Abstract
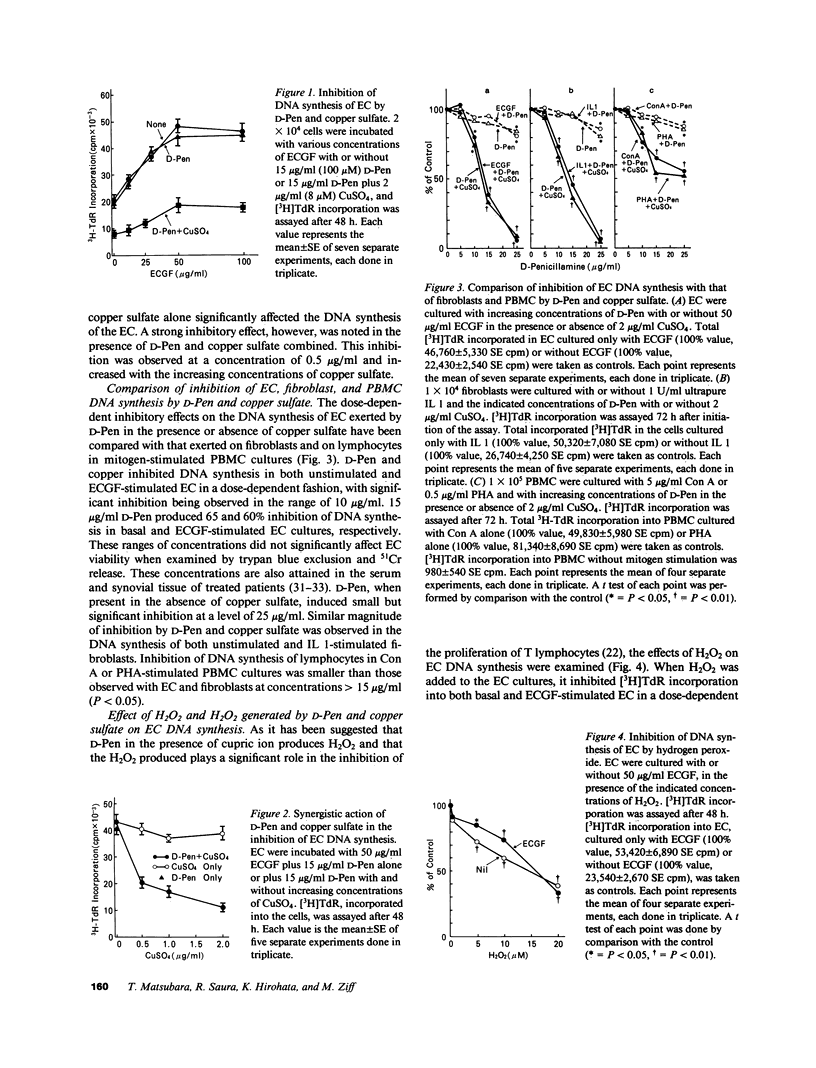
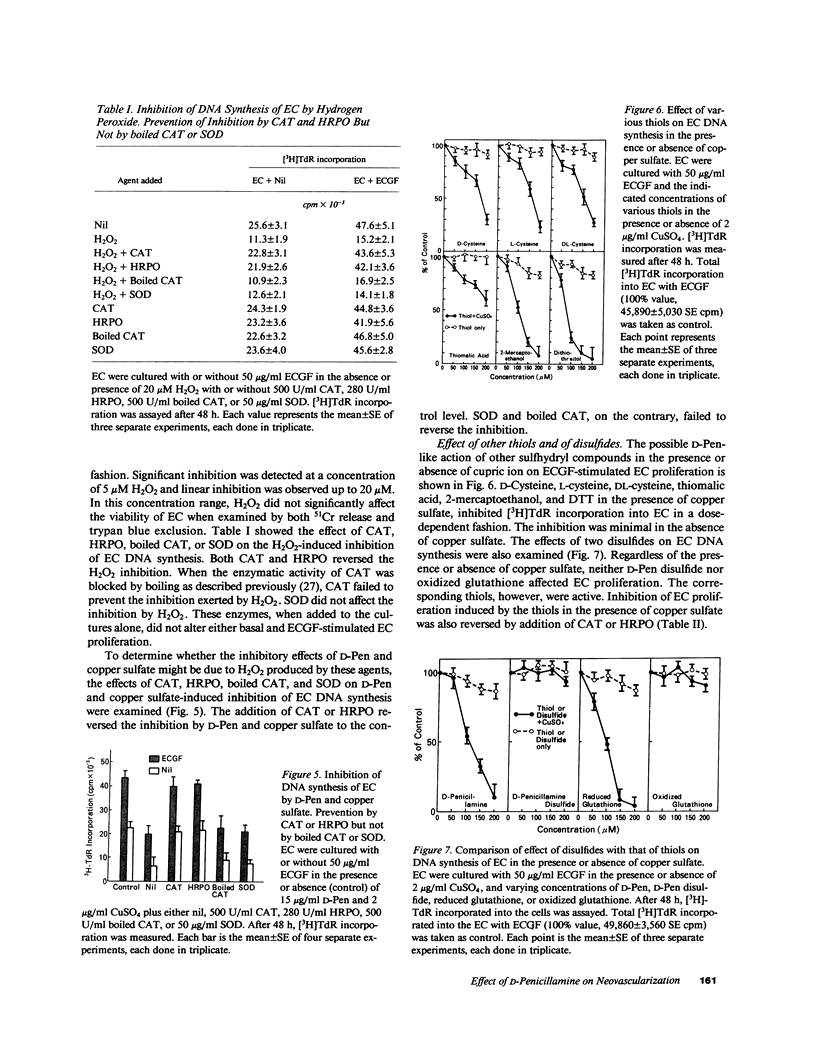
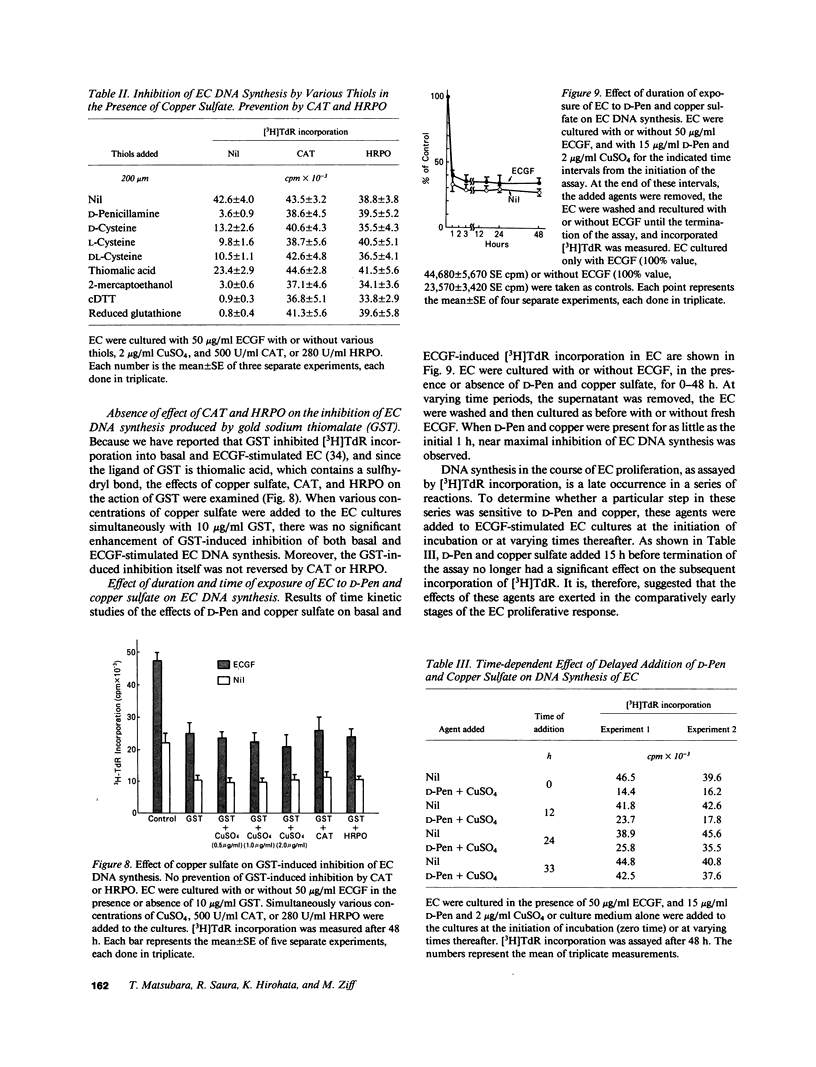
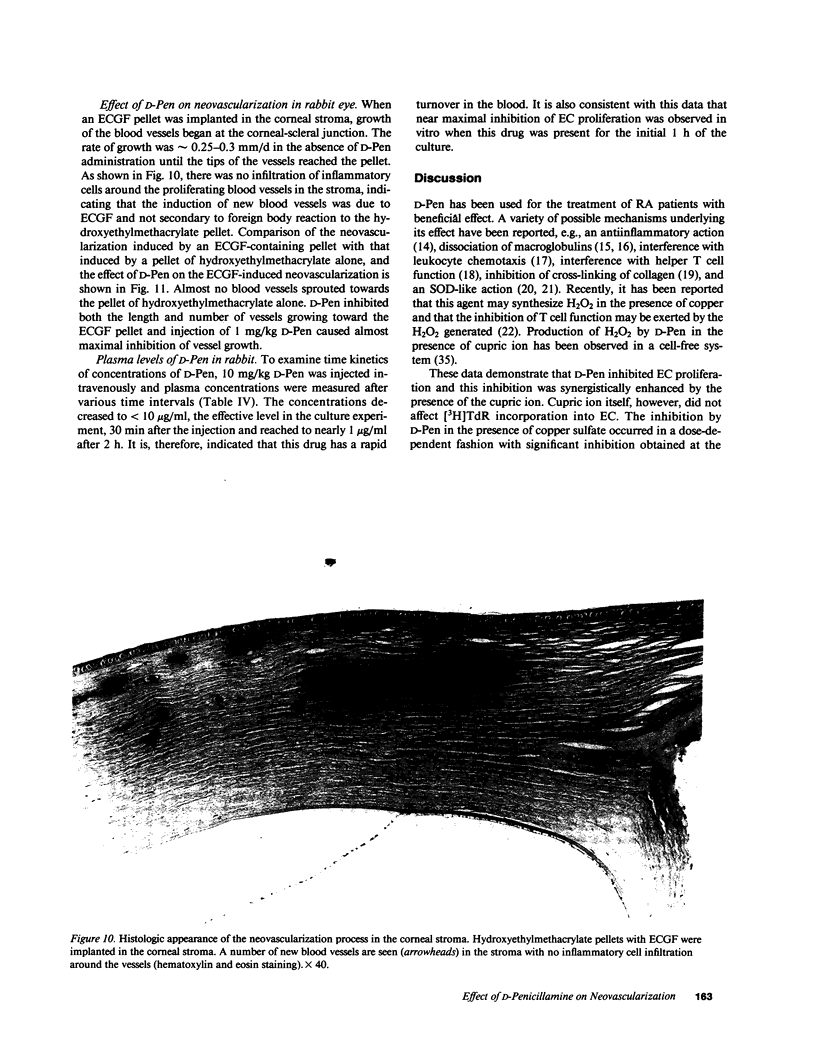
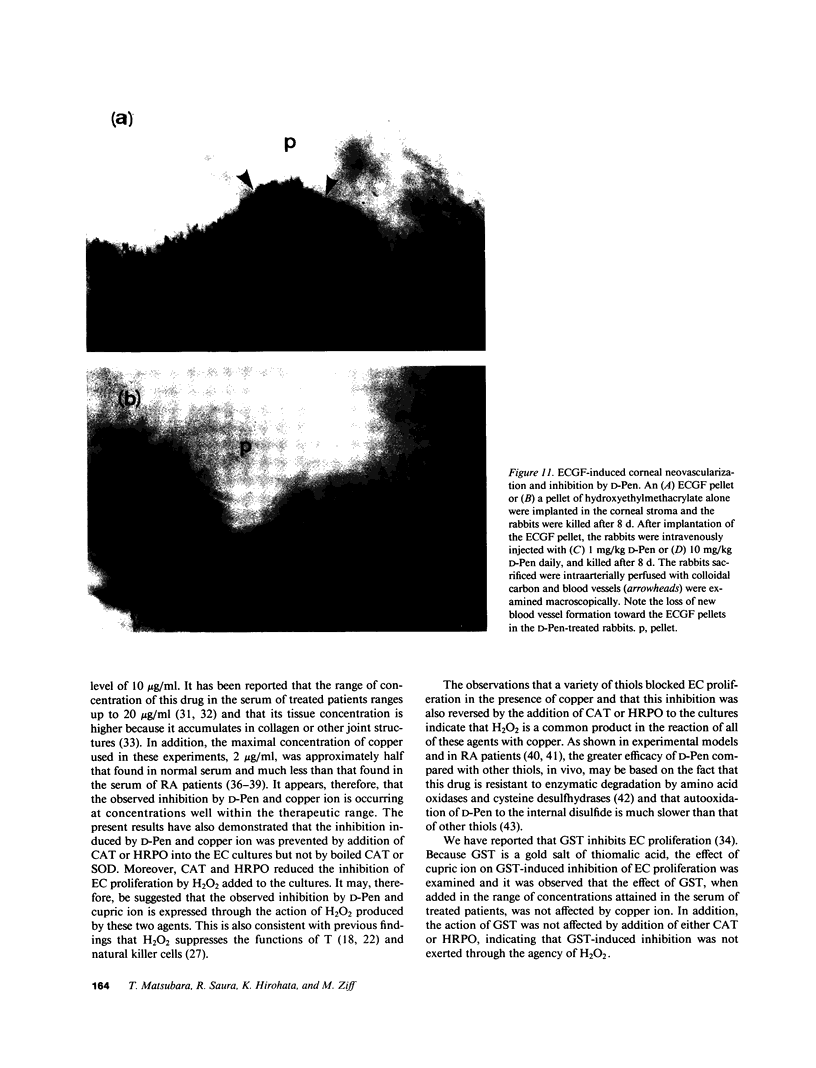
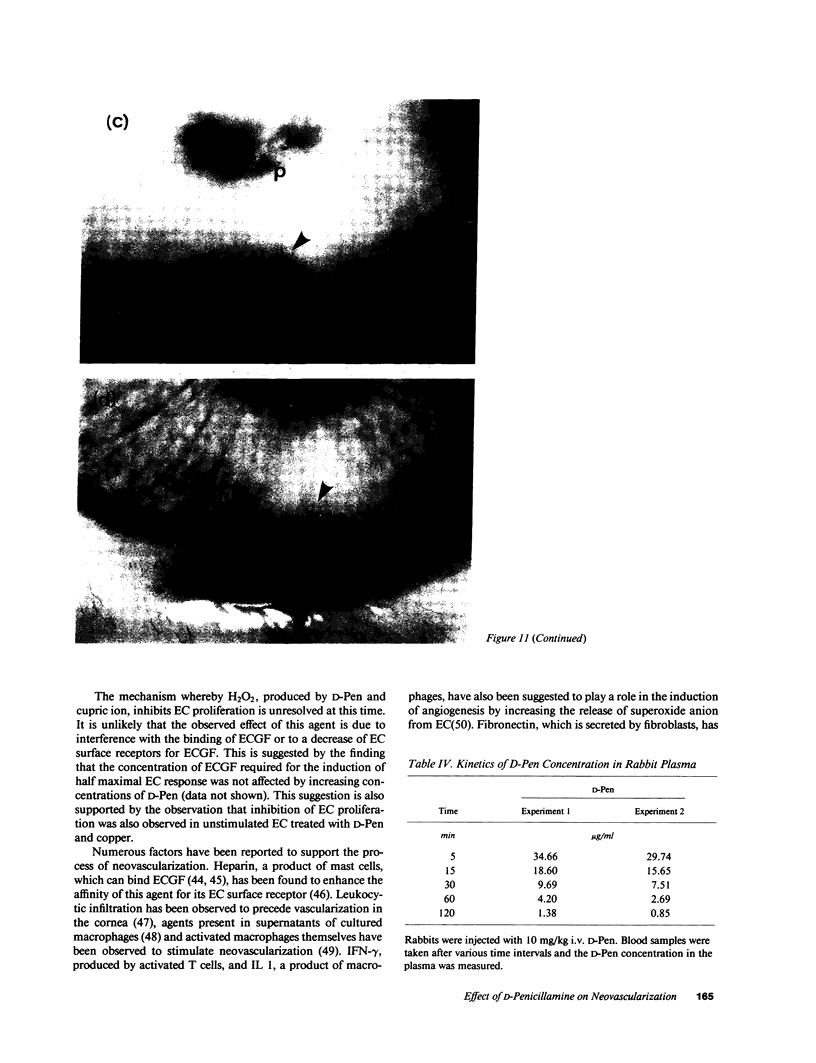
To investigate the effects of D-penicillamine (D-Pen) on angiogenesis, we have studied the effects of this drug on in vitro proliferation of human endothelial cells (EC) and in vivo corneal neovascularization. D-Pen, in the presence of copper sulfate, suppressed tritiated thymidine ([3H]TdR) incorporation into EC in a dose-dependent manner. Significant inhibition was observed with D-Pen concentrations attainable in the serum and tissues of treated patients. Neither D-Pen nor copper ion alone significantly affected [3H]TdR incorporation into EC. The inhibition by D-Pen and copper was blocked by catalase (CAT) or horseradish peroxidase but not by boiled CAT or SOD. When rabbits were daily injected intravenously with D-Pen at the per kilogram dosage administered to rheumatoid patients, neovascularization as quantitated by the proliferation of corneal new blood vessels was significantly inhibited. These results suggest that hydrogen peroxide generated by D-Pen and copper exerts a pronounced antiangiogenic effect through inhibition of EC proliferation. It is, therefore, considered that D-Pen may suppress rheumatoid synovitis by reducing the number of small blood vessels available for the emigration of chronic inflammatory cells, and the proliferation of the synovial tissue.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V., BRADHAM L. S. Metabolism in vitro of the sulphydryl amino acids, L- and D-penicillamine. Biochem Pharmacol. 1959 Dec;3:38–41. doi: 10.1016/0006-2952(59)90006-1. [DOI] [PubMed] [Google Scholar]
- Aaseth J., Munthe E., Førre O., Steinnes E. Trace elements in serum and urine of patients with rheumatoid arthritis. Scand J Rheumatol. 1978;7(4):237–240. doi: 10.3109/03009747809095662. [DOI] [PubMed] [Google Scholar]
- Ashida E. R., Johnson A. R., Lipsky P. E. Human endothelial cell-lymphocyte interaction. Endothelial cells function as accessory cells necessary for mitogen-induced human T lymphocyte activation in vitro. J Clin Invest. 1981 May;67(5):1490–1499. doi: 10.1172/JCI110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry H., Liyanage S. P., Durance R. A., Barnes C. G., Berger L. A., Evans S. Azathioprine and penicillamine in treatment of rheumatoid arthritis: a controlled trial. Br Med J. 1976 May 1;1(6017):1052–1054. doi: 10.1136/bmj.1.6017.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowersox J. C., Sorgente N. Chemotaxis of aortic endothelial cells in response to fibronectin. Cancer Res. 1982 Jul;42(7):2547–2551. [PubMed] [Google Scholar]
- Burger D. R., Ford D., Vetto R. M., Hamblin A., Goldstein A., Hubbard M., Dumonde D. C. Endothelial cell presentation of antigen to human T cells. Hum Immunol. 1981 Nov;3(3):209–230. doi: 10.1016/0198-8859(81)90019-7. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chwalinska-Sadowska H., Baum J. The effect of D-penicillamine on polymorphonuclear leukocyte function. J Clin Invest. 1976 Oct;58(4):871–879. doi: 10.1172/JCI108540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon A. J., Davies J., Dormandy T. L., Hamilton E. B., Holt P. J., Mason R. M., Thompson M., Weber J. C., Zutshi D. W. Synthetic D(-)penicillamine in rheumatoid arthritis. Double-blind controlled study of a high and low dosage regimen. Ann Rheum Dis. 1975 Oct;34(5):416–421. doi: 10.1136/ard.34.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Fromer C. H., Klintworth G. K. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. Am J Pathol. 1975 Jun;79(3):537–554. [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Leapman S. B., Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst. 1974 Feb;52(2):413–427. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- Hirschberg H., Bergh O. J., Thorsby E. Antigen-presenting properties of human vascular endothelial cells. J Exp Med. 1980 Aug 1;152(2 Pt 2):249s–255s. [PubMed] [Google Scholar]
- JAFFE I. A. Intra-articular dissociation of the rheumatoid factor. J Lab Clin Med. 1962 Sep;60:409–421. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe I. A., Merryman P. Effect of increased serum sulphydryl content on titre of rheumatoid factor. Ann Rheum Dis. 1968 Jan;27(1):14–18. doi: 10.1136/ard.27.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamer B., Kimura E. T., Makstenieks M. Effects of oral cysteine, penicillamine and N-acetyl-penicillamine on adjuvant arthritis in rats. Pharmacology. 1968 May;1(5):283–288. doi: 10.1159/000135976. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of lymphoid cells in the rheumatoid synovial membrane. Arthritis Rheum. 1973 Jul-Aug;16(4):471–486. doi: 10.1002/art.1780160407. [DOI] [PubMed] [Google Scholar]
- Kukovetz W. R., Beubler E., Kreuzig F., Moritz A. J., Nirnberger G., Werner-Breitenecker L. Bioavailability and pharmacokinetics of D-penicillamine. J Rheumatol. 1983 Feb;10(1):90–94. [PubMed] [Google Scholar]
- Lengfelder E., Elstner E. F. Determination of the superoxide dismutating activity of D-penicillamine copper. Hoppe Seylers Z Physiol Chem. 1978 Jun;359(6):751–757. doi: 10.1515/bchm.1978.359.1.751. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E. Immunosuppression by D-penicillamine in vitro. Inhibition of human T lymphocyte proliferation by copper- or ceruloplasmin-dependent generation of hydrogen peroxide and protection by monocytes. J Clin Invest. 1984 Jan;73(1):53–65. doi: 10.1172/JCI111207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Ziff M. Inhibition of human helper T cell function in vitro by D-penicillamine and CuSO4. J Clin Invest. 1980 May;65(5):1069–1076. doi: 10.1172/JCI109759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T., Mehlman T., Friesel R., Schreiber A. B. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984 Aug 31;225(4665):932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Unanue E. R., Cotran R. S. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981 Apr;126(4):1510–1515. [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol. 1986 Nov 15;137(10):3295–3298. [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Inhibition of human endothelial cell proliferation by gold compounds. J Clin Invest. 1987 May;79(5):1440–1446. doi: 10.1172/JCI112972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mery C., Delrieu F., Ghozlan R., Saporta L., Simon F., Amor B., Menkes C. J., Delbarre F. Controlled trial of D-penicillamine in rheumatoid arthritis. Dose effect and the role of zinc. Scand J Rheumatol. 1976;5(4):241–247. doi: 10.3109/03009747609099913. [DOI] [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- Muijsers A. O., Van de Stadt R. J., Henrichs A. M., Van der Korst J. K. Determination of D-penicillamine in serum and urine of patients with rheumatoid arthritis. Clin Chim Acta. 1979 Jun 1;94(2):173–180. doi: 10.1016/0009-8981(79)90010-x. [DOI] [PubMed] [Google Scholar]
- Nunez G., Ugolini V., Capra J. D., Stastny P. Monoclonal antibodies against human monocytes. II. Recognition of two distinct cell surface molecules. Scand J Immunol. 1982 Dec;16(6):515–523. doi: 10.1111/j.1365-3083.1982.tb00753.x. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- RITZMANN S. E., COLEMAN S. L., LEVIN W. C. The effect of some mercaptanes upon a macrocryogelglobulin; modifications induced by cysteamine, penicillamine and penicillin. J Clin Invest. 1960 Aug;39:1320–1329. doi: 10.1172/JCI104149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Schreiber A. B., Kenney J., Kowalski W. J., Friesel R., Mehlman T., Maciag T. Interaction of endothelial cell growth factor with heparin: characterization by receptor and antibody recognition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6138–6142. doi: 10.1073/pnas.82.18.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder P. R., Al-Timimi D., McMurray W., White A. G., Zoob B. C., Dormandy T. L. Serum copper and related variables in rheumatoid arthritis. Ann Rheum Dis. 1978 Feb;37(1):67–70. doi: 10.1136/ard.37.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder P. R., McMurray W., White A. G., Dormandy T. L. Synovial fluid copper and related variables in rheumatoid and degenerative arthritis. Ann Rheum Dis. 1978 Feb;37(1):71–72. doi: 10.1136/ard.37.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman W. E., Gindhart T. D., Blackman M. A., Dalal B., Talal N., Werb Z. Suppression of natural killing in vitro by monocytes and polymorphonuclear leukocytes: requirement for reactive metabolites of oxygen. J Clin Invest. 1982 Apr;69(4):876–888. doi: 10.1172/JCI110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiokawa Y., Horiuchi Y., Honma M., Kageyama T., Okada T., Azuma T. Clinical evaluation of D-penicillamine by multicentric double-blind comparative study in chronic rheumatoid arthritis. Arthritis Rheum. 1977 Nov-Dec;20(8):1464–1472. doi: 10.1002/art.1780200804. [DOI] [PubMed] [Google Scholar]
- Siegel R. C. Collagen cross-linking. Effect of D-penicillamine on cross-linking in vitro. J Biol Chem. 1977 Jan 10;252(1):254–259. [PubMed] [Google Scholar]
- Sorenson J. R. Copper chelates as possible active forms of the antiarthritic agents. J Med Chem. 1976 Jan;19(1):135–148. doi: 10.1021/jm00223a024. [DOI] [PubMed] [Google Scholar]
- Starkebaum G., Root R. K. D-Penicillamine: analysis of the mechanism of copper-catalyzed hydrogen peroxide generation. J Immunol. 1985 May;134(5):3371–3378. [PubMed] [Google Scholar]
- Stern D. M., Bank I., Nawroth P. P., Cassimeris J., Kisiel W., Fenton J. W., 2nd, Dinarello C., Chess L., Jaffe E. A. Self-regulation of procoagulant events on the endothelial cell surface. J Exp Med. 1985 Oct 1;162(4):1223–1235. doi: 10.1084/jem.162.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. G., Scudder P., Dormandy T. L., Martin V. M. Copper--an index of erosive activity? Rheumatol Rehabil. 1978 Feb;17(1):3–5. doi: 10.1093/rheumatology/17.1.3. [DOI] [PubMed] [Google Scholar]
- Younes M., Weser U. Superoxide dismutase activity of copper-penicillamine: possible involvement of Cu(I) stabilized sulphur radical. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1247–1253. doi: 10.1016/0006-291x(77)91427-9. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]
- van de Stadt R. J., Muijsers A. O., Henrichs A. M., van der Korst J. K. D-penicillamine: biochemical, metabolic and pharmacological aspects. Scand J Rheumatol Suppl. 1979;(28):13–20. doi: 10.3109/03009747909108229. [DOI] [PubMed] [Google Scholar]








