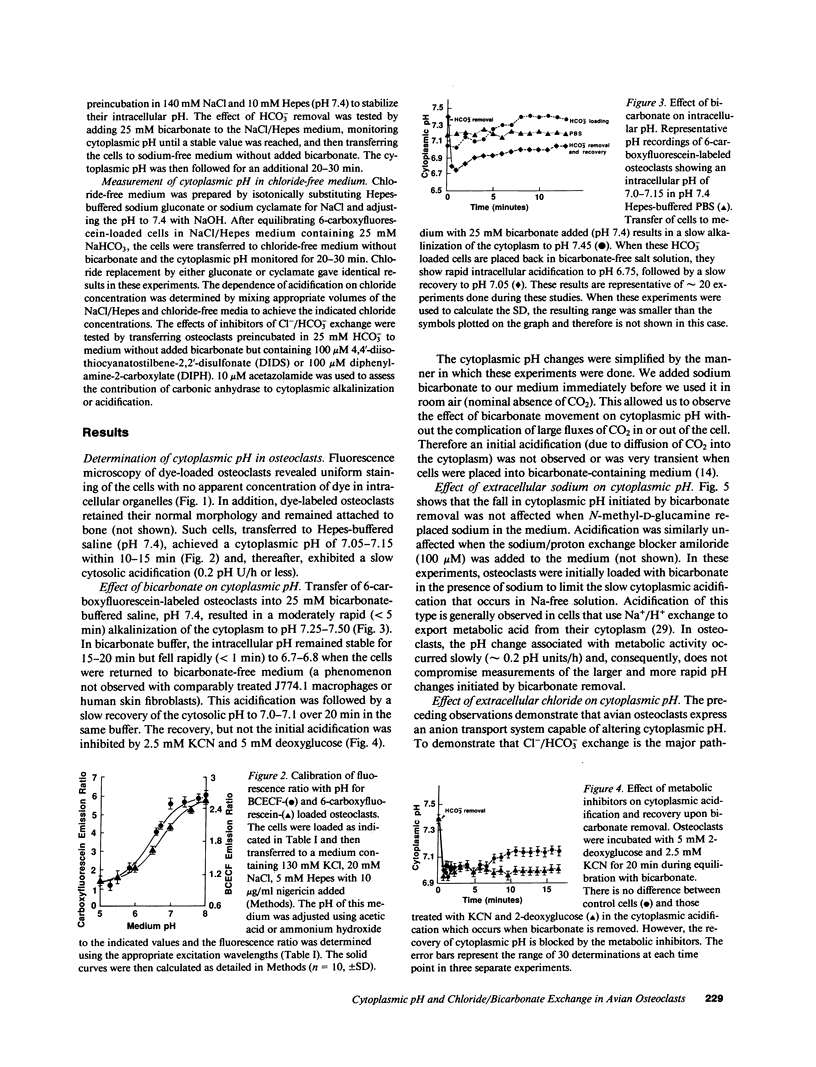
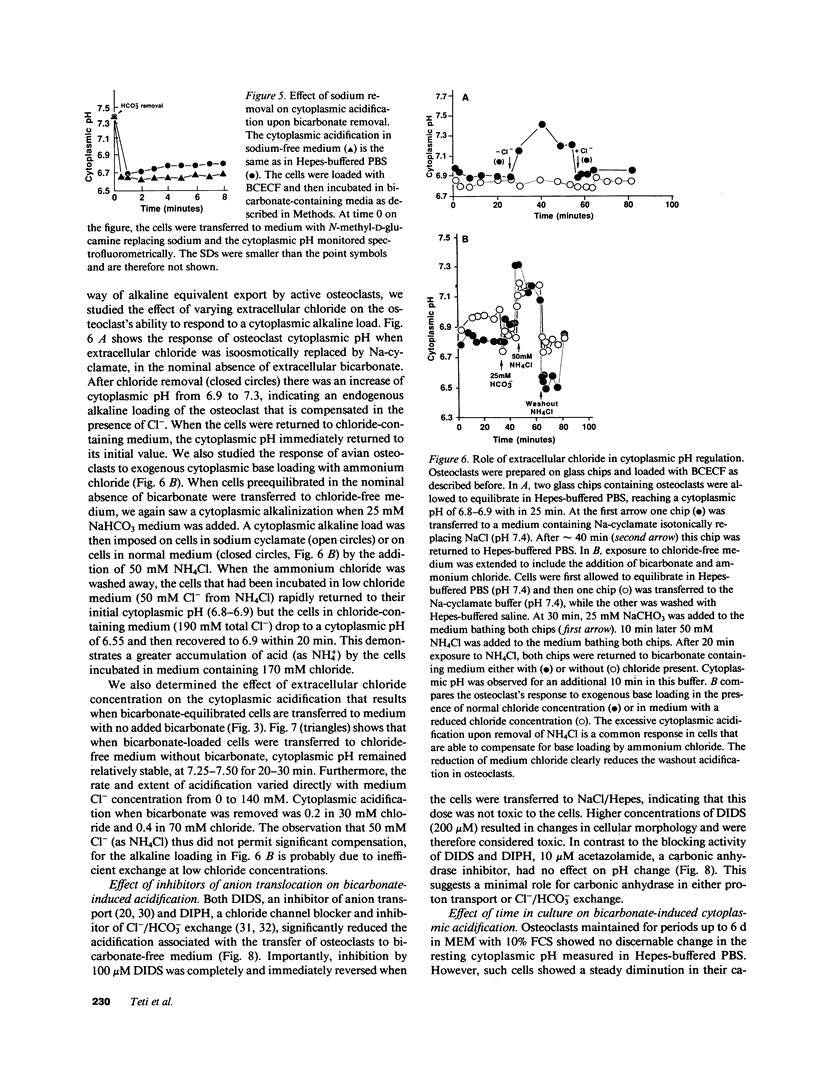
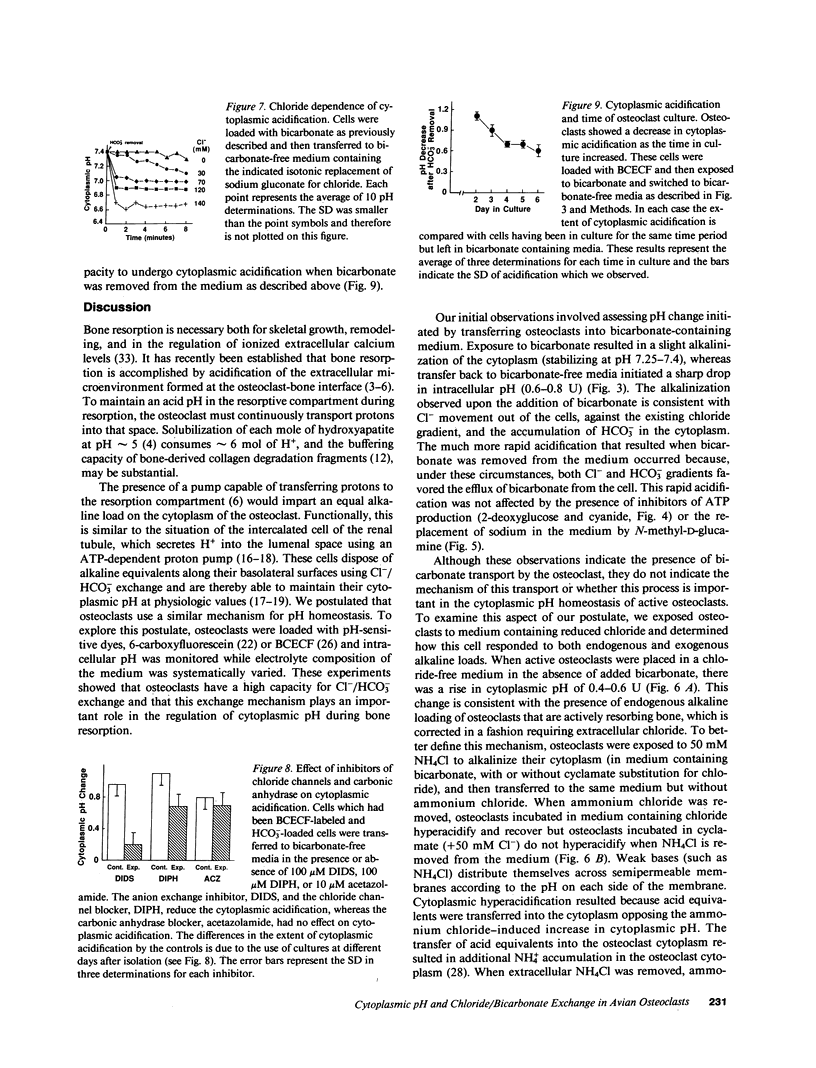
Abstract
Osteoclasts resorb bone by first attaching to the bone surface and then secreting protons into an isolated extracellular compartment formed at the cell-bone attachment site. This secretion of protons (local acidification) is required to solubilize bone hydroxyapatite crystals and for activity of bone collagen-degrading acid proteases. However, the large quantity of protons required, 2 mol/mol of calcium, would result in an equal accumulation of cytosolic base equivalents. This alkaline load must be corrected to maintain cytosolic pH within physiologic limits. In this study, we have measured cytoplasmic pH with pH-sensitive fluorescent compounds, while varying the extracellular ionic composition of the medium, to determine the nature of the compensatory mechanism used by osteoclasts during bone resorption. Our data show that osteoclasts possess a chloride/bicarbonate exchanger that enables them to maintain normal intracellular pH in the face of a significant proton efflux. This conclusion follows from the demonstration of a dramatic cytoplasmic acidification when osteoclasts that have been incubated in bicarbonate-containing medium are transferred into bicarbonate-free medium. This acidification is absolutely dependent on and proportional to medium [Cl-]. Furthermore, acidification is inhibited by the classic inhibitor of red cell anion exchange, 4,4'-diisothiocyanatostilbene-2,2'-disulfonate, and by diphenylamine-2-carboxylate, an inhibitor of chloride specific channels. However, the acidification process is neither energy nor sodium dependent. The physiologic importance of chloride/bicarbonate exchange is demonstrated by the chloride dependence of recovery from an endogenous or exogenous alkaline load in osteoclasts. We conclude that chloride/bicarbonate exchange is in large part responsible for cytoplasmic pH homeostasis of active osteoclasts, showing that these cells are similar to renal tubular epithelial cells in their regulation of intracellular pH.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron R., Neff L., Louvard D., Courtoy P. J. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J Cell Biol. 1985 Dec;101(6):2210–2222. doi: 10.1083/jcb.101.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H. C., Kahn A. J., Crouch E. C., Jeffrey J. J., Teitelbaum S. L. Isolated osteoclasts resorb the organic and inorganic components of bone. J Cell Biol. 1986 Apr;102(4):1164–1172. doi: 10.1083/jcb.102.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt B. C., Cassola A. C., Frömter E. Electrophysiological analysis of bicarbonate permeation across the peritubular cell membrane of rat kidney proximal tubule. II. Exclusion of HCO3(-)-effects on other ion permeabilities and of coupled electroneutral HCO3(-)-transport. Pflugers Arch. 1984 May;401(1):43–51. doi: 10.1007/BF00581531. [DOI] [PubMed] [Google Scholar]
- CRETIN A. Contribution histochimique à l'étude de la construction et de la destruction osseuse. Presse Med. 1951 Sep 22;59(60):1240–1242. [PubMed] [Google Scholar]
- Doty S. B., Schofield B. H. Electron microscopic localization of hydrolytic enzymes in osteoclasts. Histochem J. 1972 May;4(3):245–258. doi: 10.1007/BF01890996. [DOI] [PubMed] [Google Scholar]
- Eilon G., Raisz L. G. Comparison of the effects of stimulators and inhibitors of resorption on the release of lysosomal enzymes and radioactive calcium from fetal bone in organ culture. Endocrinology. 1978 Dec;103(6):1969–1975. doi: 10.1210/endo-103-6-1969. [DOI] [PubMed] [Google Scholar]
- Gay C. V., Ito M. B., Schraer H. Carbonic anhydrase activity in isolated osteoclasts. Metab Bone Dis Relat Res. 1983;5(1):33–39. doi: 10.1016/0221-8747(83)90048-6. [DOI] [PubMed] [Google Scholar]
- Gluck S., Al-Awqati Q. An electrogenic proton-translocating adenosine triphosphatase from bovine kidney medulla. J Clin Invest. 1984 Jun;73(6):1704–1710. doi: 10.1172/JCI111378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göthlin G., Ericsson J. L. Fine structural localization of acid phosphomonoesterase in the brush border region of osteoclasts. Histochemie. 1971;28(4):337–344. doi: 10.1007/BF00702639. [DOI] [PubMed] [Google Scholar]
- Hall G. E., Kenny A. D. Role of carbonic anhydrase in bone resorption induced by 1,25 dihydroxyvitamin D3 in vitro. Calcif Tissue Int. 1985 Mar;37(2):134–142. doi: 10.1007/BF02554832. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A., Jr, Frazier W. A., Schlesinger P. H. Transient increase in intracellular pH during Dictyostelium differentiation. J Cell Biol. 1984 Nov;99(5):1883–1887. doi: 10.1083/jcb.99.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen B. M. Conductive properties of the rabbit outer medullary collecting duct: inner stripe. Am J Physiol. 1985 Apr;248(4 Pt 2):F500–F506. doi: 10.1152/ajprenal.1985.248.4.F500. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Schlesinger P. H. A perspective on antimalarial action: effects of weak bases on Plasmodium falciparum. Biochem Pharmacol. 1986 Feb 15;35(4):547–552. doi: 10.1016/0006-2952(86)90345-x. [DOI] [PubMed] [Google Scholar]
- Lombard W. E., Kokko J. P., Jacobson H. R. Bicarbonate transport in cortical and outer medullary collecting tubules. Am J Physiol. 1983 Mar;244(3):F289–F296. doi: 10.1152/ajprenal.1983.244.3.F289. [DOI] [PubMed] [Google Scholar]
- Lucht U. Acid phosphatase of osteoclasts demonstrated by electron microscopic histochemistry. Histochemie. 1971;28(2):103–117. doi: 10.1007/BF00279855. [DOI] [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Naldini L., Primavera M. V., Teti A., Zambonin-Zallone A. Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J Cell Biol. 1984 Nov;99(5):1696–1705. doi: 10.1083/jcb.99.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Teti A., Zambonin-Zallone A., Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res. 1987 Mar;169(1):202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G. Physiologic and pharmacologic regulation of bone resorption. N Engl J Med. 1970 Apr 16;282(16):909–916. doi: 10.1056/NEJM197004162821608. [DOI] [PubMed] [Google Scholar]
- Reuss L., Costantin J. L., Bazile J. E. Diphenylamine-2-carboxylate blocks Cl(-)-HCO3- exchange in Necturus gallbladder epithelium. Am J Physiol. 1987 Jul;253(1 Pt 1):C79–C89. doi: 10.1152/ajpcell.1987.253.1.C79. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg P., Glaser L., Schlesinger P., Cassel D. Activation of Na+/H+ exchange by epidermal growth factor elevates intracellular pH in A431 cells. J Biol Chem. 1983 Oct 25;258(20):12644–12653. [PubMed] [Google Scholar]
- Sly W. S., Hewett-Emmett D., Whyte M. P., Yu Y. S., Tashian R. E. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A. 1983 May;80(9):2752–2756. doi: 10.1073/pnas.80.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz P. R., Andersen O. S. Electrogenic proton transport in epithelial membranes. J Membr Biol. 1982;65(3):155–174. doi: 10.1007/BF01869960. [DOI] [PubMed] [Google Scholar]
- Stone D. K., Seldin D. W., Kokko J. P., Jacobson H. R. Anion dependence of rabbit medullary collecting duct acidification. J Clin Invest. 1983 May;71(5):1505–1508. doi: 10.1172/JCI110905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum S. L., Stewart C. C., Kahn A. J. Rodent peritoneal macrophages as bone resorbing cells. Calcif Tissue Int. 1979 Jul 3;27(3):255–261. doi: 10.1007/BF02441194. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Vaes G. On the mechanisms of bone resorption. The action of parathyroid hormone on the excretion and synthesis of lysosomal enzymes and on the extracellular release of acid by bone cells. J Cell Biol. 1968 Dec;39(3):676–697. doi: 10.1083/jcb.39.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambonin Zallone A., Teti A., Primavera M. V. Isolated osteoclasts in primary culture: first observations on structure and survival in culture media. Anat Embryol (Berl) 1982 Dec;165(3):405–413. doi: 10.1007/BF00305576. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Silva P., Seifter J. L. Intracellular pH regulation and proton transport by rabbit renal medullary collecting duct cells. Role of plasma membrane proton adenosine triphosphatase. J Clin Invest. 1986 Jan;77(1):113–120. doi: 10.1172/JCI112264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidel M. L., Silva P., Seifter J. L. Intracellular pH regulation in rabbit renal medullary collecting duct cells. Role of chloride-bicarbonate exchange. J Clin Invest. 1986 May;77(5):1682–1688. doi: 10.1172/JCI112486. [DOI] [PMC free article] [PubMed] [Google Scholar]