Abstract
Objective: When treating acute bipolar mania, the speed of onset of anti-manic effects is crucial. Quetiapine and divalproex ER are widely used agents to treat acute mania. Rapid dose administration regimens for divalproex ER and for quetiapine have been described. We conducted a naturalistic, head-to-head, pilot study comparing the efficacy and safety of rapidly titrated divalproex ER and quetiapine in acutely manic inpatients, with the primary outcome being improvement within the first seven days.
Method: Thirty consenting bipolar patients with acute mania (Young Mania Rating Scale >17 ) needing hospitalization due to acute mania were randomized to receive rapidly loaded divalproex ER (30mg/kg/day) or rapidly titrated quetiapine (200mg Day 1, raised by 200mg/day up to 800mg as tolerated). Assessments were made on Day 1 (baseline), Day 3, Day 7, Day 14, and Day 21 and included Young Mania Rating Scale, Clinical Global Impressions-Severity, Clinical Global Impressions-Improvement, and Montgomery-Asberg Depression Rating Scale. Raters but not patients or treating physicians were blinded (single-blinded study).
Results: Subjects in both treatment groups exhibited significant and rapid improvement in their mania starting at Day 3 with few significant adverse effects; however, there were no significant differences in the degree or rate of improvement between the two treatment groups in any of the efficacy or adverse effects scales.
Conclusion: Results of this small study indicate that rapid-dose administration of both quetiapine and divalproex ER produce rapid improvement in acute mania within the first seven days and both seem to be well tolerated.
Keywords: Mania, bipolar affective disorder, quetiapine, divalproex
Introduction
Type I bipolar disorder is one of the most common, severe, and persistent mental illnesses,1 characterized by its distinct alternating periods of depression and mania. Symptoms of mania are often exhibited through destructive and sometimes life-threatening behavior, including reckless actions without concern for consequences, unrestricted shopping sprees, sexual promiscuity, and other potentially harmful actions. These periods cause devastation in the lives of patients and their loved ones. One of the challenges facing psychiatrists, with regard to patients who are exhibiting severe acute mania, is to reduce the symptoms rapidly. For this reason, it is crucial not only to identify effective treatments for this illness, but important to identify treatment options providing rapid onset of efficacious treatment.
Currently, the standard of care for the treatment of acute bipolar mania is pharmacological intervention using either lithium, atypical antipsychotics, or anticonvulsant mood stabilizers. Though all three classes of drug have proven efficacy, factors not directly related to the inherent efficacy of a drug play an important role in their ability to stabilize manic patients, in particular, the time required to onset of their therapeutic effects. In the case of many anti-mania medications, this is associated with the time required to achieve a therapeutic dose. For this reason, efforts have been made to develop rapid oral-loading regimens of anti-mania medication, and evidence suggest that such regimens produce more rapid clinical improvement.2–4
Divalproex sustained release (SR) was approved for treatment of bipolar mania in 1995 and is widely used in inpatient settings. Rapid oral loading regiments for divalproex SR were developed to achieve its therapeutic plasma level of 50 to 125mcg/mL within 24 to 48 hours. More recently, an extended release (ER) formulation of divaproex has been available and approved for bipolar mania. The product labeling dosing for divalproex is currently 25mg/kg, and studies suggest that a rapid loading of divalproex ER (30mg/kg) is well tolerated and produces target therapeutic plasma levels by Day 3 of treatment.4,5
Quetiapine is a commonly used atypical antipsychotic that is indicated as monotherapy for bipolar mania. In published studies of quetiapine in bipolar mania, doses were initiated at 100mg/day and titrated to 600 to 800mg/day by Days 5 to 6. The average dose of responders was 600mg/day,6 though a rapid-dosing regimen is not included in product labeling for quetiapine.
A recent study comparing quetiapine and divalproex sustained release (SR) in adolescents with mania7 demonstrated significantly faster therapeutic effects among subjects given quietiapine. However, this study used a rapid oral regimen for quetiapine but not a rapid loading regimen of divalproex. Moreover, the comparative benefits of quetiapine and divalproex have never been studied in adults with mania. The aim of this study was to compare the efficacy and tolerability of quetiapine versus divalproex ER in the treatment of acute episodes of mania or mixed mania in adults when both drugs are administered in a rapid oral-loading fashion.
Research Design and Methods
Subjects. Subjects between the ages of 18 and 65 years of age with a diagnosis of bipolar I disorder, most recent episode manic, or bipolar I disorder, most recent episode mixed, with or without psychotic features, as defined by DSM-IV1 were recruited. Subjects were recruited from among subjects presenting to the University of California, San Diego (UCSD) Medical Center's emergency department and deemed needing hospitalization for their mania and from those recently admitted to the inpatient psychiatry unit for treatment of acute mania. Consenting subjects were enrolled if they scored equal to or greater than 17 on the Young Mania Rating Scale (YMRS)8 as well as receiving a score of 4 (moderate) or higher on the Clinical Global Impressions-Severity (CGI-S) Scale.9 Subjects meeting inclusion criteria were hospitalized for a minimum of three days and randomized in a 1:1 ratio to one of two treatment arms, divalproex ER or quetiapine. All subjects gave written consent and were assessed for capacity to consent prior to performing any study procedures. This study was approved by the Human Research Protection Program at UCSD. Subjects were excluded if treatment with a depot antipsychotic was within one treatment cycle. Lorazepam was provided for agitation and insomnia as needed for rescue only. The maximal dose of lorazepam was 6mg in the first seven days, 4mg for the next three days, and 2mg/day for the remainder of the study. Those who required a greater amount of lorazepam were excluded. Nonpsychotropic medications were allowed as deemed necessary by the subject's treating physician.
Study medication. Divalproex ER was initiated at 30mg/kg/day orally taken at night, rounded up to the nearest 500mg dose, with adjustments made through the trial to obtain optimal serum valproic acid levels between 85 and 125µ/mL. This is based upon an oral dosing regimen for divalproex ER.4 Plasma valproate levels were taken on Day 3 and on Day 21. Upon study physicans' discretion, additional plasma valproate levels could be drawn on Days 7 and 14 if the therapeutic plasma level was not reached by Day 3. Unblinded study physicians were able to adjust the dose at any day subsequent to Day 3 with the goal of achieving therapeutic levels (85–125µ/mL) as guided by tolerance and efficacy.
Quetiapine was given orally at an initial dose of 200mg/day and titrated up to a target dose of 600 to 800mg/day based upon published rapid-loading regiments.10,11 The targeted titration for quetiapine is described in Table 1.
Table 1.
Quetiapine target titration schedule
| DAY | DOSE |
|---|---|
| 1 | 200mg |
| 2 | 400mg |
| 3 | 600mg |
| 4 | 800mg |
Study physicians had the option of slowing the titration rate only if the subject experienced significant adverse effects (e.g., excess sedation).
Efficacy assessments. Independent raters, blind to the subjects' treatment, used the following scales to assess efficacy: YMRS,8 CGI-S and CGI-Improvement (CGI-I),9 Extra Pyramidal Symptoms Rating Scale (ESRS),12 Montgomery-Asberg Depression Rating Scale (MADRS),13 and Behavioural Activity Rating Scale (BARS).14
The primary efficacy measure was the change from baseline to endpoint in YMRS. Assessments were made on Day 1 (baseline), Day 3, Day 7, Day 14, and Day 21, with the primary endpoint being Day 7 corresponding to our interest in the comparative speed of onset.
Statistical methods. Efficacy analyses were performed on the intent-to-treat (ITT) population, which included all subjects who received at least one dose of study medication and at least one post-baseline efficacy assessment (i.e., Day 3 assessment). Independent t-tests were used to compare demographic and baseline clinical variables between treatment groups. Data were analyzed using last observation carried forward (LOCF) method, in which the last collected data point is projected forward to all subsequent study assessments for subjects who dropped out before final visit (Day 21).
The primary outcome measures were tested using an independent group t-test to compare the change in YMRS scores from baseline to Day 7.
For analysis of outcome measures (i.e., YMRS, YMRS change from baseline, MADRS, and BARS), a repeated measures analysis of variance (ANOVA) was applied with the “drug treatment group” as a between-subject factor, “treatment day” as a within-subject factor, and “baseline score” as a covariate term.
Significant effects of “treatment day,” “treatment group,” or “treatment day by treatment group” interaction were followed up with appropriate post-hoc pairwise comparisons. Specifically, Dunnet's test was used to compare differences between baseline scores and scores at each assessment day when there was a significant effect of “treatment day” and independent t-test with Bonferonni corrections were used to compare differences between treatment groups at each assessment point. Data were not collapsed across “treatment group,” even when there was no significant effects of “treatment group” since potential differences in treatment groups were the a priori interest of this study. Categorical data (CGI-S, CGI-I) were analyzed using Pearson chi-square test.
All statistical tests were performed using the SPSS for Windows v. 11.0.1 Rel. 2001 (SPSS inc., Chicago, Illinois) software, and the method of last observation carried forward (LOCF) and all statistical tests were two-tailed with alpha=0.05.
Results
Thirty subjects received study medication but two subjects were discontinued prior to receiving any post-baseline efficacy assessment and therefore were not included in the analysis. One of these subjects was assigned in the divalproex ER treatment group and withdrew consent, and the other was assigned to the quetiapine group and was withdrawn for medical reasons. Of the remaining 28 subjects, 15 were randomly assigned to the quetiapine treatment group while 13 were assigned to the divalproex ER treatment group. Sixteen subjects (57.1%) were male. The majority of subjects (71%) were Caucasian, and the ages ranged between 21 and 65 years (mean=39.5). There were no significant differences between the two treatment groups regarding race, gender, or age (Table 2). At Day 7, the most common doses were 800mg of quetiapine and 2500mg of divalproex ER. Lorazepam usage was similar between treatment groups. No subjects who completed baseline efficacy assessments and were assigned to quetiapine discontinued within the first seven days. Three subjects were withdrawn from divalproex ER group between Day 3 and, the primary efficacy endpoint, Day 7; two due to poor adherence with study protocol and one due to an adverse effect (Table 3).
Table 2.
Baseline characteristics of included subjects (N=28)
| CHARACTERISTICS | NUMBER (%) DIVALPROEX ER GROUP (n=13) | NUMBER (%) QUETIAPINE GROUP (n=15) |
|---|---|---|
| GENDER | ||
| Male | 8 (61.5) | 8 (53.3) |
| Female | 5 (38.5) | 7 (46.7) |
| AGE | 42.4 | 36.9 |
| RACE | ||
| Caucasian | 11 (84.6) | 9 (60.0) |
| African American | 0 (0) | 4 (26.7) |
| Other | 2 (14.1) | 2 (13.3) |
Table 3.
Participation by treatment day
| TREATMENT GROUP | BASELINE | DAY 3 | DAY 7 | DAY 14 | DAY 21 |
|---|---|---|---|---|---|
| Divalproex ER | n=14 | n=13 | n=10 | n=10 | n=10 |
| Quetiapine | n=16 | n=15 | n=15 | n=14 | n=13 |
Primary Outcome Measures–YMRS Change Day 7. There was no significant difference between the two treatment groups in the change in YMRS scores from baseline to Day 7 among the intent-to-treat population (t(26)=1.12, p=0.275) or among observed cases (t(23)=0.80, p=0.435).
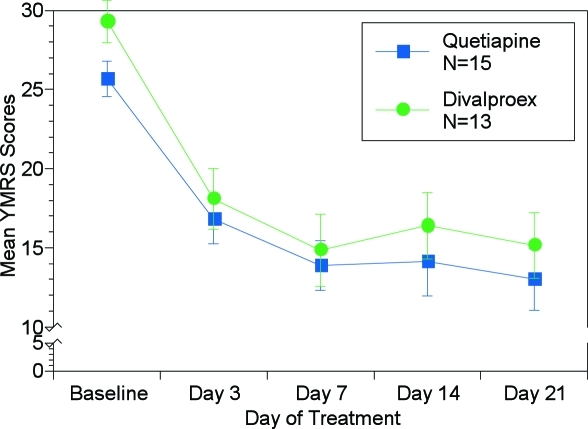
YMRS. A repeated measures ANOVA applied to the YMRS scores revealed a significant “treatment day” effect (F (4, 104)=49.44, p<0.001), as YMRS scores in both treatment groups dropped over the course of the study (Figure 1). There was no significant effect of “treatment group” (F (1, 26)=0.903, p=0.351) and no significant “treatment group by treatment day” interaction (F (4, 104)=0.424, p=0.791) YMRS scores were significantly lower (p<0.001 for all comparisons) at Days 3, 7, 14, and 21 compared to respective baseline in both the divalproex ER treatment group (baseline=29.3, Day 3=18.1, Day 7=14.8, Day 14=16.4, Day 21=15.2, p<0.001 ), as well as the quetiapine treatment group (baseline=25.7, Day 3=16.8, Day 7=13.9, Day 14=14.1, Day 21=13.0, p<0.001).
Figure 1.
Mean YMRS scores over time with LOCF method. Asterisks indicate significantly (P< 0.05) lower scores for both treatments compared to their respective baseline by Dunnetts test.
ANOVA applied to the YMRS change from baseline data revealed a significant effect of “treatment day” (F(3, 78)=4.25, p=0.008) as change from baseline increased across study visit days. There was no significant effect of “treatment groups” (F (1, 26)=0.86, p=0.364) and no significant “treatment day by treatment group” interaction (F (3, 78)=0.18, p=0.907).
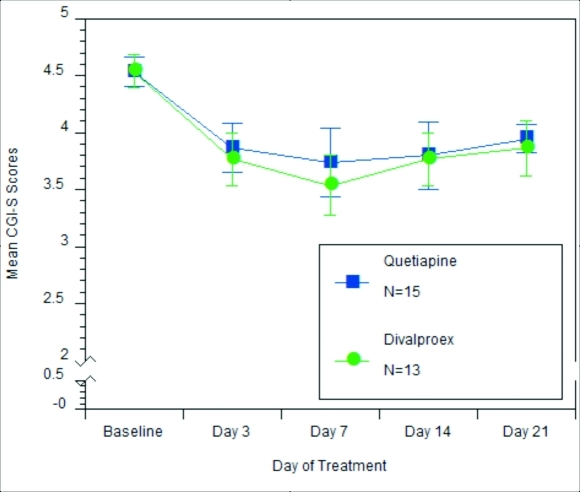
CGI-S and CGI-I. Figures 2 and 3 illustrate CGI-S and CGI-I results, respectively. Results indicate that there was no statistically significant relationship between the treatment group and CGI-S scores. At baseline, subjects in both the quetiapine and divalproax ER treatment groups were rated moderately ill or markedly ill (chi-square=0.001, p=0.978). At Treatment Day 7, 61.5 percent of subjects receiving divalproex ER versus 40 percent of subjects receiving quetiapine received a score of mildly ill or better (chi-square=3.642, p=0.303).
Figure 2.
Mean CGI-S scores over time. CGI-S scores: 0=not assessed; 1=normal, not at all ill; 2=borderline mentally ill; 3=mildly mentally ill; 4=moderately ill; 5=markedly ill; 6=severely ill; 7=among the most extremely ill subjects
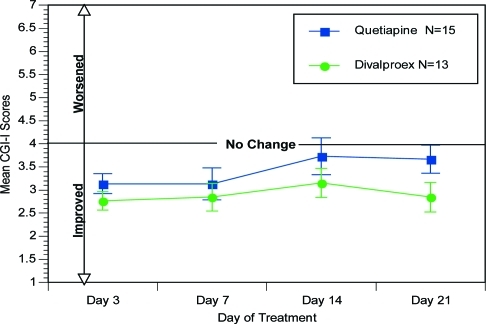
Figure 3.
Mean CGI-I scores over time. CGI:I scores: 1=very much improved; 2=much improved; 3=minimally improved; 4=no change; 5=minimally worse; 6=much worse; 7=very much worse
Analysis of the CGI-I scores indicate that at primary endpoint, Treatment Day 7, 84.7 percent receiving divalproex ER versus 60 percent of subjects receiving quetiapine were minimally improved, much improved, or very much improved.
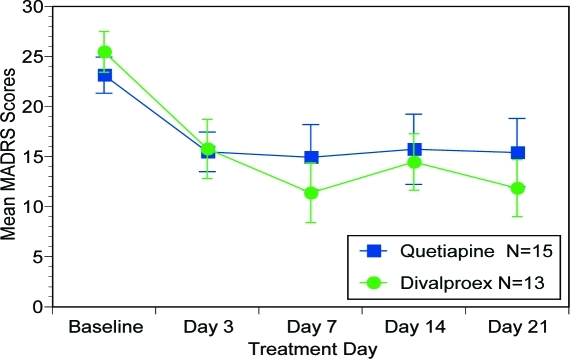
MADRS. Figure 4 illustrates MADRS scores revealed significant effect of “treatment day” (F (4, 104)=14.88, p<0.001), but not “treatment group” (F (1, 26)=0.11, p=0.740) nor “treatment group by treatment day” interaction (F (4,104)=1.16, p=0.335).
Figure 4.
Mean MADRS scores over time
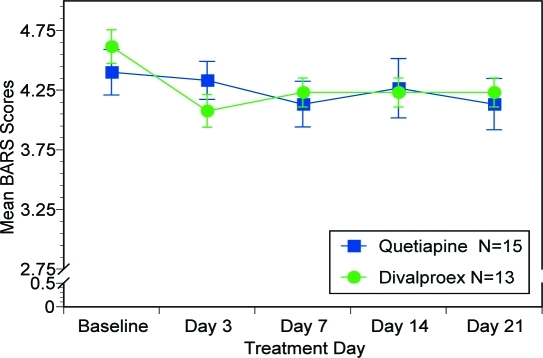
BARS. Figure 5 reveals BARS results. There was no significant effect of “treatment day” (F (4, 104)=1.81, p=0.133), “treatment group” (F (1, 26)=0.02, p=0.890) nor “treatment assignment by treatment day” interaction (F (4, 104)=0.766, p=0.550).
Figure 5.
Mean BARS scores over time
Adverse events. ESRS data revealed no significant effects of “treatment day” (F (4, 96)=1.19, p=0.321), “treatment group” (F (1, 24)=1.04, p=0.319), or “treatment group by treatment day” interaction (F (4, 96)=1.214, p=0.310.)
There were also no significant differences in safety and laboratory values from baseline to endpoints in either the divalproex ER treatment group or the quetiapine treatment group (F (1, 26)=2.792, p=0.106).While there were no statistically significant differences there were much greater incidence of adverse events regarding the head, eyes, nose, ears, and throat reported for quetiapine (33.3%) than divalproex ER (7.7%). See Table 4 for percentage of subjects experiencing adverse events.
Table 4.
Percent of subjects experiencing adverse events reported by treatment group
| DRUG GROUP | HEAD, EYES, EARS, NOSE, AND THROAT | MUSCULO/SKELETAL | GENITO-URINARY | PULMONARY | PSYCHIATRIC | NEUROLOGICAL (FAINTING, DIZZINESS, HEADACHES) | GASTRO-INTESTINAL | OTHER |
|---|---|---|---|---|---|---|---|---|
| Quetiapine n=15 | 33.30% | 6.70% | 6.70% | 13.30% | 46.70% | 17.30% | 26.70% | 0.93% |
| Divalproex ER n=13 | 7.70% | 7.70% | 7.70% | 15.40% | 30.80% | 38.50% | 30.80% | 1.38% |
Discussion
This study aimed to evaluate the relative efficacy and tolerability of quetiapine and divalproex ER in the treatment of subjects exhibiting an acute bipolar mania episode under highly naturalistic conditions. The subjects recruited in this study had already recently been admitted to an acute inpatient psychiatry unit for treatment of their mania or had presented to an emergency room due to their acute mania and thus were candidates for acute hospitalization. We found that both quetiapine and divalproex ER, administered using rapid titratation schedules, produced rapid improvements in symptoms of mania and depression. The overall degree of improvement in symptoms, the speed of improvement, and the burden of adverse effects were comparable between the two treatments. There was a nonsignificant but notably higher rate of reported adverse events associated with head, ears, eyes, nose, and throat in quietiapine-treated patients (n=5) compared to divalproex (n=1) and this might be due to the antihistaminergic properties associated with that drug. The largest improvement occurred within the first three days for both treatment regimens. YMRS item analysis also revealed no differences in individual symptom improvement.
Two major limitations of this study were the relatively small sample size and the lack of a placebo-controlled arm. The former may have precluded detection of significant differences between the groups and the latter precluded the ability to determine whether either treatment produced bioactive therapeutic effects beyond placebo. In addition, since there was no comparator arms using nonrapid titration of either drug, this study could not establish whether these aggressive titration regimens enhanced the speed of recovery. Another limitation is the fact that this study compared an extended-release formulation of divalproex to an immediate-release formulation of quietiapine. This study was initiated before the recently available extended-release formulation of quietiapine was on the market. Nevertheless, this study does provide preliminary support for the notion that acutely manic subjects treated with either rapidly titrated divalproex ER or quetiapine exhibit robust and well-tolerated improvement in their mania within a few days.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of mental disorders. Fourth Edition. Arlington, VA: American Psychiatric Press, Inc.; 2004. [Google Scholar]
- 2. Keck PE, Jr, McElroy SL, Tugrul KC, Bennett JA. Valproate oral loading in the treatment of acute mania. J Clin Psychiatry. 1993;54(8):305–308. [PubMed] [Google Scholar]
- 3. Hirschfeld RM, Baker JD, Wozniak P, et al. The safety and early efficacy of oral-loaded divalproex versus standard-titration divalproex, lithium, olanzapine, and placebo in the treatment of acute mania associated with bipolar disorder. J Clin Psychiatry. 2003;64(7):841–846. doi: 10.4088/jcp.v64n0717. [DOI] [PubMed] [Google Scholar]
- 4. Miller BP, Perry W, Moutier CY, et al. Rapid oral loading of extended release divalproex in patients with acute mania. Gen Hosp Psychiatry. 2005;27(3):218–221. doi: 10.1016/j.genhosppsych.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 5. Feifel D. Dosing of divalproex extended release. J Clin Psychiatry. 2007;68(7):1146–1147. doi: 10.4088/jcp.v68n0726b. [DOI] [PubMed] [Google Scholar]
- 6. Vieta E, Mullen J, Brecher M, et al. Quetiapine monotherapy for mania associated with bipolar disorder: combined analyses of two international, double-blind, randomised, placebo-controlled studies. Curr Med Res Opin. 2005;21(6):923–934. doi: 10.1185/030079905X46340. [DOI] [PubMed] [Google Scholar]
- 7. DelBello MP, Kowatch RA, Adler CM, et al. A double-blind, randomized, pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45(3):305–313. doi: 10.1097/01.chi.0000194567.63289.97. [DOI] [PubMed] [Google Scholar]
- 8. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiat. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 9.Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised DHEW Pub. (ADM) Rockville, MD: National Institute for Mental Health; 1976. Clinical Global Impressions; pp. 218–222. [Google Scholar]
- 10. Pae CU, Ghaemi N, Kim TS, et al. Rapid titration versus conventional titration of quetiapine in the treatment of bipolar mania: a preliminary trial. Int Clin Psychopharmacol. 2005;20(6):327–330. doi: 10.1097/00004850-200511000-00008. [DOI] [PubMed] [Google Scholar]
- 11. Hatim A, Habil H, Jesjeet SG, et al. Safety and efficacy of rapid dose administration of quetiapine in bipolar mania. Hum Psychopharmacol. 2006;21(5):313–318. doi: 10.1002/hup.771. [DOI] [PubMed] [Google Scholar]
- 12. Chouinard G, Ross-Chouinard A, Annable L, et al. Extrapyramidal Rating Scale. Can J Neurologic Sci. 1980;7:233–239. [Google Scholar]
- 13. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 14.Swift RH, Harrigan EP, Cappelleri J, et al. Validation of the Behavioural Assessment Rating Scale (BARS): a novel measure of activity in agitated patients. Jul 12-16, 1998. Collegium Internationale Neuro-Psychopharmacologium (CINP). Glasgow, Scotland. [DOI] [PubMed]