- Walker D. G. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science. 1975 Nov 21;190(4216):784–785. doi: 10.1126/science.1105786. [DOI] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbart R. H., Golde D. W., Clark S. C., Wong G. G., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. 1985 Mar 28-Apr 3Nature. 314(6009):361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- Wong G. L., Cohn D. V. Target cells in bone for parathormone and calcitonin are different: enrichment for each cell type by sequential digestion of mouse calvaria and selective adhesion to polymeric surfaces. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3167–3171. doi: 10.1073/pnas.72.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]