Abstract
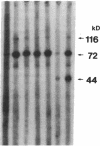
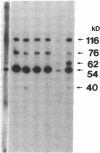
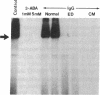
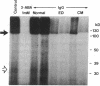
The factors responsible for the production of autoantibodies against self-components are not well understood. We have identified monospecific human autoantibodies to poly(ADP-ribose) polymerase (ADPRP) in the sera of rheumatic patients. Since this nuclear enzyme has been extensively characterized, and its entire structure is known, we could investigate in detail the epitope specificity of the human autoantibodies, and their effects on the biological functions of the enzyme. All sera with autoantibodies to ADPRP recognized the NAD-binding domain of the enzyme, as demonstrated by either immunoblotting or immunoprecipitation of partially proteolyzed ADPRP. The autoantibodies also inhibited the catalytic activity of the purified enzyme, as measured by the transfer of ADP-ribose from [32P]NAD to either histones or to ADPRP itself. Because comparative structural analyses have shown that the active sites of enzymes are often conserved during evolution, we tested the ability of the autoantibodies to react with ADPRP from lower eukaryotes. The human autoantibodies reacted with ADPRP in cellular extracts from mammalian, avian, amphibian, arthropod, and protozoan cells, and also inhibited the catalytic activity of the various enzymes. Collectively, these experiments indicate that the human autoantibodies to ADPRP recognize a distinct group of evolutionarily conserved antigenic determinants that are closely related to the catalytic site of the enzyme. The results are consistent with the hypothesis that the epitope selectivity of human autoantibodies to ADPRP is influenced by cross-reactive antigens in the external environment.
Full text
PDF

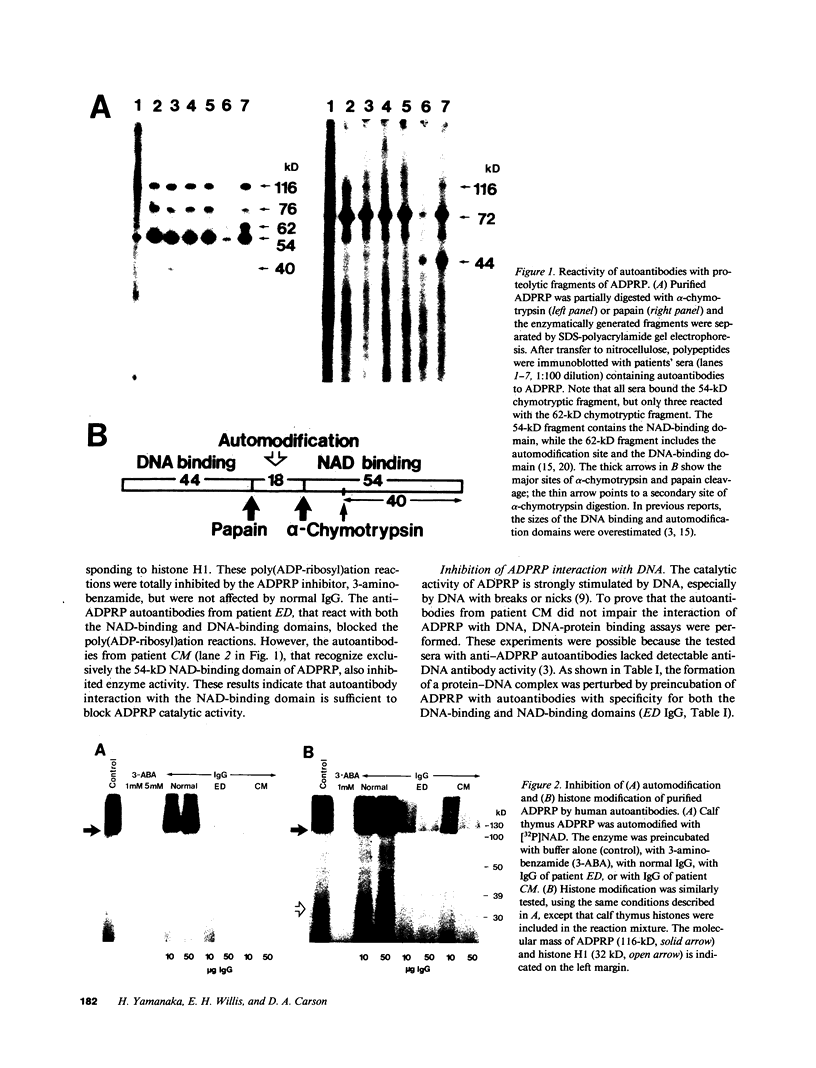




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benavente R., Rose K. M., Reimer G., Hügle-Dörr B., Scheer U. Inhibition of nucleolar reformation after microinjection of antibodies to RNA polymerase I into mitotic cells. J Cell Biol. 1987 Oct;105(4):1483–1491. doi: 10.1083/jcb.105.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin R. C., Gill D. M. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980 Nov 10;255(21):10502–10508. [PubMed] [Google Scholar]
- Berger N. A., Sikorski G. W., Petzold S. J., Kurohara K. K. Association of poly(adenosine diphosphoribose) synthesis with DNA damage and repair in normal human lymphocytes. J Clin Invest. 1979 Jun;63(6):1164–1171. doi: 10.1172/JCI109410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. C., Mathews M. B. Autoreactive epitope defined as the anticodon region of alanine transfer RNA. Science. 1987 Nov 20;238(4830):1116–1119. doi: 10.1126/science.2446387. [DOI] [PubMed] [Google Scholar]
- Chan E. K., Tan E. M. Human autoantibody-reactive epitopes of SS-B/La are highly conserved in comparison with epitopes recognized by murine monoclonal antibodies. J Exp Med. 1987 Dec 1;166(6):1627–1640. doi: 10.1084/jem.166.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney B. W., McBride O. W., Chen D. F., Alkhatib H., Bhatia K., Hensley P., Smulson M. E. cDNA sequence, protein structure, and chromosomal location of the human gene for poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8370–8374. doi: 10.1073/pnas.84.23.8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C. Anionic regions in nuclear proteins. J Cell Biol. 1987 Oct;105(4):1479–1482. doi: 10.1083/jcb.105.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W. Genes-in-pieces revisited. Science. 1985 May 17;228(4701):823–824. doi: 10.1126/science.4001923. [DOI] [PubMed] [Google Scholar]
- Hardin J. A. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986 Apr;29(4):457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- Harth G., Haidaris C. G., So M. Neuraminidase from Trypanosoma cruzi: analysis of enhanced expression of the enzyme in infectious forms. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8320–8324. doi: 10.1073/pnas.84.23.8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone A. P., Williams G. T. Role of DNA breaks and ADP-ribosyl transferase activity in eukaryotic differentiation demonstrated in human lymphocytes. Nature. 1982 Nov 25;300(5890):368–370. doi: 10.1038/300368a0. [DOI] [PubMed] [Google Scholar]
- Kameshita I., Matsuda Z., Taniguchi T., Shizuta Y. Poly (ADP-Ribose) synthetase. Separation and identification of three proteolytic fragments as the substrate-binding domain, the DNA-binding domain, and the automodification domain. J Biol Chem. 1984 Apr 25;259(8):4770–4776. [PubMed] [Google Scholar]
- Kurosaki T., Ushiro H., Mitsuuchi Y., Suzuki S., Matsuda M., Matsuda Y., Katunuma N., Kangawa K., Matsuo H., Hirose T. Primary structure of human poly(ADP-ribose) synthetase as deduced from cDNA sequence. J Biol Chem. 1987 Nov 25;262(33):15990–15997. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Blake C. C., Evans S. T., Orkin S. H. Structure of the human phosphoglycerate kinase gene and the intron-mediated evolution and dispersal of the nucleotide-binding domain. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6965–6969. doi: 10.1073/pnas.82.20.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Hardin J. A. Mechanism of interaction between Ku protein and DNA. J Biol Chem. 1986 Aug 5;261(22):10375–10379. [PubMed] [Google Scholar]
- Okolie E. E., Onyezili N. I. ADP-ribosyltransferase in Plasmodium (malaria parasites). Biochem J. 1983 Mar 1;209(3):687–693. doi: 10.1042/bj2090687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekala P. H., Moss J. Poly ADP-ribosylation of protein. Curr Top Cell Regul. 1983;22:1–49. doi: 10.1016/b978-0-12-152822-5.50005-1. [DOI] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M., Hillyard D., Olivera B. M. Turnover at nicotinamide adenine dinucleotide in cultures of human cells. J Cell Physiol. 1976 Jun;88(2):207–217. doi: 10.1002/jcp.1040880210. [DOI] [PubMed] [Google Scholar]
- Scovassi A. I., Izzo R., Franchi E., Bertazzoni U. Structural analysis of poly(ADP-ribose)polymerase in higher and lower eukaryotes. Eur J Biochem. 1986 Aug 15;159(1):77–84. doi: 10.1111/j.1432-1033.1986.tb09835.x. [DOI] [PubMed] [Google Scholar]
- Shero J. H., Bordwell B., Rothfield N. F., Earnshaw W. C. High titers of autoantibodies to topoisomerase I (Scl-70) in sera from scleroderma patients. Science. 1986 Feb 14;231(4739):737–740. doi: 10.1126/science.3003910. [DOI] [PubMed] [Google Scholar]
- Stanford D. R., Rohleder A., Neiswanger K., Wieben E. D. DNA sequence of a human Sm autoimmune antigen. The multigene family contains a processed pseudogene. J Biol Chem. 1987 Jul 25;262(21):9931–9934. [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Reimer G., Sullivan K. Intracellular autoantigens: diagnostic fingerprints but aetiological dilemmas. Ciba Found Symp. 1987;129:25–42. doi: 10.1002/9780470513484.ch3. [DOI] [PubMed] [Google Scholar]
- Uchida K., Morita T., Sato T., Ogura T., Yamashita R., Noguchi S., Suzuki H., Nyunoya H., Miwa M., Sugimura T. Nucleotide sequence of a full-length cDNA for human fibroblast poly(ADP-ribose) polymerase. Biochem Biophys Res Commun. 1987 Oct 29;148(2):617–622. doi: 10.1016/0006-291x(87)90921-1. [DOI] [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Yokoyama Y., Shizuta Y. Purification and characterization of poly (ADP-ribose) synthetase from human placenta. J Biol Chem. 1987 Feb 15;262(5):2352–2357. [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H., Penning C. A., Willis E. H., Wasson D. B., Carson D. A. Characterization of human poly(ADP-ribose) polymerase with autoantibodies. J Biol Chem. 1988 Mar 15;263(8):3879–3883. [PubMed] [Google Scholar]
- Yamanaka H., Willis E. H., Penning C. A., Peebles C. L., Tan E. M., Carson D. A. Human autoantibodies to poly(adenosine diphosphate-ribose) polymerase. J Clin Invest. 1987 Sep;80(3):900–904. doi: 10.1172/JCI113150. [DOI] [PMC free article] [PubMed] [Google Scholar]