Abstract
The evolution of the avian wing has long fascinated biologists, yet almost no work includes the length of primary feathers in consideration of overall wing length variation. Here we show that the length of the longest primary feather ( ) contributing to overall wing length scales with negative allometry against total arm (ta = humerus+ulna+manus). The scaling exponent varied slightly, although not significantly so, depending on whether a species level analysis was used or phylogeny was controlled for using independent contrasts:
) contributing to overall wing length scales with negative allometry against total arm (ta = humerus+ulna+manus). The scaling exponent varied slightly, although not significantly so, depending on whether a species level analysis was used or phylogeny was controlled for using independent contrasts: 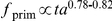 . The scaling exponent was not significantly different from that predicted (0.86) by earlier work. It appears that there is a general trend for the primary feathers of birds to contribute proportionally less, and ta proportionally more, to overall wingspan as this dimension increases. Wingspan in birds is constrained close to mass (M
1/3) because of optimisation for lift production, which limits opportunities for exterior morphological change. Within the wing, variations in underlying bone and feather lengths nevertheless may, in altering the joint positions, permit a range of different flight styles by facilitating variation in upstroke kinematics.
. The scaling exponent was not significantly different from that predicted (0.86) by earlier work. It appears that there is a general trend for the primary feathers of birds to contribute proportionally less, and ta proportionally more, to overall wingspan as this dimension increases. Wingspan in birds is constrained close to mass (M
1/3) because of optimisation for lift production, which limits opportunities for exterior morphological change. Within the wing, variations in underlying bone and feather lengths nevertheless may, in altering the joint positions, permit a range of different flight styles by facilitating variation in upstroke kinematics.
Introduction
The total length of the avian wing derives from the underlying wing bones (humerus, radius/ulna and manus) and the functional primary feathers (Fig. 1). Although scaling exponents vary slightly depending upon whether the effects of common ancestry are controlled for using independent contrasts or not (M 0.35 and M 0.39 respectively, table 1 in [1]), it is well established that wingspan (b) in birds scales with slightly positive allometry with respect to body mass (M >1/3) [1]–[4]. This positive allometry, however, appears related to size dependent variation in flight behaviour [1]. Specifically, the line of best fit is depressed at lower body masses and elevated at high body masses, because slow speed flapping flight styles seen in smaller birds are associated with short-wings, while the soaring flight styles of larger birds favour longer wings [4]. Surprisingly, and in spite of variations in flight behavior, the relative contribution of the primary feathers to overall wing length has received little attention from ornithologists.
Figure 1. Diagram showing the measurements taken from the museum specimens and used in the analyses (adapted from figure 1 in [1]).
Table 1. The primary feather and total arm data set.
| Number in figure 2a | Species | n | Total-arm (m) | Primary feather (m) |
| 1 | Aegolius acadicus | 1 | 0.131 | 0.108 |
| 2 | Anas americana | 2 | 0.223 | 0.200 |
| 3 | Anas crecca | 1 | 0.150 | 0.165 |
| 4 | Anas platyrhynchos | 1 | 0.245 | 0.233 |
| 5 | Anthus spinoletta | 2 | 0.066 | 0.066 |
| 6 | Ardea herodias | 1 | 0.611 | 0.378 |
| 7 | Aythya marila | 1 | 0.225 | 0.159 |
| 8 | Bombycilla garrulus | 2 | 0.081 | 0.086 |
| 9 | Bubulcus ibis | 4 | 0.252 | 0.182 |
| 10 | Butorides striatus | 1 | 0.205 | 0.160 |
| 11 | Cathartes aura | 1 | 0.465 | 0.428 |
| 12 | Ceryle alcyon | 10 | 0.147 | 0.139 |
| 13 | Dendragapus obscurus | 3 | 0.190 | 0.184 |
| 14 | Dendroica coronata | 2 | 0.053 | 0.054 |
| 15 | Dendroica magnolia | 1 | 0.045 | 0.044 |
| 16 | Egretta thula | 1 | 0.245 | 0.187 |
| 17 | Eremophila alpestris | 2 | 0.079 | 0.084 |
| 18 | Gavia adamsii | 1 | 0.518 | 0.252 |
| 19 | Gavia immer | 2 | 0.510 | 0.217 |
| 20 | Glaucidium gnoma | 1 | 0.091 | 0.070 |
| 21 | Mniotilta varia | 2 | 0.051 | 0.058 |
| 22 | Oceanodroma furcata | 4 | 0.108 | 0.120 |
| 23 | Parus hudsonicus | 2 | 0.045 | 0.054 |
| 24 | Passerculus sandwichensis | 2 | 0.064 | 0.061 |
| 25 | Phalacrocorax pelagicus | 1 | 0.308 | 0.207 |
| 26 | Pheuctitis ludovicianus | 2 | 0.077 | 0.078 |
| 27 | Pipilio erythrophthalmus | 2 | 0.066 | 0.069 |
| 28 | Piranga ludovicianus | 2 | 0.068 | 0.072 |
| 29 | Puffinus griseus | 14 | 0.298 | 0.194 |
| 30 | Puffinus tenurostris | 1 | 0.248 | 0.189 |
| 31 | Seiurus aurocapillus | 2 | 0.058 | 0.058 |
| 32 | Setophaga ruticilla | 2 | 0.045 | 0.050 |
| 33 | Sitta canadensis | 2 | 0.050 | 0.049 |
| 34 | Sphyrapicus ruber | 3 | 0.091 | 0.107 |
Curiously, total-arm (ta = humerus+ulna+manus) length does not scale with unity against wing semi-span b
semi and instead appears to scale with positive allometry ( ), indicating that larger birds have longer wings relative to their M, but also have longer ta relative to their b
[1]. An explanation forwarded to explain this disproportionate increase in ta with b
[1] was that primary feather length (
), indicating that larger birds have longer wings relative to their M, but also have longer ta relative to their b
[1]. An explanation forwarded to explain this disproportionate increase in ta with b
[1] was that primary feather length ( ) is relatively shorter in longer winged birds (i.e.,
) is relatively shorter in longer winged birds (i.e.,  ). The only data available, however, suggested
). The only data available, however, suggested  scaled as M
0.32, which was not significantly different from the exponent predicted for isometry (M
1/3) [5]. Worcester's [5] study was, however, limited in taxa (n = 13) and, because the relationship between b and ta was not determined, whether increasing M in the sample correlated with a relatively longer ta was not known. Therefore, a trend towards shorter primaries in birds with longer ta remains a possibility [1] and required further investigation. Nudds [1] also acknowledged that if elbow angle varied with b it would influence how close to parallel the leading edge of the humerus and ulna was and hence the relationship between ta and b (Fig. 1). Elbow angle is extremely difficult to measure in live birds, however, because bones are not visible from the wing-surface. Plucking of feathers is unethical and undesirable, and x-ray not necessarily practical, but if negative allometry was found between
scaled as M
0.32, which was not significantly different from the exponent predicted for isometry (M
1/3) [5]. Worcester's [5] study was, however, limited in taxa (n = 13) and, because the relationship between b and ta was not determined, whether increasing M in the sample correlated with a relatively longer ta was not known. Therefore, a trend towards shorter primaries in birds with longer ta remains a possibility [1] and required further investigation. Nudds [1] also acknowledged that if elbow angle varied with b it would influence how close to parallel the leading edge of the humerus and ulna was and hence the relationship between ta and b (Fig. 1). Elbow angle is extremely difficult to measure in live birds, however, because bones are not visible from the wing-surface. Plucking of feathers is unethical and undesirable, and x-ray not necessarily practical, but if negative allometry was found between  and ta then the effect of elbow angle could be ruled out.
and ta then the effect of elbow angle could be ruled out.
‘Stretched’ or ‘flat’ wing preservations are rare in museum collections and those including the humerus intact within the skin are even more so (personal observations). However, a small collection of suitably stretched wing specimens was located in the Royal British Columbia Museum, Victoria, BC, Canada (RBCM). Even these had the humerus removed from the wing, but fortunately kept separately to permit all wing-bone measurements to be recorded from a homogenous specimen. These skins allowed us to test the hypothesis that  scales with negative allometry against ta (i.e.,
scales with negative allometry against ta (i.e., 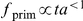 ) as proposed by Nudds [1]. More specifically it was predicted that, over the range of wing semi-spans (b
semi = 0.075 to 1.622 m) used in Nudds [1], the predicted scaling exponent between
) as proposed by Nudds [1]. More specifically it was predicted that, over the range of wing semi-spans (b
semi = 0.075 to 1.622 m) used in Nudds [1], the predicted scaling exponent between 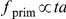 should approximate to 0.86, because
should approximate to 0.86, because  and,
and,  , so
, so 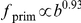 and therefore
and therefore  . A predicted exponent of 0.86 assumes that size dependent variation in
. A predicted exponent of 0.86 assumes that size dependent variation in 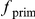 is entirely responsible for the positive allometry seen in ta (i.e., elbow angle is constant across all wingspans).
is entirely responsible for the positive allometry seen in ta (i.e., elbow angle is constant across all wingspans).
Methods
Humerus, ulna and manus lengths were measured using Vernier calipers to the nearest mm from the ‘spread wing’ bird skin collection at the RBCM. ‘Total-arm’ is the sum of humerus, ulna and manus length [3], [6]. Primary feather length (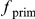 ) was measured from the distal end of digit 2 of the manus to the feather tip, parallel to the feather shaft (Fig. 1). The primary feather chosen was that contributing the most distal point of the wing representing maximum b.
) was measured from the distal end of digit 2 of the manus to the feather tip, parallel to the feather shaft (Fig. 1). The primary feather chosen was that contributing the most distal point of the wing representing maximum b.
Because the data set comprises interspecific measures (Table 1), the effects of common ancestry must be considered to prevent spurious correlations resulting from common descent rather than from independent evolution. Here a comparative analysis using standardized independent contrasts, conducted in CAIC version 2.6.9 [7], was used. The analyses were implemented in three ways. Initially the scaling relationships were calculated using species as independent data points. The analysis was then repeated using CAIC and the phylogenetic hypotheses of Sibley and Ahlquist [8], and finally CAIC was implemented using the phylogenetic hypotheses of Livezey and Zusi [9]. A punctuated model of evolution was used in both cases: the branch length estimates of Sibley and Ahlquist [8] are disputed and none are available for the phylogeny of Livezey and Zusi [9]. The topological disagreement between these two hypotheses [8], [9] is useful, because if phylogeny is going to affect the results, then using two different phylogenies is likely to have a greater effect than changes to branch lengths within a single phylogeny. Use of two different phylogenies should therefore indicate whether the scaling relationships determined are likely to be affected by future refinements of phylogenetic topology.
The relationship between 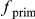 and ta was investigated using the empirical scaling formula
and ta was investigated using the empirical scaling formula  where α is the allometric exponent (slope) and k is the allometric coefficient (intercept), which was in turn determined using a Model II reduced major axis (RMA) regression [10]–[12]. Regression analyses using independent contrasts were performed through the origin [7]. The RMA slope was calculated as the ordinary least squares (OLS) Model I slope (regression coefficient) divided by the OLS correlation coefficient, and 95% confidence limits were calculated following Sokal and Rohlf [13]. The standard error (s.e.) of the RMA slope was taken as equal to that of the s.e. of the OLS slope. Two-tailed t-tests were used to test for differences between calculated slopes and the slopes predicted for geometric similarity (α = 1) or predicted from Nudds [1] (α = 0.86).
where α is the allometric exponent (slope) and k is the allometric coefficient (intercept), which was in turn determined using a Model II reduced major axis (RMA) regression [10]–[12]. Regression analyses using independent contrasts were performed through the origin [7]. The RMA slope was calculated as the ordinary least squares (OLS) Model I slope (regression coefficient) divided by the OLS correlation coefficient, and 95% confidence limits were calculated following Sokal and Rohlf [13]. The standard error (s.e.) of the RMA slope was taken as equal to that of the s.e. of the OLS slope. Two-tailed t-tests were used to test for differences between calculated slopes and the slopes predicted for geometric similarity (α = 1) or predicted from Nudds [1] (α = 0.86).
Results
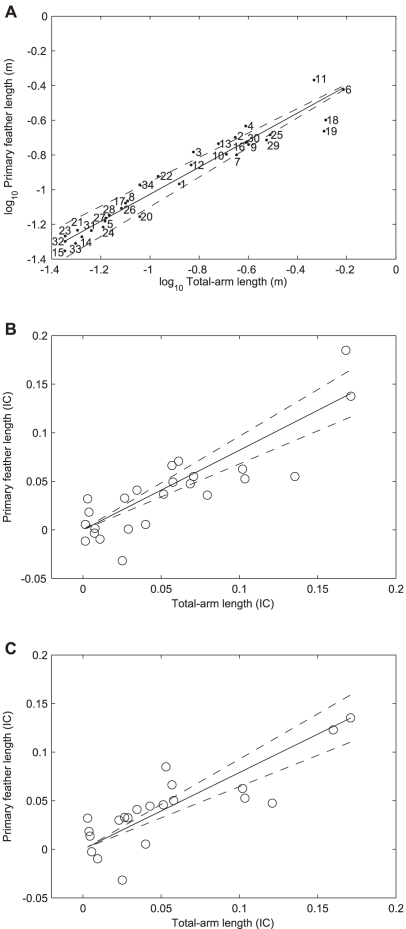
In all three analyses the relationship between  and ta was statistically significant, with the scaling exponent dependent upon the analysis used (Fig. 2). The scaling exponent determined using species as independent data points was significantly below (t = −6.50, p<0.001) that predicted for geometric similarity (α = 1). Similarly, both CAIC using the phylogeny of Livezey and Zusi [9] and the phylogeny of Sibley and Ahlquist [8] produced slopes significantly below 1 (t = −2.72, p<0.05 and t = −3.12, p<0.05 respectively). In all three cases the scaling exponents were below, yet not significantly different from, that predicted (i.e.,
and ta was statistically significant, with the scaling exponent dependent upon the analysis used (Fig. 2). The scaling exponent determined using species as independent data points was significantly below (t = −6.50, p<0.001) that predicted for geometric similarity (α = 1). Similarly, both CAIC using the phylogeny of Livezey and Zusi [9] and the phylogeny of Sibley and Ahlquist [8] produced slopes significantly below 1 (t = −2.72, p<0.05 and t = −3.12, p<0.05 respectively). In all three cases the scaling exponents were below, yet not significantly different from, that predicted (i.e., 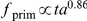 ) by Nudds [1] as demonstrated by the 95% confidence intervals (Fig. 2).
) by Nudds [1] as demonstrated by the 95% confidence intervals (Fig. 2).
Figure 2. Scatter plots of log10 primary feather length (m) against log10 total-arm length (sum of humerus, ulna and manus length in m).
The regression lines (dashed lines are 95% C.I.s) describing the relationship were A) species treated as independent data points: y = 0.57x 0.78 (0.71–0.85), t = 23.35, n = 34, r 2 = 0.94, p<0.001, B) phylogenetic independent contrasts (IC) using the phylogeny of Livezey and Zusi [9]: y = x 0.82 (0.68–0.96), t = 12.26, n = 24, r 2 = 0.84, p<0.001 and C) phylogenetic independent contrasts (IC) using the phylogeny of Sibley and Ahlquist [8]: y = x 0.79 (0.64–0.93), t = 11.46, n = 21, r 2 = 0.84, p<0.001. See table 1 for the species corresponding to the numbers in panel A.
Discussion
As predicted by Nudds [1] and contrary to that suggested by the data of Worcester [5], there is a general trend for the primary feathers of birds to contribute proportionally less to overall wing-length as b
semi increases. The sample size here was relatively small (n = 34) compared to the sample sizes (n = 306) used to investigate the scaling of ta
[1], which precludes any analysis of flight style or ecologically driven variation in 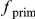 /ta ratio. The wingspan of birds is constrained close to M
1/3, because of optimisation for lift, limiting the opportunities for exterior morphological change. Within the wing, however, variations in underlying bone ratios may permit a range of different flight styles, by possibly facilitating variation in upstroke kinematics [6]. It is not unreasonable to expect the relationship between
/ta ratio. The wingspan of birds is constrained close to M
1/3, because of optimisation for lift, limiting the opportunities for exterior morphological change. Within the wing, however, variations in underlying bone ratios may permit a range of different flight styles, by possibly facilitating variation in upstroke kinematics [6]. It is not unreasonable to expect the relationship between  and ta to also vary depending upon the ecology or flight style of the bird.
and ta to also vary depending upon the ecology or flight style of the bird.
The scaling relationship determined here between 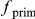 and ta does not entirely exclude the possibility of size dependent variation in elbow angle. Although there were no statistical differences between the calculated scaling exponents (Fig. 2) and the 0.86 predicted [1], they were lower (0.78–0.82) and the 95% confidence intervals broad. Of course, the angle at the elbow in a stretched out wing when a bird is having its wingspan measured [14] is not necessarily functional. Instead, it could just be an artefact of how the bird is held by the researcher. Indeed, the elbow angle is likely varied in flight and during a wing-stroke [15], [16]. This, of course, begs the question of exactly what we measure when we measure b in a bird: it may be maximum extended wingspan, but is this used during flight? In hummingbirds, span in flight is effectively the width of the body plus the distances from the wrists to wing tips [17], but in other birds the portions of the wings between the wrists and the body need to be considered [18]. Measurements of functional wingspan from birds in flight are long overdue.
and ta does not entirely exclude the possibility of size dependent variation in elbow angle. Although there were no statistical differences between the calculated scaling exponents (Fig. 2) and the 0.86 predicted [1], they were lower (0.78–0.82) and the 95% confidence intervals broad. Of course, the angle at the elbow in a stretched out wing when a bird is having its wingspan measured [14] is not necessarily functional. Instead, it could just be an artefact of how the bird is held by the researcher. Indeed, the elbow angle is likely varied in flight and during a wing-stroke [15], [16]. This, of course, begs the question of exactly what we measure when we measure b in a bird: it may be maximum extended wingspan, but is this used during flight? In hummingbirds, span in flight is effectively the width of the body plus the distances from the wrists to wing tips [17], but in other birds the portions of the wings between the wrists and the body need to be considered [18]. Measurements of functional wingspan from birds in flight are long overdue.
In conclusion, there is a general trend for  to contribute relatively less to overall wingspan in larger birds. Conversely, ta contributes more to the overall length with increasing b. Why this trend exists is not immediately obvious. Although tentative at this stage, the scaling of ta and
to contribute relatively less to overall wingspan in larger birds. Conversely, ta contributes more to the overall length with increasing b. Why this trend exists is not immediately obvious. Although tentative at this stage, the scaling of ta and  may be the product of an as yet unidentified optimum ratio for feathers to wing-skeleton length within the biomechanical and aerodynamic constraints acting upon the scaling of b (M
1/3) [1]. Similarly, whether the scaling is driven by aerodynamics, feather biomechanical properties or a combination of both requires further investigation.
may be the product of an as yet unidentified optimum ratio for feathers to wing-skeleton length within the biomechanical and aerodynamic constraints acting upon the scaling of b (M
1/3) [1]. Similarly, whether the scaling is driven by aerodynamics, feather biomechanical properties or a combination of both requires further investigation.
Acknowledgments
We thank M. McNall for collections access in Victoria. Comments by three reviewers, X. Wang and A. Farke improved the clarity of this paper.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Nudds RL. Wing-bone length allometry in birds. Journal of Avian Biology. 2007;38:515–519. [Google Scholar]
- 2.Greenewalt CH. Dimensional relationships for flying animals. Smithsonian Miscellaneous Collection. 1962;144:1–46. [Google Scholar]
- 3.Norberg UM. Vertebrate flight: Mechanics, physiology, morphology, ecology and evolution. Berlin: Springer - Verlag; 1990. [Google Scholar]
- 4.Rayner JMV. Form and function in avian flight. In: Johnston RF, editor. Current Ornithology. New York: Plenum Press; 1988. pp. 1–66. [Google Scholar]
- 5.Worcester SE. The scaling of the size and stiffness of primary flight feathers. Journal of Zoology London. 1996;239:609–624. [Google Scholar]
- 6.Nudds RL, Dyke GJ, Rayner JMV. Avian brachial index and wing-kinematics: putting movement back into bones. Journal of Zoology London. 2007;272:218–226. [Google Scholar]
- 7.Purvis A, Rambaut A. Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analysing comparative data. Computer Applications in the Biosciences. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- 8.Sibley CG, Ahlquist JE. Phylogeny and classification of birds: a study in molecular evolution. New Haven and London: Yale University Press; 1990. [Google Scholar]
- 9.Livezey BC, Zusi RL. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. II. Analysis and discussion. Zoological Journal Of The Linnean Society. 2007;149:1–95. doi: 10.1111/j.1096-3642.2006.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McArdle BH. The structural relationship: regression in biology. Canadian Journal of Zoology. 1988;66:2329–2339. [Google Scholar]
- 11.Rayner JMV. Linear relations in biomechanics: the statistics of scaling functions. Journal of Zoology London. 1985;206:415–439. [Google Scholar]
- 12.Ricker WE. Linear regressions in fishery research. Journal of the Fisheries Research Board of Canada. 1973;30:409–434. [Google Scholar]
- 13.Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. New York: W. H. Freeman and Company; 1995. p. 887 p. [Google Scholar]
- 14.Pennycuick CJ. Bird flight performance: a practical calculation manual. Oxford: Oxford University Press; 1989. [Google Scholar]
- 15.Dial KP, Goslow GE, Jenkins FA. The functional anatomy of the shoulder in the European starling (Sturnus vulgaris). Journal of Morphology. 1991;207:327–344. doi: 10.1002/jmor.1052070309. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins FA, Dial KP, Goslow GE. A cineradiographic analysis of bird flight: the wishbone in starlings is a spring. Science. 1988;241:1495–1497. doi: 10.1126/science.241.4872.1495. [DOI] [PubMed] [Google Scholar]
- 17.Tobalske BW, Warrick DR, Clark CJ, Powers DR, Hedrick TL, et al. Three dimensional kinematics of hummingbird flight. Journal of Experimental Biology. 1996;210:2368–2382. doi: 10.1242/jeb.005686. [DOI] [PubMed] [Google Scholar]
- 18.Greenewalt CH. The flight of birds. Transactions of the American Philosophical Society. 1975;65:1–67. [Google Scholar]